Diet in childhood and adolescence has been suggested to have a potential lifelong effect on the development of many chronic diseases such as obesity( Reference Dietz 1 ) and CVD( Reference McGill 2 ). Additionally, evidence suggests that dietary patterns established during childhood and adolescence persist into adulthood at least to some extent( Reference te Velde, Twisk and Brug 3 , Reference Bertheke Post, de Vente and Kemper 4 ). Thus, investigation of dietary behaviours that contribute to healthier dietary patterns in children and adolescents is a high public health priority. In this regard, eating frequency (EF), i.e. sum of meal frequency (MF) and snack frequency (SF), has received increased attention because of an increasing trend in EF in representative samples of US children and adolescents between the 1970s and 2000s( Reference Popkin and Duffey 5 ), in parallel with an increasing prevalence of overweight and obesity( Reference Fryar, Carroll and Ogden 6 ). Nevertheless, research on the association between EF and dietary intake is extremely limited in young populations( Reference Evans, Jacques and Dallal 7 ).
Investigation of EF in relation to dietary intake in adult populations has also produced inconsistent associations( Reference Titan, Bingham and Welch 8 – Reference Leech, Worsley and Timperio 14 ). However, several methodological limitations make it difficult to interpret these studies. First, EF has often been estimated using a series of self-report questions( Reference Titan, Bingham and Welch 8 – Reference Berteus Forslund, Torgerson and Sjostrom 11 , Reference Marin-Guerrero, Gutierrez-Fisac and Guallar-Castillon 15 – Reference Howarth, Huang and Roberts 19 ), the validity of which has not been examined or reported. Only a limited number of studies have estimated EF with the use of information on actual dietary behaviours (based on dietary record or 24 h recall)( Reference Drummond, Crombie and Cursiter 20 – Reference Murakami and Livingstone 24 ). Second, there is no consensus about what constitutes a snack, a meal or an eating occasion. While some studies have relied on respondents’ self-identification of meals, snacks or eating occasions( Reference Titan, Bingham and Welch 8 – Reference Berteus Forslund, Torgerson and Sjostrom 11 , Reference Marin-Guerrero, Gutierrez-Fisac and Guallar-Castillon 15 – Reference van der Heijden, Hu and Rimm 18 , Reference Hartline-Grafton, Rose and Johnson 25 , Reference Mills, Perry and Reicks 26 ), others have tried to apply more objective criteria( Reference Howarth, Huang and Roberts 19 – Reference Murakami and Livingstone 24 , Reference Ruidavets, Bongard and Bataille 27 – Reference Yannakoulia, Melistas and Solomou 30 ). Third, the associations between EF and dietary intake may be confounded by the under-reporting of EF accompanied by the under-reporting of energy intake (EI), particularly by obese or overweight individuals( Reference Zizza, Arsiwalla and Ellison 12 , Reference Zizza and Xu 13 ). Taken together, the discrepant findings are not surprising and merit more robust data analyses than hitherto to resolve this issue.
Furthermore, potentially different effects of MF and SF have not been investigated using different definitions of meals and snacks. An accurate distinction between meals and snacks is important, because they are hypothesized to exert different effects on EI and dietary intake( Reference Leech, Worsley and Timperio 14 ). This is also important for the development of science-based recommendations on snack and meal patterns for consumers( Reference Johnson and Anderson 31 ). Moreover, in the absence of a universally accepted definition of meals and snacks, an understanding of the influence of different meal and snack definitions on the associations with diet quality may facilitate the interpretation of the existing literature and help establish consensus on the most appropriate research definition for meals and snacks( Reference Leech, Worsley and Timperio 14 ).
The aim of the present cross-sectional study in a representative sample of US children and adolescents based on the data from the National Health and Nutrition Examination Survey (NHANES) was to examine the relationship of MF and SF with diet quality, by focusing on the confounding of EI misreporting and the use of different definitions of meals and snacks.
Methods
Survey design and analytic sample
The present cross-sectional analysis was based on public domain data from NHANES, a continuing population-based survey that uses a complex, stratified multistage probability sample design to create a representative sample of the non-institutionalized civilian US population( Reference Zipf, Chiappa and Porter 32 , Reference Johnson, Paulose-Ram and Ogden 33 ). The survey examines about 5000 persons each year and the data are released in two-year cycles. The unweighted response rates for the examined persons aged 6–19 years for NHANES 2003–2004, 2005–2006, 2007–2008, 2009–2010 and 2011–2012 were 84 %, 83 %, 83 %, 86 % and 77 %, respectively( 34 ). The documentation and data for each of these surveys used were downloaded from the NHANES website( 35 ).
The analytic sample was limited to children and adolescents aged 6–19 years with two complete and reliable, self-reported 24 h dietary recall data determined by the National Center for Health Statistics (n 11712). After excluding pregnant (n 96) and lactating (n 15) respondents as well as those with missing information on the variables of interest (n 672 for family poverty income ratio; n 446 for education of household head; n153 for body height or weight; n 3 for some EF variables; some respondents had more than one missing value), the final analytic sample included 10 462 respondents from NHANES 2003–2012.
Dietary assessment
All surveys collected dietary information with the use of two 24 h dietary recalls (one by face-to-face interview and one by telephone 3–10 d after the first recall). The dietary data were collected with the use of an automated five-step multiple pass approach, namely the US Department of Agriculture’s Automated Multiple-Pass Method( 35 – Reference Conway, Ingwersen and Moshfegh 39 ). Participants were asked to report the time each food and beverage was eaten and to classify each eating occasion from a predefined list of categories, which were used to define meals and snacks, as described later. Proxies, most commonly a parent, assisted with the dietary interview for children aged 6–11 years; dietary intake was self-reported by adolescents aged 12–19 years. Estimates of intakes of energy and selected nutrients from all reported foods and beverages were calculated by using the US Department of Agriculture’s Food and Nutrient Database for Dietary Studies( 35 ). The mean of dietary intake over the 2 d for each participant was used for the present analysis.
Assessment of diet quality
As a measure of diet quality, the Healthy Eating Index (HEI)-2010 was applied. Food group and nutrient component standards and the development and evaluation of the HEI-2010 have been described previously( Reference Guenther, Casavale and Reedy 40 , Reference Guenther, Kirkpatrick and Reedy 41 ). Briefly, the HEI-2010 is an index that reflects conformance/adherence to the 2010 Dietary Guidelines for Americans( 42 ), including twelve components. Of these, nine components assess adequacy of the diet (total fruit; whole fruit; total vegetables; greens and beans; whole grains; dairy; total protein foods; seafood and plant proteins; fatty acids as the ratio of PUFA and MUFA to SFA), with higher scores reflecting better diet quality. The remaining three components assess moderation of the diet (refined grains; sodium; empty calories, i.e. energy from solid fats, alcohol and added sugars), with higher scores reflecting better diet quality (lower consumption). A density approach is used to set standards (i.e. per 4184 kJ of energy or as a percentage of energy). The scores of the twelve components are summed to a total score (i.e. HEI-2010), the maximum of which is 100. The SAS code used to calculate HEI-2010 was downloaded from the Center for Nutrition Policy and Promotion’s website( 43 ).
Definition of eating frequency, meal frequency and snack frequency
Data from the two 24 h dietary recalls were also used to calculate the average number of eating occasions per day, i.e. EF. Eating occasions were defined as any occasion when any food or drink was consumed( Reference Murakami and Livingstone 24 – Reference Mills, Perry and Reicks 26 , Reference Yannakoulia, Melistas and Solomou 30 ). In many previous studies, if two eating occasions occurred in ≤15 min, both events were counted as a single eating occasion; when >15 min separated two eating occasions, these were considered distinct eating occasions( Reference Drummond, Crombie and Cursiter 20 , Reference Ma, Bertone and Stanek 21 , Reference Duval, Strychar and Cyr 23 , Reference Murakami and Livingstone 24 , Reference Yannakoulia, Melistas and Solomou 30 ). In the present study, however, all foods and beverages reported at one discrete clock time were considered as part of one eating occasion, because almost all eating episodes (>99·5 %) occurred at least 15 min apart in NHANES( Reference Kant and Graubard 44 ). EF was calculated based on all eating occasions except for those providing <210 kJ of energy. This calculation method has been used in several previous studies( Reference Ma, Bertone and Stanek 21 , Reference Murakami and Livingstone 24 – Reference Mills, Perry and Reicks 26 , Reference Yannakoulia, Melistas and Solomou 30 ) and was chosen to avoid giving undue weight to eating occasions that included only water, low-calorie beverages or small quantities of foods.
All eating occasions were divided into either meals or snacks with the use of three different published definitions: on the basis of (i) contribution to total EI( Reference Ritchie 45 ); (ii) self-reported name of eating occasion( Reference Kant and Graubard 44 ); and (iii) clock time( Reference Jennings, Cassidy and van Sluijs 46 ). For the first definition( Reference Ritchie 45 ), a meal was defined as any eating episode comprising ≥15 % of total EI, regardless of the time of day or composition of foods or beverages consumed. All other eating episodes were classified as a snack. For each participant, MF and SF determined based on percentage contribution to total EI were thus calculated (hereafter referred to as MFenergy% and SFenergy%, respectively). For the second definition( Reference Kant and Graubard 44 ), any eating occasions with self-reported name of ‘breakfast’, ‘brunch’, ‘lunch’, ‘supper’ and ‘dinner’ or their equivalents in Spanish were considered meals. All other self-reported eating events (i.e. ‘snack’, ‘drink’ and ‘extended consumption’ or their equivalents in Spanish, as well as ‘other’ and ‘don’t know’) were considered snacks. For each participant, MF and SF determined based on self-report were thus calculated (hereafter referred to as MFself-report and SFself-report, respectively). For the third definition( Reference Jennings, Cassidy and van Sluijs 46 ), meals were defined as eating events reported during selected times of the day, i.e. 06.00–09.00, 12.00–14.00 and 17.00–20.00 hours. All other eating occasions were considered snacks. For each participant, MF and SF determined based on the time consumed were thus calculated (hereafter referred to as MFtime and SFtime, respectively).
Assessment of non-dietary variables
Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican American, and others. As indicators of socio-economic status, we considered family income as a percentage of the federal poverty threshold and years of education of the household head. The family poverty income ratio was categorized as <130 %, 130–349 % and ≥350 %( Reference Kant and Graubard 47 ). The educational level of the household head was categorized as <12 years, 12 years, some college, and college degree or more. Information on household size (≤2, 3–4 or ≥5) was also collected. The physical activity variable was created as follows. For children aged 6–11 years, the response to the question on the number of times per week that play or exercise was hard enough to induce sweat in the past 7 d (NHANES 2009–2012) or without a specified period (NHANES 2003–2008): 0–3 times=low; 4–6 times=moderate; and 7 times=active. For adolescents aged 12–19 years, the responses to two different questions on any leisure-time moderate or vigorous activities lasting ≥10 min in the past 30 d (NHANES 2003–2006) or without a specified period (NHANES 2007–2012): no to both two questions=low; yes to one question=moderate; and yes to both two questions=active. The hours of screen time were determined from questions on television/video watching (h/d) or computer use (h/d) over the past 30d (except for adolescents aged 12–19 years in NHANES 2007–2008 and 2009–2010, for whom information on sedentary activity was used), which were categorized as <2, ≥2 to <4, ≥4 to <6 and ≥6 h/d. BMI (kg/m2) was calculated as weight (in kilograms) divided by height (in metres) squared. The percentile of BMI-for-age was calculated using the SAS program for growth charts available from the Centers for Disease Control and Prevention( Reference Kuczmarski, Ogden and Guo 48 , 49 ). Weight status was defined based on the percentile of BMI-for-age as follows( 50 ): underweight (<5th percentile); normal (≥5th to <85th percentile); overweight (≥85th to <95th percentile); and obese (≥95th percentile).
Misreporting of EI was evaluated based on the ratio of EI to estimated energy requirement (EER). EER was calculated using sex-, age- and weight status-specific equations published from the US Dietary Reference Intakes, based on sex, age, body height and weight, and physical activity( 51 ). Because of a lack of an objective measure of physical activity in the present study, we assumed ‘low active’ level of physical activity (i.e. physical activity level ≥1·4 to <1·6)( 51 ) for all participants during this calculation. The 95 % confidence limits of the expected EI:EER of 1·0 (i.e. 0 on the natural log scale) were calculated, taking into account coefficients of variation in intakes and other components of energy balance (i.e. the within-subject variation in EI=23 %; the error in the EER equations=4·8 %; the day-to-day variation in total energy expenditure=8·2 %)( Reference Huang, Howarth and Lin 52 ). Consequently, under-, acceptable and over-reporters were defined as having EI:EER <0·69, 0·69–1·46 and >1·46, respectively.
Statistical analysis
Statistical analyses were performed for children aged 6–11 years (n4269) and adolescents aged 12–19 years (n 6193) separately, using the statistical software package SAS version 9·2. All of the analyses used the NHANES-provided sampling weights that were calculated to take into account unequal probabilities of selection resulting from the sample design, non-response and planned oversampling of selected subgroups, so that the results are representative of the US community-dwelling population( Reference Johnson, Paulose-Ram and Ogden 33 , 53 ). Linear regression analyses were performed to explore the associations of EF, MF and SF (independent variables) with dietary intake variables (dependent variables). EF, MF and SF were analysed continuously after confirming the linearity of relationships using tertile, quartile and quintile categories. With the use of the PROC SURVEYREG procedure, we calculated the adjusted regression coefficients (with standard errors) of variation of dietary intakes by one increase of EF, MF and SF. Potential confounding factors considered were sex, age, race/ethnicity, family poverty income ratio, education of household head, household size, physical activity, watching television and computer use, weight status, dietary reporting status and survey cycle. We repeated the analyses without adjustment for dietary reporting status as well as those after excluding under- and over-reporters. All reported P values are two-tailed, and P<0·01 was considered statistically significant to minimize the chance of a type 1 error arising from multiple testing.
Results
Basic characteristics of the participants are shown in Table 1. The mean value of EF was higher in children than in adolescents. The mean values of MF and SF were also higher in children than in adolescents irrespective of the definition applied, except for no difference in SFtime.
Table 1 Basic characteristics of the sample of US children and adolescents: National Health and Nutrition Examination Survey 2003–2012Footnote *
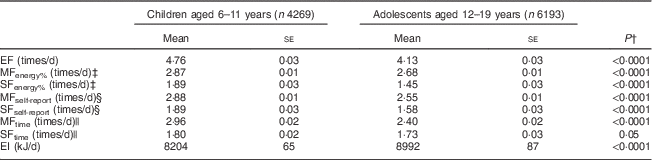
EF, eating frequency; MFenergy%, meal frequency (MF) determined based on percentage contribution to total EI; SFenergy%, snack frequency (SF) determined based on percentage contribution to total EI; MFself-report, MF determined based on self-report; SFself-report, SF determined based on self-report; MFtime, MF determined based on the time consumed; SFtime, SF determined based on the time consumed; EI, energy intake.
* Analyses are based on participants with complete data on two 24 h dietary recalls as well as complete information on the variables of interest. All dietary variables are based on mean values of two 24 h dietary recalls.
† P values for differences between children and adolescents based on independent t test.
‡ A meal was defined as any eating episode comprising ≥15 % of total EI, regardless of the time of day or composition of foods and beverages consumed; all other eating episodes were classified as snacks.
§ Self-reports of breakfast, brunch, lunch, supper and dinner or their equivalents in Spanish were considered as meals; all other self-reported eating events were considered as snacks.
|| Meals were defined as eating events reported during selected times of the day (0.600–09.00, 12.00–14.00 and 17.00–20.00 hours); all other eating occasions were considered as snacks.
Table 2 shows EF, MFself-report and SFself-report (as examples) according to categories of participants’ characteristics. In both age groups, there was no sex difference in any measures. For children EF and SFself-report were the highest in non-Hispanic whites and MFself-report was the highest in Mexican Americans, with the lowest in non-Hispanic blacks. For adolescents EF was the highest in others and MFself-report was the highest in non-Hispanic whites, with the lowest in non-Hispanic blacks. Only in adolescents, there were positive associations between family poverty income ratio and EF and MFself-report. Education of the household head was positively associated with MFself-report in both children and adolescents. Household size showed an association with MFself-report only in adolescents, with the highest value in the ‘3–4’ group and the lowest in the ‘≤2’ group. All EF, MFself-report and SFself-report were positively associated with physical activity in adolescents. Watching television and computer use was inversely associated with MFself-report only in children and with EF only in adolescents. SFself-report was highest in the ‘≥4 to <6 h/d’ group and lowest in the ‘≥6 h/d’ group only in adolescents. Weight status was inversely associated with all EF, MFself-report and SFself-report in adolescents, while in children MFself-report was the highest in those who were underweight and the lowest in those who were overweight. Over-reporters had the highest values for all measures, with under-reporters having the lowest values. In adolescents, survey cycle was associated positively with MFself-report and inversely with SFself-report.
Table 2 Eating frequency, meal frequency and snack frequency according to categories of participants’ characteristics: US children and adolescents, National Health and Nutrition Examination Survey 2003–2012Footnote *

EF, eating frequency; MFself-report, meal frequency determined based on self-report; SFself-report, snack frequency determined based on self-report.
* Analyses are based on participants with complete data on two 24 h dietary recalls as well as complete information on the variables of interest. All dietary variables are based on mean values of two 24 h dietary recalls.
† Self-reports of breakfast, brunch, lunch, supper and dinner or their equivalents in Spanish were considered as meals; all other self-reported eating events were considered as snacks.
‡ Based on Wald’s F test.
§ The physical activity variable was created as follows. For children aged 6–11 years, the response to the question on the number of times per week that play or exercise was hard enough to induce sweat in the past 7 d (NHANES 2009–2012) or without a specified period (NHANES 2003–2008): 0–3 times=low; 4–6 times=moderate; and 7 times=active. For adolescents aged 12–19 years, the responses to two different questions on any leisure-time moderate or vigorous activities lasting ≥10 min in the past 30 d (NHANES 2003–2006) or without a specified period (NHANES 2007–2012): no to both two questions=low; yes to one question=moderate; and yes to both two questions=active.
|| Defined based on the percentile of BMI-for-age( 50 ): <5th percentile for underweight; ≥5th to <85th percentile for normal; ≥85th to <95th percentile for overweight; and ≥95th percentile for obese.
¶ Defined based on ratio of reported energy intake to estimated energy requirement: <0·69 for under-reporting; 0·69–1·46 for acceptable reporting; and >1·46 for over-reporting.
Associations of EF, MF and SF with HEI-2010 and its component intakes are presented in Table 3. Irrespective of the definition of meals, MF was positively associated with HEI-2010 in both age groups. In children, one additional meal per day defined by MFenergy%, MFself-report and MFtime increased HEI-2010 by 1·45, 3·59 and 1·72 points, respectively. The corresponding value in adolescents was 1·74, 3·56 and 1·99 points, respectively. All measures of MF also showed positive associations with whole grains. There was a positive association between MFenergy% and total fruit in children, while in adolescents MFenergy% was associated positively with dairy and inversely with total vegetables, fatty acids ratio and sodium. MFself-report was positively associated with total fruit, whole fruit (adolescents only) and dairy, and inversely with the fatty acids ratio (adolescents only) and empty calories. MFtime showed positive associations with total fruit, whole fruit, dairy (adolescents only), and seafood and plant proteins (adolescents only), and inverse associations with total protein foods, fatty acids ratio (adolescents only), sodium (children only) and empty calories (children only).
Table 3 Associations of eating frequency, meal frequency and snack frequency with the Healthy Eating Index (HEI)-2010 and its component intakes: US children and adolescents, National Health and Nutrition Examination Survey 2003–2012Footnote *

EF, eating frequency; MFenergy%, meal frequency (MF) determined based on percentage contribution to total energy intake; SFenergy%, snack frequency (SF) determined based on percentage contribution to total energy intake; MFself-report, MF determined based on self-report; SFself-report, SF determined based on self-report; MFtime, MF determined based on the time consumed; SFtime, SF determined based on the time consumed; eq., equivalent.
* Analyses are based on participants with complete data on two 24 h dietary recalls as well as complete information on the variables of interest. All dietary variables are based on mean values of two 24 h dietary recalls. Adjustment was made for sex (boys or girls), age (years, continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, or others), family poverty income ratio (<130 %, 130–349 % or ≥350 %), education of household head (<12 years, 12 years, some college, or college degree or more), household size (≤2, 3–4 or ≥5), physical activity (low, moderate or active), watching television and computer use (<2, ≥2 to <4, ≥4 to <6 or ≥6 h/d), weight status (underweight, normal, overweight or obese), dietary reporting status (under-reporting, acceptable reporting or over-reporting) and survey cycle (2003–2004, 2005–2006, 2007–2008, 2009–2010 or 2011–2012).
† A meal was defined as any eating episode comprising ≥15 % of total energy intake, regardless of the time of day or composition of foods and beverages consumed; all other eating episodes were classified as snacks.
‡ Self-reports of breakfast, brunch, lunch, supper and dinner or their equivalents in Spanish were considered as meals; all other self-reported eating events were considered as snacks.
§ Meals were defined as eating events reported during selected times of the day (0.600–09.00, 12.00–14.00 and 17.00–20.00 hours); all other eating occasions were considered as snacks.
|| Regression coefficients mean the change of dietary variables with one additional eating occasion per day.
¶ Ratio of PUFA and MUFA to SFA.
** Energy from solid fats, alcohol and added sugars.
The associations for SF varied depending on the definition of snacks. While SFenergy% was positively associated with HEI-2010 in both children and adolescents (0·70 and 1·00 point increase by one additional snack, respectively), SFself-report and SFtime showed no associations. SFenergy% was associated positively with total fruit, whole fruit, and seafood and plant proteins (adolescents only), and inversely with total protein foods and sodium. SFself-report showed positive associations with total fruit, whole fruit and empty calories, and inverse associations with total vegetables, dairy (adolescents only), total protein foods, refined grains and sodium. SFtime was positively associated with empty calories and inversely with total protein foods and sodium. EF was positively associated with HEI-2010, total fruit, whole fruit, whole grains, and seafood and plant proteins (adolescents only), and inversely with total protein foods, fatty acids ratio (adolescents only), refined grains and sodium.
All analyses were conducted without adjustment for dietary reporting status (data not shown) or after excluding under- and over-reporters (n 447 and 397 in children and 2115 and 326 in adolescents, respectively; see online supplementary material, Supplemental Table 1), providing similar results.
Discussion
To our knowledge, the present study is the first to examine associations of different measures of MF and SF with diet quality, after taking into account the confounding of EI misreporting. In a representative sample of US children and adolescents, higher MF was associated with higher diet quality as assessed by HEI-2010. This was not dependent on the definition of meals. The associations were not confounded by misreporting of EI, as the results did not change before and after adjustment for EI misreporting and when only those with plausible EI were analysed. However, the associations for SF varied depending on the definition of snacks. While SF based on energy contribution was positively associated with diet quality, there were no associations for SF based on self-report or based on time. Thus, the study suggests the importance of the use of different definition of meals and snacks to better understand the effects of different dietary patterns on health.
While many epidemiological studies which have investigated the association between EF (i.e. sum of MF and SF) and dietary intakes have yielded inconsistent outcomes( Reference Titan, Bingham and Welch 8 – Reference Leech, Worsley and Timperio 14 ), there have been few attempts to investigate the effects of MF and SF separately. In Swedish adults, SF assessed by a questionnaire showed positive associations with intakes of confectionery, fat, sugar and alcohol, and inverse associations with intakes of protein and dietary fibre( Reference Berteus Forslund, Torgerson and Sjostrom 11 ). In contrast, SF calculated based on self-report was associated with higher intakes of vitamins A, C and E, β-carotene, Mg and K in US elderly people( Reference Zizza, Arsiwalla and Ellison 12 ). SF calculated based on self-report was also associated with a higher score for HEI-2005 in US adults( Reference Zizza and Xu 13 ). A higher MF was associated with higher diet quality as assessed by the Canadian HEI among Canadian elderly people, although no clear definition of meal was provided( Reference Shatenstein, Gauvin and Keller 54 ). A previous US study showed that SF calculated based on self-report, but not MF, was positively associated with diet quality (as assessed by the HEI-2005) in children (aged 9–11 years)( Reference Evans, Jacques and Dallal 7 ). Conversely, in adolescents (aged 12–15 years) SF was inversely associated with diet quality, while MF showed a positive association( Reference Evans, Jacques and Dallal 7 ). In the present study, while MF was associated with better diet quality irrespective of the definition of meals, the associations for SF varied depending on the definition of snacks. These inconsistent observations may be, at least partly, due to differences in the characteristics and lifestyles of the populations, definitions of MF and SF, dietary assessment methods and potential confounding factors considered.
While MF was more strongly associated with diet quality than SF based on any definition of meals and snacks we applied, the difference in the strength of the associations was the smallest when meals and snacks were defined based on EI contribution, suggesting that this definition may not appropriately differentiate meals and snacks in terms of nutritional quality of eating occasions. This may explain why only SFenergy% (but not SFself-report or SFtime) was positively associated with diet quality. Conversely, the difference in the strength of the associations was the largest when meals and snacks were defined based on participants’ self-report. Of particular interest is that higher MFself-report did not contribute to overconsumption of (unhealthy) foods but rather decreased the consumption of empty calories. Thus the classification of meals and snacks based on self-report, rather than contribution to total EI and time, may be more informative for investigating the effect of MF and SF on dietary intakes, at least in US children and adolescents. However, oversimplification should be avoided because there is no consensus about what constitutes a snack or a meal. For example, MF and SF based on self-report are subject to inconsistencies due to differences in individual perception( Reference Johnson and Anderson 31 ). In accordance with a previous analysis based on NHANES( Reference Kant and Graubard 44 ), eating occasions reported as ‘extended consumption’, ‘other’ and ‘don’t know’ were considered as snacks, but this may not be appropriate at least for some individuals; further research is needed to clarify the kind and the amount of foods consumed in such eating occasions. Additionally, MF and SF based on time may be problematic because eating patterns vary according to lifestyle (e.g. shift workers, individuals who consistently eat their meals at non-traditional times of day) as well as the cultural environment( Reference Johnson and Anderson 31 ). Furthermore, MF and SF based on energy contribution (≥15 % or <15 %) was made on the basis of the US adults’ national averages of the distribution of energy from (self-defined) meals compared with (self-defined) snacks (breakfast ≈ 16 %; lunch ≈ 25 %; dinner ≈ 37 %; and snack ≈ 22 % from two occasions)( Reference Kant and Graubard 44 ), but this may not be suitable in children and adolescents. Thus, results may possibly differ on the basis of other definitions. In any case, as research explicitly examining the impact of these different definitions is limited, further research using different definitions of meals and snacks is warranted.
The strengths of the present study include the use of a variety of published definitions of MF and SF based on detailed dietary information obtained from two 24 h dietary recalls and the use of individualized measures of EER to assess misreporting of EI in a large representative sample of US children and adolescents. However, there are also several limitations. First, due to the cross-sectional design of the present study, the assessment of causality cannot be addressed. Considering the advice frequently made by individuals in the health-care professions and in the lay press that those who want to lose weight should eat small, frequent meals( Reference Hartline-Grafton, Rose and Johnson 25 , Reference Mattson 55 ), overweight and obese individuals may increase their MF and EF. Alternatively, overweight and obese individuals may simply decrease their MF, SF and EF in an attempt to lose weight. To minimize the potential effect, we included weight status as a potential confounding factor.
All self-reported dietary assessment methods are subject to both random and systematic measurement errors( Reference Livingstone and Black 56 ). Given the day-to-day variability in eating patterns of free-living individuals, estimates of dietary intakes and behaviours derived from two 24 h dietary recalls unlikely represent the usual intakes or behaviours of individual respondents. Nevertheless, as this kind of random error would tend to result in bias towards attenuating rather than enhancing the relationship, more recall days would have provided stronger associations. A recent analysis based on NHANES( Reference Murakami and Livingstone 57 ) has shown that misreporting (under- and over-reporting) of EI is prevalent particularly in adolescents aged 12–19 years (32·6 % and 4·8 %, respectively), although the prevalence of under- and over-reporting in children aged 6–11 years (8·7 % and 9·6 %, respectively) was not high (this may, at least partly, be due to the parental help in the recall( Reference Murakami, Miyake and Sasaki 58 )). This kind of bias may distort true relationships between dietary behaviours and intakes or even create spurious ones. However, we believe that the associations observed here were not seriously influenced by misreporting of EI, because the results did not change before and after adjustment for EI misreporting and when only participants with plausible EI were analysed.
At present, the only way to obtain unbiased information on energy requirements in free-living settings is to use doubly labelled water( Reference Livingstone and Black 56 ). This technique is expensive and impractical for application in large-scale epidemiological studies. Instead, in the present study, EER was calculated using equations from the US Dietary Reference Intakes, which have been developed based on a large number of measurements of total energy expenditure by the doubly labelled water method and are highly accurate (R 2≥0·95)( 51 ). In the absence of actual, measured total energy expenditure, these equations should serve as the best proxy. Because of constraints within the data set, we did not have a validated and individualized measure of physical activity. Although some measures of self-reported physical activity were available, we decided not to use such variables in the present study because self-report of physical activity suffers from significant reporting bias mainly attributable to a combination of social desirability bias and the cognitive challenge associated with estimating frequency and duration of physical activity, particularly for children and adolescents( Reference Troiano, Berrigan and Dodd 59 ). Instead, we assumed ‘low active’ level of physical activity for all participants during the calculation of EER. This seems adequate for most US children and adolescents, based on accelerometer-based data in NHANES 2003–2006( Reference Troiano, Berrigan and Dodd 59 , Reference Belcher, Berrigan and Dodd 60 ). Nevertheless, in very active individuals (e.g. those aged 6–11 years, as has been reported)( Reference Belcher, Berrigan and Dodd 60 ), EER would be underestimated, resulting in an overestimation of EI:EER. Further, although we adjusted for a variety of potential confounding variables, residual confounding could not be ruled out. Finally, in view of the multiple analyses, it is possible that some of the findings in the present study occurred by chance.
Conclusion
In the present cross-sectional study in a representative sample of US children and adolescents based on NHANES 2003–2012, we showed positive associations between MF and diet quality as assessed by HEI-2010. These associations were observed whatever definition of meals, i.e. based on EI contribution, based on self-report or based on time, was applied and was not confounded by EI misreporting. In contrast, the associations for SF varied depending on the definition of snacks. While SF based on energy contribution was positively associated with diet quality, SF based on self-report or based on time showed no associations. In any case, replacing snack occasions to meal occasions may be a good strategy for improving diet quality. Future studies should divide EF into MF and SF based on a variety of definitions of meals and snacks so that any effects of different dietary patterns on health can be properly investigated.
Acknowledgements
Financial support: This work was supported in part by the Grants-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (K.M., grant number 15K16213). The Ministry of Education, Culture, Sports, Science and Technology of Japan had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: K.M. contributed to the concept and design of the study, statistical analysis, data interpretation and manuscript writing. M.B.E.L. critically reviewed the manuscript. Both authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by National Center for Health Statistics Research Ethics Review Board. Written informed consent was obtained from all participants or proxies.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980016000069






