The increasing of life expectancy has led to a rise in the prevalence and incidence of cognitive decline, dementia and Alzheimer’s disease, among other age-related diseases. In 2015, the number of people with dementia worldwide was estimated at 47 million and it is expected to increase to 152 million by 2050(1).
Due to the lack of disease-modifying treatments for any common dementia, in recent decades, there has been a steady increase in research into preventive measures, including lifestyle factors. In this context, dietary habits have been proposed as a potential alternative to prevent dementia and much attention of researchers and clinicians has focused on the Mediterranean diet (MedDiet)(Reference Petersson and Philippou2).
A number of clinical, epidemiological and experimental studies have suggested that the MedDiet or some of its components may reduce the incidence of neurodegenerative diseases and other conditions related to oxidative stress and chronic inflammation such as CVD, which are closely associated with cognitive decline(Reference Gardener and Caunca3,Reference Martínez-González, Gea and Ruiz-Canela4) . These effects can be partially attributed to different bioactive compounds present in the MedDiet such as polyphenols.
Polyphenols comprise a wide and diverse family of more than 8000 bioactive compounds containing phenol rings(Reference Tsao5). They are mainly grouped into flavonoids, phenolic acids, stilbenes, lignans and other polyphenols, and they are largely found in plant foods (whole cereals, fruits, nuts, legumes, extra virgin olive oil, etc.) and beverages (wine, tea, coffee, cocoa, etc.)(Reference Li, Tan and Wang6).
Due to the lipophilic nature of polyphenols, they have the ability to cross the blood–brain barrier and they may exert a series of beneficial effects on brain ageing(Reference Figueira, Tavares and Jardim7) through the maintenance of cerebral mass and mitochondrial integrity, prevention of the age-dependent decrease of monoaminergic neurotransmitters, modulation of some anti-ageing proteins and enzymes, anti-inflammatory and antioxidant capacities, and stimulation of factors involved in adult neurogenesis process(Reference Sarubbo, Moranta and Pani8). Thus, polyphenols may constitute a promising target for the prevention of cognitive impairment and Alzheimer’s disease. In fact, several intervention studies have shown the favourable effect of different subtypes of polyphenols on cognitive function and memory(Reference Evans, Howe and Wong9–Reference Whyte, Cheng and Fromentin11). Nevertheless, evidence on the association between total polyphenol intake and its main subclasses and cognitive function from prospective cohort studies is still limited.
In this context, the aim of this work was to address the association between total polyphenol and subclasses of polyphenols intake and 6-year change in cognitive function in the ‘Seguimiento Universidad de Navarra’ (SUN) Project, a prospective cohort of university graduates.
Materials and methods
Study population
The SUN Project is a prospective, dynamic, cohort study which started in December 1999 and includes more than 22 000 Spanish university graduates. Its aim is to assess the association between diet and other lifestyles with chronic diseases and mortality. The study design, methods and cohort profile have been previously reported in detail elsewhere(Reference Carlos, De La Fuente-Arrillaga and Bes-Rastrollo12).
Until May 2008, a total of 19 919 participants had completed the baseline questionnaire. Out of these, 1921 participants over 55 years of age were invited to participate in a sub-study designed to evaluate the effect of dietary and environmental factors in the development of cognitive impairment(Reference Muñoz-García, Martínez-González and Martín-Moreno13). A subsample of 1069 agreed to participate.
For the analysis, we then excluded 145 participants without follow-up visit at 6 years, 103 participants who did not provide the saliva sample to determine the APOE haplotype or were not correctly genotyped, fourteen participants who reported values of energy intake outside of predefined values (1st–99th percentiles), and one participant due to implausible value for the difference between baseline and final Spanish version of the modified Telephone Interview of Cognitive Status (STICS-m) scores (the change in score was >4 sd). Therefore, the final sample size was 806 participants (Fig. 1).
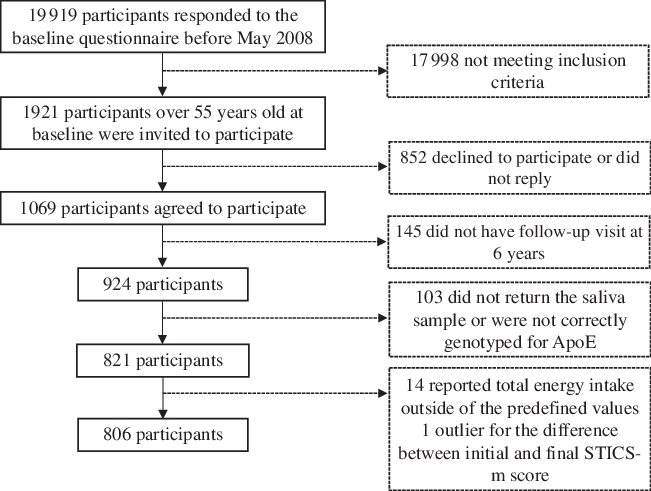
Fig. 1. Flowchart of participants in the SUN (‘Seguimiento Universidad de Navarra’) cognitive function subproject. STICS-m, Spanish version of the modified Telephone Interview of Cognitive Status.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the Institutional Review Board of the University of Navarra. Participants in the cognitive function subproject of the SUN cohort provided a specific written informed consent.
Cognitive function assessment
The cognitive function was evaluated with the validated STICS-m(Reference Muñoz-García, Cervantes and Razquin14). It was administered over the phone at baseline, and after 2 and 6 years of follow-up. The questionnaire is composed of eleven items with a maximum score of 41. It assesses four cognitive domains: orientation, memory, attention/calculation and language(Reference Welsh, Breitner and Magruder-Habib15). In order to assess long-term changes in cognitive function, we addressed changes between STICS-m scores at baseline and at 6 years of follow-up.
A total of fourteen subjects in the baseline and one subject in the 6-year STICS-m showed missing data for some of the items (n 13, one item missing; n 1, two items missing; n 1, three items missing). These missing values were imputed based on the participants’ sex and age, the attained scores in the other items of the questionnaire, and the attained scores for the item in the 2- and 6-year STICS-m for subjects with missing data in the baseline questionnaire, and in the baseline and 2-year STICS-m for those with missing data in the 6-year questionnaire(Reference Muñoz-García, Martínez-González and Martín-Moreno13).
Polyphenol intake and dietary assessment
A validated 136-item semi-quantitative FFQ was used to assess dietary habits(Reference Martin-Moreno, Boyle and Gorgojo16,Reference de la Fuente-Arrillaga, Vázquez Ruiz and Bes-Rastrollo17) . Both the foods (dairy products; eggs, meat and fish; vegetables; fruits; legumes and cereals; oils and fats; pastries; drinks and others) and consumption frequencies were grouped into nine categories (never or seldom; 1–3 times/month; once weekly; 2–4 times/week; 5–6 times/week; once daily; 2–3 times/d; 4–6 times/d; 6 or more times/d). To calculate the nutrient composition of the diet, we used Spanish food composition tables once the daily food consumption was calculated for each food item multiplying the consumption frequency by its typical portion size(Reference Moreiras, Carbajal and Cabrera18,Reference Mataix Verdú19) .
Information on the polyphenol content in foods was obtained from the Phenol-Explorer database (www.phenol-explorer.eu)(Reference Neveu, Perez-Jiménez and Vos20). For those foods not included in Phenol-Explorer (leek, thistle and honey), the United States Department of Agriculture database (www.ars.usda.gov/nutrientdata) was used(Reference Ahuja, Moshfegh and Holden21). Foods with only traces of polyphenols, such as meat-based foods, were removed.
When an item of the FFQ included more than one food, we used a weighted average according to the typical relative frequency of consumption in the Spanish population(22). For recipes and processed foods, polyphenol content was calculated according to their ingredients. Finally, the retention factors from Polyphenol-Explorer were applied to consider food cooking and processing to calculate the polyphenol content(Reference Rothwell, Medina-Remón and Pérez-Jiménez23).
Total polyphenol and specific major subclasses of polyphenols (flavonoids, lignans stilbenes, phenolic acids and other polyphenols – alkylphenols, tyrosols, hydroxybenzaldehydes, hydroxybenxoketones, hydroxycoumarins and methoxyphenols) were calculated as the sum of all individual polyphenol intakes from food sources reported in the FFQ. Moreover, we separately analysed the following six major subclasses of flavonoids: anthocyanidins, flavones, flavonols, flavan-3-ols, isoflavonoids and flavanones.
Assessment of covariates
The baseline questionnaire provides information about participants’ sociodemographic characteristics (sex, age and years of university education), medical history (hypertension, hypercholesterolaemia, low HDL-cholesterol, diabetes and CVD), lifestyles (smoking habit) and anthropometric measurements (weight and height). Physical activity was evaluated with a previously validated questionnaire(Reference Martínez-González, López-Fontana and Varo24), and metabolic equivalents (METs-h/week) scores were estimated for each participant. The adherence to the MedDiet was assessed with the MedDiet Score developed by Trichopoulou et al.(Reference Trichopoulou, Costacou and Bamia25).
Statistical analysis
Intake of total and subclasses of polyphenols was adjusted for total energy intake using the residuals method(Reference Willett26), and participants were subsequently categorised into quintiles.
We used inverse probability weighting to adjust the means or proportions of baseline characteristics of our participants for age and sex across quintiles of total polyphenol intake.
To analyse the association between the intake of polyphenols and changes in cognitive function, we applied multiple linear regression models. The dependent variable was the change in STICS-m from baseline to 6-year follow-up. The linear regression models were adjusted for several potential confounders: model 1: age, sex and years of university education (continuous); model 2: model 1 + APOEϵ4 haplotype, physical activity (tertiles), baseline BMI (kg/m2) (continuous), follow-up time between baseline and cognitive evaluation (continuous), smoking status (current, former and never smoker), package-years among ever smokers (continuous), energy intake (quartiles), sugar-sweetened beverage consumption (continuous), prevalent hypertension, prevalent hypercholesterolaemia, low HDL-cholesterol, prevalent diabetes and prevalent CVD and model 3: model 2 + adherence to the MedDiet (tertiles). When we considered stilbenes as exposure, we additionally adjusted our final model for alcohol intake from sources other than wine. The analyses were repeated using change in each of the four domains of the STICS-m (orientation, immediate memory, attention/calculation and language) as the dependent variable. To investigate linear trends across the quintiles of intake of total and subclasses of polyphenols, we assigned the median value to each category and treated them as continuous. We explored if the rate of changes in cognitive function over time was different across the intake of polyphenols (as continuous variable) using mixed model analysis. Moreover, we tested (likelihood ratio test) the interaction between the intake of total polyphenols and subclasses of polyphenols (dichotomised at the median) and sex and age (cut-off point = 65 years at the time of recruitment in the SUN cohort) and changes in cognitive function, once adjusted for potential confounders.
After stepwise-selection regression analyses, we conducted a series of nested regression models to establish the contribution of each food item to the between-person variability in polyphenol intake. The cumulative R 2 change reflects the additional contribution of each food. Finally, to estimate the contribution of each food to the total intake of polyphenols, we calculated the ratio between the polyphenol content of each food over the total polyphenol intake and multiplied this amount by 100.
Statistical analyses were performed with STATA/SE 12.0. All P values were two tailed, and a P value <0·05 was considered as statistically significant.
Results
Mean total polyphenol intake was 849 (sd 534) mg/d, of which 478 (sd 406) mg/d were flavonoids, 0·7 (sd 0·4) mg/d lignans, 321 (sd 202) mg/d phenolic acids, 2·0 (sd 3·2) mg/d stilbenes and 48 (sd 50) mg/d other polyphenols. The mean follow-up time in the cohort until the first cognitive evaluation was 5·6 (sd 2·6) years.
Of the 806 individuals, 69·7 % were male and 30·3 % female. The mean age of participants at recruitment was 60·7 (sd 5·6) years, at the time of the initial cognitive evaluation 66·3 (sd 5·2) years and at the time of the final cognitive evaluation 72·3 (sd 5·3) years. Baseline characteristics of participants according to quintiles of total polyphenol intake adjusted for sex and age are shown in Table 1. Compared with subjects in the first quintile, those in the fifth quintile were more likely to be more physically active and current smokers, to show a lower energy intake and to present a higher prevalence of hypercholesterolaemia and diabetes. For the total population, the mean STICS-m score at baseline was 34·0 (sd 2·4). No relevant differences were observed in baseline characteristics of participants according to the median in the baseline STICS-m score (online Supplementary Table S1).
Table 1. Baseline characteristics* of the participants across sex-specific energy-adjusted quintiles (Q) of polyphenol intake
(Percentages; mean values and standard deviations)

STICS-m, Spanish version of the modified Telephone Interview of Cognitive Status; MET, metabolic equivalents; SUN, Seguimiento Universidad de Navarra.
* Adjusted for inverse probability weight by sex and age at baseline questionnaire of the SUN project.
† Includes stroke, myocardial infarction, CHD, coronary artery surgery or angioplasty.
‡ Score proposed by Trichopoulou et al.(22).
§ To convert kcal to kJ, multiply by 4·184.
|| From sources other than wine.
¶ Presence of at least one APOE ϵ4 allele.
We found no significant association between total polyphenol intake and changes in cognitive function after 6 years of follow-up (Table 2). However, when we analysed subclasses of polyphenols, we observed in the multivariable-adjusted analyses that a higher intake of lignans (β Q5 v. Q1 0·81; 95 % CI 0·12, 1·51; P trend = 0·020) and stilbenes (β Q5 v. Q1 0·82; 95 % CI 0·15, 1·49; P trend = 0·028) was associated with more favourable changes in cognitive function over time (P for trend = 0·020 and 0·028, respectively) (Fig. 2). Additional adjustment for alcoholic drinks other than wine did not change the results for stilbenes (P for trend = 0·031). In mixed models testing for rates of changes in cognitive function over time across the intake of different polyphenols, we found a significant time × polyphenol interaction on rates of changes in cognitive function for lignans (P for interaction = 0·045) and stilbenes (P for interaction = 0·049). Online Supplementary Tables S3 and S4 show the analysis by subtypes of lignans and stilbenes, respectively. No significant association was found between the intake of flavonoids and its subclasses (anthocyanidins, flavones, flavonols, flavan-3-ols, isoflavonoids and flavanones), phenolic acids, and other polyphenols and changes in cognitive function.
Table 2. Differences in cognitive function change after 6 years according to quintiles (Q) of polyphenol intake* (β Values and 95 % confidence intervals)
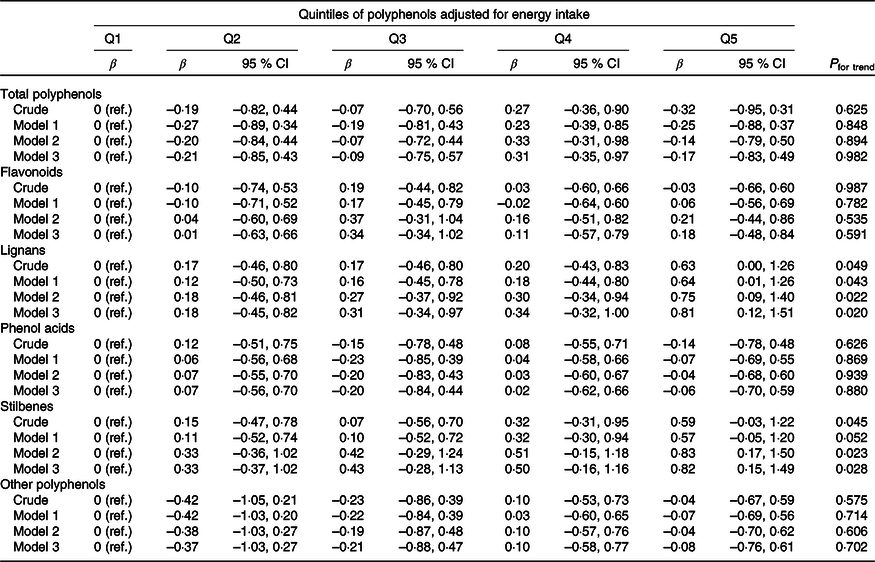
ref., Reference.
* Model 1: adjusted for age, sex and years of university education (continuous). Model 2: model 1 + APOE haplotype, physical activity (tertiles), baseline BMI (kg/m2) (continuous), follow-up time between baseline and cognitive evaluation (continuous), smoking status (current, former and never smoker), package-years among ever smokers (continuous), energy intake (quartiles), sweetened beverages consumption (continuous), prevalent hypertension, prevalent hypercholesterolaemia, low HDL-cholesterol, prevalent diabetes and prevalent CVD. Model 3: model 2 + adherence to the Mediterranean diet (tertiles).

Fig. 2. Adjusted means and 95 % confidence intervals for the association between subclasses of polyphenol intake and 6-year change in cognitive function. Q, quintile; STICS-m, Spanish version of the modified Telephone Interview of Cognitive Status. Adjusted for age, sex, years of university education (continuous), STICS-m score at the initial evaluation, APOE haplotype, physical activity (tertiles), baseline BMI (kg/m2) (continuous), follow-up time between baseline and cognitive evaluation (continuous), smoking status (current, former and never smoker), package-years among ever smokers (continuous), energy intake (quartiles), sweetened beverage consumption (continuous), prevalent hypertension, prevalent hypercholesterolaemia, low HDL-cholesterol, prevalent diabetes, prevalent CVD and adherence to the Mediterranean diet (tertiles).
When we explored the potential interaction with sex or age on the association between intake of total polyphenols and subclasses of polyphenols (dichotomised at the median) and cognitive function decline, we found no significant interactions (all P for interaction values > 0·05).
By domains of the STICS-m, the multivariable-adjusted analysis showed a possible direct association between higher intake of lignans and favourable 6-year changes in immediate memory and language domains (P for trend = 0·036 and 0·028, respectively) (Table 3). For stilbenes, a positive association was observed for the domain immediate memory (P for trend = 0·020) (Table 4). When the interaction between each subclass of polyphenols and sex or age was explored, we found that the interaction was not statistically significant (all P for interaction values > 0·05).
Table 3. Differencces in cognitive function change by domains after 6 years according to quintiles (Q) of lignan intake* (β Values and 95 % confidence intervals)

ref., Reference.
* Model 1: adjusted for age, sex, years of university education (continuous) and Spanish version of the modified Telephone Interview of Cognitive Status score at the initial evaluation. Model 2: model 1 + APOE haplotype, physical activity (tertiles), baseline BMI (kg/m2) (continuous), follow-up time between baseline and cognitive evaluation (continuous), smoking status (current, former and never smoker), package-years among ever smokers (continuous), energy intake (quartiles), sweetened beverages consumption (continuous), prevalent hypertension, prevalent hypercholesterolaemia, low HDL-cholesterol, prevalent diabetes and prevalent CVD. Model 3: model 2 + adherence to the Mediterranean diet (tertiles).
Table 4. Differences in cognitive function change by domains after 6 years according to quintiles (Q) of stilbene intake* (β Values and 95 % confidence intervals)

ref., Reference.
* Model 1: adjusted for age, sex, years of university education (continuous) and Spanish version of the modified Telephone Interview of Cognitive Status score at the initial evaluation. Model 2: model 1 + APOE haplotype, physical activity (tertiles), baseline BMI (kg/m2) (continuous), follow-up time between baseline and cognitive evaluation (continuous), smoking status (current, former and never smoker), package-years among ever smokers (continuous), energy intake (quartiles), sweetened beverage consumption (continuous), prevalent hypertension, prevalent hypercholesterolaemia, low HDL-cholesterol, prevalent diabetes and prevalent CVD. Model 3: model 2 + adherence to the Mediterranean diet (tertiles).
Table 5 shows the contribution of different foods to lignan and stilbene intake. Olive oil (44·7 %) and nuts (17·2 %) were the main foods contributing to lignan intake and wine (52·7 %) to stilbene intake.
Table 5. Sources of variability (cumulative R 2) and main sources in total lignan and stilbene intake according to each food included in the FFQ*

* Cumulative R 2 values were determined from nested regression analyses after stepwise selection.
Discussion
The results of the present prospective cohort study suggest that the intake of lignans and stilbenes is associated with more favourable changes in cognitive function over a 6-year period as measured by the STICS-m, particularly with regard to immediate memory and language domains. Contrary to expectations, we observed that the absolute values of the STICS-m score improved over time, an observation which was likely to be due to the learning effect of repeated testing of participants with the same questionnaire. However, our comparisons relied only on relative changes. The mean intake of lignans and stilbenes of participants in the highest quintile was 1·3 (sd 0·5) mg/d and 6·9 (sd 4·2) mg/d, respectively. In terms of foods, and taking into account the main sources of lignans and stilbenes in the SUN cohort, for example, the consumption of 40 g of olive oil and 60 g of nuts per d ensures the intake of 1·3 mg/d of lignans, and the consumption of 150 ml of red wine per d ensures the consumption of 6·5 mg/d of stilbenes.
Some longitudinal studies on lignans and cognition in humans have been published recently. According to our results, Greendale et al.(Reference Greendale, Huang and Leung27) observed that higher lignan intake was associated with a slower decline in verbal episodic memory during late perimenopause. Also, Nooyens et al.(Reference Nooyens, Milder and Van Gelder28) reported that a greater intake of lignans was associated with less decline in global cognitive function, memory and processing speed. As far as we know, there are three further cross-sectional studies on the association between lignan intake and cognitive function. On one hand, Franco et al. showed a positive association between a high dietary intake of lignans and a higher probability of intact cognitive function among postmenopausal women(Reference Franco, Burger and Lebrun29). In contrast, Kreijkamp-Kaspers et al.(Reference Kreijkamp-Kaspers, Kok and Grobbee30) did not find a significant association between lignan intake and cognitive function. When the authors studied the relationship between polyphenols intake and different domains of cognitive function, they found that a higher lignan intake was associated with a better performance in processing speed and executive function, but unlike our study, not in memory tasks (verbal episodic memory and visual memory). However, as we found in our study, Rosli et al. showed a positive correlation between lignan intake and punctuation in immediate memory measured by the Rey auditory verbal learning test(Reference Rosli, Shahar and Din31). The evidence is more limited about total stilbenes and cognitive function. In contrast to our results, a previous study did not find a significant correlation between stilbene intake and immediate or delayed recall(Reference Rosli, Shahar and Din31).
These inconsistent results could be due to the differences in study population, patterns of food consumption and the heterogeneity in the instruments used to assess cognitive function(Reference Rosli, Shahar and Din31). It should be mentioned that the STICS-m is a screening questionnaire and not a diagnostic one and it is mainly focused on determine the verbal episodic memory.
Lignans and stilbenes display oestrogenic activity; therefore, they have been reported as phyto-oestrogens(Reference Michel, Halabalaki and Skaltsounis32). The most studied phyto-oestrogens are isoflavones. Isoflavone intake is higher in Asian populations due to the consumption of soya and soya-derived products, whereas in Western populations, the relative contribution of lignans to the total amount of phyto-oestrogen intake is much larger(Reference Fletcher33). Phyto-o estrogens may act on cognition because they bind to α- and β-oestrogen receptors and share some of the genomic and non-genomic effects of oestrogens through the central nervous system(Reference Soni, Rahardjo and Soekardi34). However, a previous review of observational studies and randomised controlled clinical trials concluded that approximately 50 % of the studies showed positive effects of phyto-oestrogens, specifically isoflavones, on cognitive function, and the other half showed negative/null findings(Reference Soni, Rahardjo and Soekardi34).
By domains of the STICS-m, we observed that both phyto-oestrogens, lignans and stilbenes were inversely related to immediate memory performance over time. This idea is plausible given the distribution of oestrogen receptors, α and β, in the brain. These receptors are mainly expressed in areas such as the hippocampus and prefrontal cortex, which are important for learning, memory and higher-order cognitive function, suggesting that phyto-oestrogens might particularly benefit episodic memory(Reference González, Cabrera-Socorro and Pérez-García35,Reference Montague, Weickert and Tomaskovic-Crook36) . According to our results, this suggests that phyto-oestrogens might particularly benefit episodic memory. Moreover, tasks involving the prefrontal cortex including verbal learning and verbal fluency, among others, may be particularly sensitive to phyto-oestrogens according to observational studies and randomised control trials(Reference Soni, Rahardjo and Soekardi34).
Lignans are widely distributed, and the main sources in our study were olive oil and nuts. In this sense, the PREDIMED study, a randomised intervention trial, showed that a MedDiet enriched with extra virgin olive oil or nuts was associated with improved cognitive function(Reference Valls-Pedret, Sala-Vila and Serra-Mir37–Reference Martinez-Lapiscina, Clavero and Toledo39). These findings are in line with the results of a recent systematic review in which adherence to the MedDiet was associated with better cognitive performance(Reference Petersson and Philippou2). Nevertheless, it should be noted that most of the included studies in this review were observational, and more controlled trials are needed to establish a cause–effect relationship(Reference Petersson and Philippou2). The positive association that we found between lignan intake and cognitive function over time was independent to MedDiet adherence. This finding suggests that the potential beneficial effect of lignans on cognitive function may be additive but independent to the MedDiet.
In our work, when subclasses of stilbenes were analysed, we observed that resveratrol and different isoforms, one of the best-known stilbenes, were associated with a better cognitive function over time. Resveratrol is extensively present in many edible fruits, although red wine represents the most substantial source in the human diet of the Spanish population, according to our results(Reference de la Fuente-Arrillaga, Vázquez Ruiz and Bes-Rastrollo17,Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventós40) . The results of the studies conducted to identify potential beneficial effects of resveratrol on cognitive function in humans are inconclusive mainly due to its poor bioavailability(Reference Farzaei, Rahimi and Nikfar41,Reference Huhn, Witte and Watson42) . However, studies in animal models reported the neuroprotective potential effect of resveratrol through different mechanisms, such as inhibition of tauopathy, brain proinflammatory responses, H2O2-induced neurotoxicity and Aβ plaque synthesis; enhancement of long-term memory formation and neuroprotective sirtuin-1 activity; astrocyte inactivation, antioxidant activity and prevention of neuronal cell death(Reference Ahmed, Javed and Javed43).
Finally, it should be mentioned that cognitive impairment and dementia have been related to CVD and its risk factors(Reference Angermann, Frey and Ertl44). Therefore, the association between the intake of polyphenols and cognitive function may be partially mediated by the cardioprotective effect of polyphenols(Reference Serino and Salazar45).
We acknowledge that the present work has some limitations that should be mentioned. First, we used self-reported data for the dietary information instead of biomarkers, which might have led to some degree of misclassification. This misclassification is most likely to be non-differential and it can attenuate the true associations. Second, the FFQ was not specifically designed to collect data regarding polyphenols; therefore, some foods rich in polyphenols such as herbs, spices and seeds (including flax seeds rich in secoisolariciresinol) were not specifically recorded. However, some validation studies have shown that FFQ are reasonable valid for estimating polyphenol intake(Reference Burkholder-Cooley, Rajaram and Haddad46), our FFQ has been previously validated and includes the main foods consumed by the study population(Reference Muñoz-García, Martínez-González and Martín-Moreno13,Reference Muñoz-García, Cervantes and Razquin14) and the average consumption of some of these foods such as seeds is 0·03 (sd 0·49) g/d in the general Spanish population, according to the last National Food Survey(47). In addition, this potential misclassification would be non-differential and would most likely shift our results towards the null. On the other hand, some items in the FFQ included several foods because they have similar nutritional characteristics according to the amount of macro- and micronutrients, as it is usual in the design of all FFQ(Reference Willett26). Thus, we applied a weighted average according to the typical relative frequency of consumption in the Spanish population(47) in order to obtain a more accurate estimation of the intake of polyphenols. Third, the intake of polyphenols was assessed at the time of inclusion in the cohort, and it was not updated by the time of the second cognitive assessment. Fourth, although the STICS-m has been widely used in other epidemiological studies, and previously validated in the Spanish population(Reference Muñoz-García, Cervantes and Razquin14), like the Mini-Mental State Examination(Reference Folstein, Folstein and McHugh48), it has been designed as a screening tool. A comprehensive battery of neuropsychological tests could be more suitable to evaluate cognitive performance over the time, but difficult to implement in practice. Fifth, the volunteers of the SUN project are university graduates, and therefore, the generalisability of the results may be limited. Nevertheless, it is plausible that the observed association would also apply to subjects with lower educational level. Sixth, the homogeneity of the population might have reduced the between-subject variability in dietary exposures and therefore the sensitivity to detect significant differences on cognitive function across polyphenol intake groups. However, this fact can also be considered as an advantage. This restriction could also reduce the potential for confounding by socio-economic and education level. In any case, we adjusted our results for a wide array of potential confounders, which have been included in the multivariable analyses and enhances the internal validity of our results. Finally, since education is considered a protective factor against dementia and Alzheimer’s disease(Reference Xu, Tan and Wang49), the homogenous high educational level of the sample could minimise the beneficial effects of lignans and stilbenes on cognitive function and partially contribute to explain the lack of efficacy of certain classes of polyphenols on cognitive function changes.
In conclusion, our results suggest that a high dietary intake of phyto-oestrogens, lignans and stilbenes is associated with favourable cognitive changes over time. Our findings support the presumed neuroprotective effects of polyphenols on cognitive function. Nevertheless, more studies are needed to confirm that polyphenols could really constitute a promising target for cognitive impairment and dementia prevention.
Acknowledgements
We thank all the SUN participants for their continued cooperation and participation. We also thank the other SUN Project investigators (Alonso A, Álvarez-Álvarez I, Balaguer A, Barbagallo M, Barrio-López MT, Basterra-Gortari FJ, Battezzati A, Bazal P, Bertoli S, Bes-Rastrollo M, Beulen Y, Beunza JJ, Buil-Cosiales P, Carlos S, Carmona L, Cervantes S, Cristobo C, de Irala J, de la Fuente-Arrillaga C, de la O V, de la Rosa PA, Delgado-Rodríguez M, Díaz-Gutiérrez J, Díez Espino J, Domínguez L, Donat-Vargas C, Donazar M, Eguaras S, Fernandez-Montero F, Fresán U, Galbete C, García-Arellano A, García López M, Gardeazábal I, Gea A, GutiérrezBedmar M, Gomes-Domingos AL, Gómez-Donoso C, Gómez-Gracia E, Goñi E, Guillén F, Henríquez P, Hernández-Hernández A, Hershey MS, Hidalgo-Santamaría M, Hu E, Leone A, Llorca J, López del Burgo C, Marí A, Marques I, Martí A, Martín Calvo N, Martín-Moreno JM, Martínez JA, Martínez-Lapiscina EH, Mendonça R, Menéndez C, Molendijk M, Murphy K, Muñoz M, Núñez-Córdoba JM, Pajares R, Papadaki A, Parletta N, Pérez de Ciriza P, Pérez-Cornago A, Pérez de Rojas J, Pimenta AM, Pons J, Ramallal R, Razquin C, Rico-Campà A, Ruano C, Ruiz-Estigarribia L, Ruiz Zambrana A, Salgado E, San Julián B, Sánchez D, Sánchez-Bayona R, Sanchez-Villegas A, Santiago S, Sayón-Orea C, Schlatter J, Serrano-Martinez M, Toledo J, Tortosa A, Valencia F, Vázquez Z, Zarnowiecki D and Zazpe I).
The SUN Project has received funding from the Instituto de Salud Carlos III, and the European Regional Development Fund (FEDER) (grant number RD 06/0045), CIBER-OBN (grant numbers PI10/02658, PI10/02293, PI13/00615, PI14/01668, PI14/01798, PI14/01764 and G03/140), the Navarra Regional Government (grant numbers 45/2011 and 122/2014) and the University of Navarra.
L. G. and E. T. wrote the original draft of the manuscript; L. G., M. F.-M., A. R.-N. and E. T. were responsible for formal analysis; M. A. M.-G., C. R., M. R.-C. and E. T. were responsible for conceptualisation and methodology, investigation and funding acquisition. All co-authors revised the manuscript critically and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S000711452000392X










