INTRODUCTION
With the total reconstruction of the European Food Law beginning in 2001 and other corresponding laws following in 2003, German farmers and veterinarians alike had to consider, for the first time, the concepts of food safety on the farm itself. Help came from the German QS System, a company dedicated to creating a ‘farm-to-fork’ monitoring system for animal-derived as well as other agricultural products [1]. In this monitoring system, herds are divided into three categories:
Category I: less than 20% of all samples taken are Salmonella spp. antibody positive.
Category II: 20–40% of all samples taken are Salmonella spp. antibody positive.
Category III: more than 40% of all samples taken are Salmonella spp. antibody positive [i.e. >40% optical density (OD)].
As a rule, 60 slaughtered pigs per herd each year are sampled, and the sampling must be spread out over a 12-month period [1]. The samples for this serological monitoring are either meat juice samples taken at slaughter or blood serum samples taken not earlier than 14 days prior to slaughter. Either kind of sample is then analysed via enzyme-linked immunosorbent assay (ELISA). It is a requirement to find the cause of the Salmonella problem for herds in category III and implement measures against it [1]. This is not a requirement for herds in category II, but farmers are encouraged to do so [1, Reference Blaha2]. In 2007 the German government made participation in the described Salmonella monitoring system mandatory for all farmers supplying slaughter pigs.
Although many risk factors, such as more than three supplier herds [Reference Lo Fo Wong3], rodent infestation [Reference Letellier4] and contaminated feed [Reference Harris5] have been discussed for a long time and many reports on studies into these risk factors have been frequently published [Reference Funk and Gebreyes6], there is still a great deal of uncertainty in the field about how to overcome the problem. This is in marked contrast to the situation found in layer hens, were the critical points of residual Salmonella contamination are well known and the strategy of prevention is well defined [Reference Namata7–Reference Davies and Breslin9].
In the pig industry, however, the veterinary practitioners and their clients often feel lost in a multitude of suggestions and options. Frustration runs high, when, as in many cases, herds are categorized into category III although many known measures against Salmonella infections, e.g. a strict all-in/all-out management [Reference Farzan10, Reference Stege11], acidifying of feed and/or coarsely ground feed [Reference Visscher12] and correct external biosecurity measures [Reference Funk, Davies and Gebreyes13], are already in place. Further complications arise when the farmer does not fully understand what the risk categorization of his herd signifies. ‘My animals aren't ill, why is their meat a risk for human consumption?’ is a question often encountered by veterinary practitioners. The frustration is regularly exacerbated by the fact that even the experts themselves rarely agree on which management or hygienic factors are key to a successful prevention of Salmonella infections in pigs [Reference Stärk14].
Keeping this frustration of the veterinarians and farmers in mind, a hypothesis was postulated: the usually discussed critical points in the animal environment such as ventilation [Reference Letellier4], floors and feeders [Reference Funk, Davies and Nichols15] are apparently not the only points of Salmonella contamination. Several other points, for instance the driving boards, central aisles of the barn and the compartment aisles, are possibly major ‘retreat’ areas for residual Salmonella as well.
This hypothesis also exemplifies one of the unique points of our study, because the mentioned areas have not (to the best of our knowledge) been the focus of previous research in the pig industry.
Another unique point, from the German perspective, is that due to the new monitoring system, it was possible for the first time to differentiate between herds of high and low seroprevalence and compare them on a large scale.
The objectives for this study were therefore the following:
(1) To utilize the possibilities of the monitoring system by including a large number of herds in the study.
(2) To confirm or refute the hypothesis that there are critical points for residual Salmonella which have been previously underestimated.
MATERIAL AND METHODS
Study farms
The farms examined in the study are situated in the northwestern part of Germany with the highest pig density within Europe (spread over eight counties). All farms participated voluntarily and were chosen because of their serological classification and geographical distribution. In total 142 herds with 61 herds in category I and 81 herds in category III participated. The herds were either just finishing or farrow-to-finishing units. The names of the counties in which the herds were located are listed in Table 1. Beyond the clear risk categorization in one of the previously mentioned categories and the locations of the herds in Lower Saxony, no other requirements were made. The study period lasted from July 2007 to December 2009. During this period, each herd was visited at least once to obtain the samples. All herds were visited by the same examiner.
Table 1. Herd types by category and county

F, Finisher herds; F–F, farrow-to-finisher herds.
Sampling
To determine the major ‘retreat areas’ of residual Salmonella, the animals' environment was divided into ‘direct’ and ‘indirect’ environments. Samples of the direct environment were defined as having been taken from objects to which the pigs were able to have continuous or repeated physical contact: pen floors, pen walls, walls, feeders and troughs, drinking nipples (only those outside the feeders), and toys. The walls were only sampled up to the level of the back height of the animals. Samples of the indirect environment were defined as having been taken from areas with which the pigs have no physical contact such as anterooms, ceilings, gas heaters, pipes, ventilation (fans), or very rare physical contact such as the compartment aisles, driving boards, the central aisle of the barn, boots/shoes of the farmer, animal scales, loading ramps, transporters and other miscellaneous objects.
The number of samples taken per herd was 14–20. If possible, samples were taken from three separate places:
(1) A cleaned and disinfected compartment.
(2) A compartment in which the youngest animals of the herd were housed (excluding suckling piglets).
(3) A compartment in which the oldest animals of the herd were housed (excluding sows).
Pig toys, central aisles, boots and shoes, anterooms, gas heaters, ventilation, ceilings, animal scales and transporters could not be sampled in every herd, either because the object was not present or it was out of reach of the examiner.
The environmental samples were taken via swabs. A swab is a 10×30 cm gauze tissue (tg® Size 7, Lohmann & Rauscher International GmbH Germany) drenched, i.e. thoroughly wet but not dripping, in buffered peptone water (BPW; Oxoid Ltd, UK) and sterilized in a sealed heat-resistant plastic envelope. These swabs were prepared by the examiner 2–3 days prior to the investigation of a farm. The swabs were stored at 7°C until required.
All objects with a small surface (pig toys, drinking nipples, driving boards) were swabbed entirely. Other objects with areas too large to be completely swabbed (central aisles, anterooms, ceilings, compartment aisles, animal scales, loading ramps, transporters), were swabbed in 3–5 locations of ~1 m2 depending on the overall size. From the feeders, pig toys and drinking nipples at least 50% of those present in the compartment were swabbed with one swab, since it was not important to find out which specific feeder or toy was contaminated, only the type of environment which was contaminated. On the day of sampling, all samples were transported in cooling boxes to the laboratory and were cultured in BPW at 37°C for 18±1 h.
Testing
Each sample was tested by a standardized real-time polymerase chain reaction (PCR) protocol. The PCR was performed with the TaqMan® Salmonella Detection kit (Applied Biosystems, USA) according to the manufacturer's instructions. Reactions were performed using a real-time PCR System 7500 and the results were analysed with SDS software package v. 1.4 (all Applied Biosystems, USA). The laboratory personnel conducting the analyses were blinded with respect to the category and the type of sample. A sample was categorized as positive if the crossing point value was less than 45 cycles. Due to the fact that the samples were incubated before analysis, the results in the tables are only given as positive or negative and not as a quantitative result.
Data handling
The odds ratios (OR) of the cumulative results were calculated with WinEpiscope 2.0® (CLIVE, UK); for small sample sizes Fisher's exact test was used, these values were calculated with SAS v. 9.0 (SAS Institute, USA). The confidence interval (CI) level was set at 95% and the cut-off of the P value was set at 0·05.
RESULTS
Because all herds participated voluntarily in the study, a matching of herds, although initially attempted, was not possible.
Although samples were, when possible, taken from three separate locations in the herd (a cleaned and disinfected compartment, a compartment with the youngest animals of the herd and a compartment of the oldest animals of the herd), the results were only calculated in two groups, i.e. before cleaning and disinfection (C+D) and after. This was because there was too much missing information regarding the age of the pigs on the day of sampling, so that satisfactory subgroups could not be established. Because of time constraints, it was not possible to sample an empty compartment prior to C+D and then sample it again afterwards.
The percentage of positive samples from the total sample size before C+D (n=2325) was 22·97%. Differentiating between direct (n=1105) and indirect (n=1220) environments showed little difference between the groups (22·90% vs. 23·03% respectively). Of the samples taken after C+D (n=222) 17·57% were positive.
Table 2 shows the results of samples of the direct environment. Listed are the different kinds of samples with their respective total amounts, the percentage of positive samples within each category (as a percentage of the amount of samples in that particular category, not the total), as well as the OR and 95% CI. Of the direct environment, the only significant difference between the categories was found between the samples from the pen floor (OR 3·69, 95% CI 1·82–7·48).
Table 2. Results of the direct environment samples
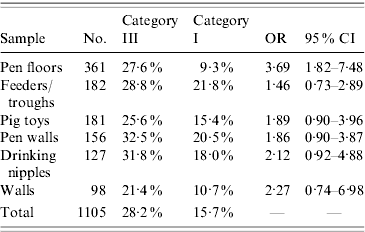
OR, Odds ratio; CI, confidence interval.
Parts of the results from the indirect environment are illustrated in Table 3 a. They are given in the same manner as the results in Table 2. In this case, three significant differences could be found in the samples: they were the compartment aisles (OR 3·45, 95% CI 1·61–7·41), driving boards (OR 3·06, 95% CI 1·34–6·92) and central aisles of the barn (OR 3·03, 95% CI 1·35–6·83).
Table 3 a. Results of the indirect environment samples (part 1)
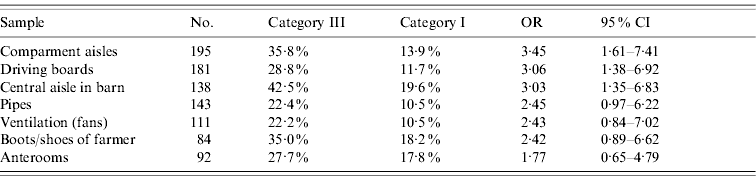
OR, Odds ratio; CI, confidence interval.
As in Tables 2 and 3 a, Table 3 b shows the different kinds of samples from the direct and indirect environments with their respective total amounts, and the percentage of positive samples within each category (again as a percentage of the amount of samples in that particular category, not the total). However, due to very small sample sizes Fisher's exact test had to be used to calculate a two-sided P value (cut-off 0·05) for the ceilings, gas heaters, animal scales, loading ramps, transporters and other miscellaneous objects. The P value for the ceilings was 0·04, the others were not statistically significant.
Table 3 b. Results of the indirect environment samples of (part 2)
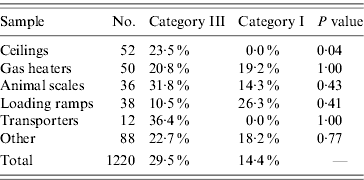
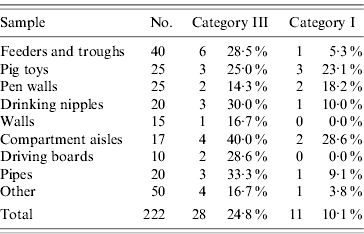
Table 4 lists the results of the sampling after C+D; these samples are not included in the total of the samples in Table 2. Because of the small sample sizes, only an overall OR and CI between the categories was calculated (OR 2·94, 95% CI 1·38–6·25). No further calculations were made and the values given in the table are the total of the samples, the number of positives within each category and the percentage of positive samples with respect to the number of samples taken in that category.
Table 4. Results of the sampling after cleaning and disinfection
DISCUSSION
Although we were aware of a recruiting bias, since voluntary participating herds were more likely to be aware of the nature of the problem and may have already implemented measures against it, it was impossible to exclude this bias, due to the nature of voluntary recruiting per se. In reference to the first objective, the utilization of the possibilities of the monitoring system, success was partial. While it was simple to assign the fairly large number of recruited herds (n=142) to one of the two study categories, we were unable to determine two further points of interest, as official data was unavailable:
(1) Is the distribution of the herds as recruited with respect to category representative of the true distribution in each county?
(2) Is the distribution of the herds as recruited with respect to type of farm representative of the true distribution in each county?
Knowledge of these points would considerably increase the value of the statistical results as well as the conclusions that could be drawn from them.
Sampling bias was excluded as far as possible by having a fixed pattern of where and how the sampling was executed. If possible a cleaned and disinfected compartment, a compartment with the youngest animals of the herd and a compartment of the oldest animals of the herd was sampled. Objects with a clearly defined small surface were swabbed entirely; the others were swabbed in 3–5 locations of ~1 m2 depending on the overall size. Naturally, even this approach has its faults: for example, a central aisle (20 m long×5 m wide) was found to be ‘negative’, simply because the ‘correct’ (i.e. those with Salmonella) five locations were not swabbed – therefore it may be said that if a sample was negative, it was negative with respect to the area swabbed.
A possible bias of analysis was excluded by blinding the laboratory personnel to the category of the herd and type of sample submitted for examination at a given time.
One drawback of the utilized PCR is the fact that it was not clear whether the detected Salmonella were viable and therefore likely to infect a pig or not. Similarly, this particular PCR does not differentiate which serovar of Salmonella was detected. However, for the purposes of this study it was assumed that even if the detected Salmonella were not viable, they must have been so at one point and therefore every positive sample represented a risk of infection. Moreover, since pigs in Germany are most frequently infected with Salmonella Typhimurium [16], a differentiation of isolates via culture was deemed unnecessary. The PCR was therefore a suitable time- and labour-saving alternative to the culture method usually employed in such studies.
Regarding the second objective, it was possible to confirm the hypothesis that there are indeed previously underestimated critical points of residual Salmonella infection.
As the results show, category III herds have a higher risk of residual Salmonella in the environment compared to herds in category I.
This finding once more demonstrates the usefulness of serological monitoring as a means for estimating the risk that herds pose for carrying Salmonella into the slaughterhouse, which has been previously established by several authors [Reference Nielsen17–Reference de Vos, Saatkamp and Ehlers19]. This is of great importance, since occasionally farmers are sceptical about the validity of the assumption that many animals with antibodies against Salmonella in a herd really means a higher risk of the occurrence of Salmonella in slaughter pigs – especially farmers with an apparently high level of hygiene.
Statistically significant differences between category I and category III samples from individual sampling sites were found in the pen floor, compartment aisles, central aisle of the barn as well as the driving boards. As these areas are likely to be contaminated with faeces, it supports the general knowledge that the faecal–oral route is the most important form of transmission, which is also consistent with previous studies [Reference Fedorka-Cray20, Reference Lo Fo Wong21]. Three of the sampling sites with significant differences (compartment aisle, central aisle, driving board) are part of the indirect environment of the pigs as defined in the Materials and Methods section.
The fact that Salmonella can be isolated from samples taken after C+D has also been observed by other authors [Reference Funk, Davies and Nichols15, Reference Madec22]. However, the objective of such studies was to evaluate C+D measures in general [Reference Davies and Breslin8, Reference Funk, Davies and Nichols15, Reference Madec22–Reference van der Wolf24], but areas outside the compartment in which the pigs are held were not the focus of such studies, i.e. no differentiation of areas with a different intensity of the animal contacts was undertaken. In view of the fact that the current C+D protocols had been developed for controlling mostly pig-associated pathogens, it is understandable that routine C+D measures, even in very well-managed herds, focus mainly on the animal contact areas and spaces (pens and compartments), but not so much on areas out of reach of the animals. Herein lays a possible explanation for the validity of the presented hypothesis: the areas not included in the traditional C+D routine are the areas of previously underestimated critical points. A similar assumption can be made with respect to the result of the sampling of the ceilings. Even though located inside the compartment, the ceilings may not be as thoroughly cleaned and disinfected as the rest, because of two reasons:
(1) Especially in old buildings, it may not be possible to clean the ceiling with water via a high-pressure cleaner because the ceiling is made of weak materials (thin wood, straw).
(2) The farmers assume that since the pigs never reach the ceiling, nothing there could be harmful to the animals and therefore the ceiling is not cleaned at all.
The fact, however, that dust is a well-known ‘bearer’ of Salmonella [Reference Letellier4, Reference Berends23, Reference Liebana25], implies that dust from the ceiling is as much a cause for residual Salmonella that are able to infect Salmonella-free piglets after their introduction into a herd.
The overwhelming importance of C+D is emphasized in this study by the fact that of samples taken after C+D, category III herds had significantly more positive samples after C+D than category I herds (OR 2·94 CI 1·38–6·25). There are three possible explanations for this phenomenon:
(1) The management of hygiene in category III herds is not as elaborate as in category I herds and/or lacks a standardized protocol. A literature review and an exploratory study have both identified this as an aspect of deficient biosecurity and a main risk factor for Salmonella infections [Reference Fosse, Seegers and Magras26, Reference Fosse27].
(2) A protocol for correct C+D does exist in these herds, but its execution is deficient. This particular explanation has also been suggested previously by other authors [Reference Berends23].
(3) In contrast to category III herds, there are other management and working procedures in category I herds that are obviously capable of permanently minimizing the dissemination of Salmonella spp. throughout the herd.
Which of these three explanations is the most important needs further research.
To summarize with respect to the hypothesis of the study: the indirect environmental areas of pigs in any pig farm are a thus far underestimated cause of residual Salmonella. This is probably due to the fact that they are not included in routine C+D measures.
A comprehensive strategy against residual Salmonella contaminations must therefore begin with the implementation and correct execution of biosecurity measures from the threshold of the barn door.
ACKNOWLEDGEMENTS
The authors thank the participating farmers and laboratory personnel at the Field Station without whom this demanding project could not have been accomplished. Thanks are due to FAEN (Forschungsverbund Agar- und Ernährungswissenschaften Niedersachsen) for funding.







