Introduction
The Southern Ocean and sub-Antarctic islands are among the regions in the world that are the most exposed to the impacts of climate change (Turner et al. Reference Turner, Barrand, Bracegirdle, Convey, Hodgson and Jarvis2014, Auger et al. Reference Auger, Morrow, Kestenare, Sallée and Cowley2021). The multiple and synergistic effects of climate change (e.g. seawater temperature increase (Mélice & Servain Reference Mélice and Servain2003, Ansorge et al. Reference Ansorge, Durgadoo and Pakhomov2009, Reference Ansorge, Durgadoo and Treasure2014, Auger et al. Reference Auger, Morrow, Kestenare, Sallée and Cowley2021), ocean acidification (McNeil & Matear Reference McNeil and Matear2008), more extreme climatic events and intense seasonality (Turner et al. Reference Turner, Comiso, Marshall, Lachlan-Cope, Bracegirdle and Maksym2009, Turner et al. Reference Turner, Barrand, Bracegirdle, Convey, Hodgson and Jarvis2014, Blanchard-Wrigglesworth et al. Reference Blanchard-Wrigglesworth, Roach, Donohoe and Ding2021)) on Southern Ocean ecosystems are already perceptible (Morley et al. Reference Morley, Abele, Barnes, Cárdenas, Cotté and Gutt2020). Alterations to the structure and functioning of marine ecosystems are particularly expected in response to these major environmental changes (Gutt et al. Reference Gutt, Bertler, Bracegirdle, Buschmann, Comiso and Hosie2015, Morley et al. Reference Morley, Abele, Barnes, Cárdenas, Cotté and Gutt2020, Lelièvre et al. Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023), leading to shifts in species distribution patterns and community compositions (Doney et al. Reference Doney, Ruckelshaus, Emmett Duffy, Barry, Chan and English2012). Nearshore marine habitats of sub-Antarctic islands are particularly at risk because shallow-water species do not necessarily have the opportunity to migrate to more favourable areas. In addition, new conditions such as warmer waters, together with direct anthropogenic impacts and the ever-increasing maritime traffic (i.e. fisheries, tourism and science), may favour the expansion of non-indigenous and invasive species (Smith Reference Smith2002, Allan et al. Reference Allan, Froneman, Durgadoo, McQuaid, Ansorge and Richoux2013, Kargel et al. Reference Kargel, Bush, Cogley, Leonard, Raup, Smiraglia, Kargel, Leonard, Bishop, Kääb and Raup2014, Garciá Molinos et al. Reference Garciá Molinos, Halpern, Schoeman, Brown, Kiessling and Moore2015, Byrne et al. Reference Byrne, Gall, Wolfe and Agüera2016), a major threat to polar ecosystems usually characterized by high endemism levels (McCarthy et al. Reference McCarthy, Peck, Hughes and Aldridge2019, Hughes et al. Reference Hughes, Pescott, Peyton, Adriaens, Cottier-Cook and Key2020).
The ecological uniqueness, endemicity, and prominent diversity of sub-Antarctic islands assign to these isolated territories a high conservation value (Chown et al. Reference Chown, Rodrigues, Gremmen and Gaston2001) as they represent pristine marine ecosystems, still little impacted by direct anthropogenic disturbance (Lecomte et al. Reference Lecomte, Beall, Chat, Davaine and Gaudin2013), and they are priceless sentinels of climate change. For many of these islands, benthic ecosystems have been scarcely studied or remain undescribed. The few studies conducted on the benthic communities of sub-Antarctic islands highlighted high levels of species richness, diversity and biomass, such as in the Prince Edward Islands (Branch et al. Reference Branch, Attwood, Gianakouras and Branch1993), the sub-Antarctic islands of New Zealand (Snares, Auckland, Campbell, Antipodes and Bounty Islands; Chown et al. Reference Chown, Rodrigues, Gremmen and Gaston2001, Freeman et al. Reference Freeman, Cooper, Funnell and Neale2011, Clark et al. Reference Clark, Pastorino, Marzinelli, Turney, Fogwill and Johnston2019), South Georgia Island and the South Sandwich Islands (Barnes et al. Reference Barnes, Linse, Waller, Morely, Enderlein, Fraser and Brown2006, Hogg et al. Reference Hogg, Barnes and Griffiths2011), the Kerguelen Islands (Arnaud Reference Arnaud1974, Féral et al. Reference Féral, Poulin, González-Wevar, Améziane, Guillaumot, Develay and Saucède2019, Reference Féral, Verlaque, Rosenfeld, Poulin, Chenuil and Saucède2021) and the Crozet archipelago (Lelièvre et al. Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023). These studies have highlighted the importance and the role of the substrate in shaping the composition and distribution of benthic communities (Branch et al. Reference Branch, Attwood, Gianakouras and Branch1993, Barnes et al. Reference Barnes, Linse, Waller, Morely, Enderlein, Fraser and Brown2006, Freeman et al. Reference Freeman, Cooper, Funnell and Neale2011). Species richness and diversity can be closely related to habitat heterogeneity and complexity, which may be reflective of substrate type (Levin et al. Reference Levin, Sibuet, Gooday, Smith and Vanreusel2010, Schlacher et al. Reference Schlacher, Williams, Althaus and Schlacher-Hoenlinger2010). Therefore, mapping substrate types and investigating the indicator species of these different habitats would be helpful for biodiversity management and conservation.
The increasing development and recent advances in imagery system equipment, such as remotely operated vehicles (ROVs), autonomous underwater vehicles (AUVs) or towed camera systems, have made imagery and digital video important components of benthic monitoring research programmes (Durden et al. Reference Durden, Schoening, Althaus, Friedman, Garcia and Glover2016). In contrast to physical sampling, this repeatable and non-destructive approach provides researchers with a better understanding of benthic communities at a wide spatial scale. These techniques contribute to preserving the integrity of marine habitats and constitute suitable tools to study vulnerable and fragile communities such as sub-Antarctic benthic communities, characterized by a slow growth rate and long recovery time (Gutt & Starmans Reference Gutt and Starmans2001, Teixidó et al. Reference Teixidó, Garrabou, Gutt and Arntz2004, Smale & Barnes Reference Smale and Barnes2008). Underwater imagery has already proved effective for studying the structure and composition of benthic communities in a wide range of shallow marine ecosystems such as coral reefs (Moyer et al. Reference Moyer, Riegl, Banks and Dodge2003, Edgar & Stuart-Smith Reference Edgar and Stuart-Smith2014), kelp forests (Włodarska-Kowalczuk et al. Reference Włodarska-Kowalczuk, Kukliński, Ronowicz, Legeżyńska and Gromisz2009), artificial reefs (Walker et al. Reference Walker, Schlacher and Schlacher-Hoenlinger2007, Higgins et al. Reference Higgins, Scheibling, Desilets and Metaxas2019) and natural rocky substrata (van Rein et al. Reference van Rein, Schoeman, Brown, Quinn and Breen2011, Lelièvre et al. Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023). Along with the progress in these technologies, the development of new softwares for benthic image analysis (Kohler & Gill Reference Kohler and Gill2006, Teixidó et al. Reference Teixidó, Albajes-Eizagirre, Bolbo, Le Hir, Demestre and Garrabou2011, Trygonis & Sini Reference Trygonis and Sini2012, Langenkämper et al. Reference Langenkämper, Zurowietz, Schoening and Nattkemper2017) is constantly increasing, facilitating and encouraging the use of imagery in marine ecological studies. Imagery and digital video therefore provide new gateways and powerful approaches to study benthic communities in sub-Antarctic regions.
Studies on the marine ecosystems of the Crozet Islands remain limited (Sicinski & Gillet Reference Sicinski and Gillet2002, Lelièvre et al. Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023, Jossart et al. Reference Jossart, Lelièvre, Kelch, Figuerola, Moreau and Di Franco2024). Benthic fauna data are rare and restricted to few campaigns undertaken on the plateau at the end of the nineteenth (HMS Challenger, 1872) and twentieth centuries (R/V Marion Dufresne MD08 1976, and MD30 1982) along with recent fishery bycatch analysis (Koubbi et al. Reference Koubbi, Hulley, Raymond, Penot, Gasparini and Labat2016). At Baie du Marin (Ile de la Possession), Lelièvre et al. (Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023) provided a first bentho-ecological baseline of the composition and structure of nearshore Crozet benthic communities associated with hard substrates. A total of 50 faunal and 14 algal taxa were identified using underwater imagery, with a distribution characterized by a high level of spatial heterogeneity. Through a trait-based approach, this study reported the high vulnerability of faunal benthic communities to current and future environmental changes (Lelièvre et al. Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023). A thorough knowledge of the biodiversity of these remote ecosystems is urgently needed to predict and subsequently monitor the influence of global change on these communities and to contribute to the protection and conservation of these habitats. Since December 2016, the maritime domain surrounding the Crozet archipelago has been classified as a nature reserve. Among the Crozet Islands, Ile de la Possession hosts a hydroacoustic monitoring station installed by the Preparatory Commission for the Comprehensive Nuclear-Test-Ban Treaty Organization (CTBTO) in December 2016. The French Southern and Antarctic Territories (TAAF) and CTBTO signed a contract for ‘Nearshore Cable Inspection and Environmental Survey at IMS Hydroacoustic Station HA04 Crozet, France’ in September 2021, and a first campaign took place on Ile de la Possession at Crozet in November 2021. The inspection of the HA04 station cable was completed by environmental surveys of nearshore habitats for conservation purposes and improving our knowledge of Crozet marine ecosystems. In this context, the aims of this study were 1) to provide an assessment and a comparison of the coastal benthic faunal and algal diversity associated with different habitat types between two sites of Ile de la Possession: Baie du Marin and Crique du Sphinx; 2) to investigate the relationships between habitat types, algal cover and faunal composition; and 3) to determine the indicator species associated with each of these habitats in order to establish a baseline for future monitoring of these sites.
Materials and methods
Study areas
The Crozet archipelago (45°48′S–46°26′S, 50°14′E–52°15′E) is composed of five main volcanic islands (from west to east: Ile aux Cochons, Ile des Pingouins, Ilots des Apôtres, Ile de la Possession and Ile de l’Est) located in the Southern Ocean, 2400 km north of the Antarctic continent and 2400 km south-east of the South African coast (Fig. 1a). Ile de la Possession (46°25′S, 51°45′E; Fig. 1a) is the largest island of the Crozet archipelago, with a total surface area of ~156 km2; it culminates at 934 m above sea level (Pic du Mascarin). The western part of the island is dominated by rugged terrain, while the eastern part is characterized by plateaus and large valleys. The present study was focused on two sites located on the eastern coast of Ile de la Possession: Baie du Marin and Crique du Sphinx (Fig. 1b).

Figure 1 a. Topographic map of Ile de la Possession (Crozet archipelago, Southern Ocean) with the location of the two study sites: Baie du Marin and Crique du Sphinx (black diamonds). b. Imagery sampling design conducted at Baie du Marin and Crique du Sphinx with black transects corresponding to scuba dives and yellow transects to remotely operated vehicle (ROV) dives; Photographs of c. Baie du Marin (© P. Salvatico) and d. Crique du Sphinx (© T. Saucède).
Baie du Marin (46°25′54"S, 51°52′11"E) is a marine inlet ~500 m long and 200 m wide in its shallowest part (down to 20 m); it opens to the ocean in a larger embayment of ~2 km wide at 40 m depth (Fig. 1c). The coast is mainly a rocky shore but for the sandy beach present at the end of the bay. The site is characterized by the presence of a large colony of over 10 000 king penguins Aptenodytes patagonicus (Miller, 1778) along with some elephant seals Mirounga leonina (Linnaeus, 1758) that occupy most of the beach inside the bay in spring. The bay has long been visited by vessels for the supply of Alfred Faure scientific base. Located next to Baie du Marin, at 2 km to the north, Crique du Sphinx (46°25’08"S, 51°52’44"E) is a small cove of ~250 m long and 150 m wide opening to the north-east (Fig. 1d). The site is mainly bordered by a rocky shore, with a small beach of pebbles and gravels at the end. Crique du Sphinx is a shelter for a handful of king penguins and elephant seals. It is never visited by ships and shows no anthropic activities.
Diving surveys and imagery acquisition
Imagery data acquisition was undertaken from the R/V Marion Dufresne II calling off Ile de la Possession from 4 to 9 November 2021. Operations were conducted by the TAAF in response to Contract No. 2021-0882 with the CTBTO for ‘Nearshore Cable Inspection and Environmental Survey at IMS Hydroacoustic Station HA04 Crozet, France’. Video imagery transects were performed using a setting composed of three cameras, two GoPro® HERO7 cameras (4K video and 20 megapixel photo resolution) with 80% field overlap and one underwater Paralenz Vaquita camera (4K resolution) mounted on a plexiglass board. Two lights and three 10 cm scaling lasers were also installed to estimate the filmed surface. To limit variations in video imaging acquisition, scuba divers scanned transects at a distance of ~50 cm from the sea bottom with a constant swimming speed.
Five video transects were conducted: three at Baie du Marin and two at Crique du Sphinx (Table I). At Baie du Marin, a first video transect (BDM-T1) was performed along the track of the cable, a second transect (BDM-T2) was carried 10 m south of the cable and a third one (BDM-T3) was conducted across the bay, from the centre of the bay at 10 m depth to the north coast (Table I). At Crique du Sphinx, two video transects (CdS-T1 and CdS-T2) were performed along the cove (Table I). Imagery surveys deeper than 20 m were conducted using the ROV SAAB Seaeye Cougar-XT Compact. Six ROV transects were aligned with transect videos performed during scuba dives. Four ROV transects were realized at Baie du Marin: two over the cable (BDM-T4 and BDM-T5) and two to the north of the bay (BDM-T6 and BDM-T7). These were complemented by one deeper transect of a restricted area at 60 m depth (BDM-T8; Table I). Finally, two ROV dives were conducted in the south and north parts of Crique du Sphinx (CdS-T3 and CdS-T4, respectively; Table I). All video imagery data are available at the dat@UBFC repository (https://doi.org/10.25666/DATAUBFC-2024-03-15; Lelièvre et al. Reference Lelièvre, Motreuil, Specq, Marschal, Dubois and Wauters2024a,Reference Lelièvre, Motreuil, Specq, Marschal, Dubois and Wautersb).
Table I. Summary of transect information for each dive carried out at Baie du Marin (BDM) and Crique du Sphinx (CdS).

ROV = remotely operated vehicle.
Benthic imagery processing
For each transect, still images were equally extracted from video imagery every 5 m along the transect. A total of 594 images were manually analysed using the open-source web platform BIIGLE 2.0 (Benthic Image Indexing and Graphical Labelling Environment; Langenkämper et al. Reference Langenkämper, Zurowietz, Schoening and Nattkemper2017). BIIGLE 2.0 was developed with a special focus on benthic imagery and provides the advantage of a collaborative and interactive work with taxonomists who can contribute to the identification of organisms from images (Langenkämper et al. Reference Langenkämper, Zurowietz, Schoening and Nattkemper2017). For each image, all faunal organisms were counted and expressed in terms of density (number of individuals m-2), and algae cover was also measured (m-2). In agreement with the taxonomists, organisms were identified to the lowest possible taxonomic level. Additionally, the substrate type was categorized into seven habitats, defined by the predominant features that create structural complexity in the environment (Airoldi & Beck Reference Airoldi and Beck2007) and annotated for each image: 1) sand, 2) sand-cables, 3) sand-pebbles, 4) sand-pebbles-cables, 5) pebbles, 6) rock and 7) suspended cables (Fig. 2).

Figure 2. Classification of the different benthic habitats: a. sand, b. sand-cables, c. sand-pebbles, d. sand-pebbles-cables, e. pebbles, f. suspended cables and g. & h. rock habitats. Laser beams for image scaling are 10 cm spaced.
Statistical analyses
To assess the sampling effort at Baie du Marin and Crique du Sphinx, individual-based rarefaction curves were computed for faunal communities and image-based rarefaction curves were computed for algal communities. Diversity metrics corresponding to Hill’s number of order q were calculated for the faunal and algal communities of each transect performed at Baie du Marin and Crique du Sphinx: species richness (q = 0), Shannon diversity (q = 1), Simpson’s inverse (q = 2; Jost Reference Jost2006) and Pielou’s evenness. These metrics were also calculated to compare the richness and diversity values between shallow (0–20 m depth) and deeper waters (20–60 m depth) at both sites.
To investigate the compositional benthic community variations between transects and sites, a principal component analysis (PCA) was performed on Hellinger-transformed faunal and algal cover densities at the transect scale. The Hellinger transformation was applied to minimize the high weight given to rare species (Legendre & Gallagher Reference Legendre and Gallagher2001). A redundancy analysis (RDA) was also performed on these Hellinger-transformed data to evaluate the relationships between substrate type, algal cover and faunal composition in shallow waters. Finally, indicator species analyses (Dufrêne & Legendre Reference Dufrêne and Legendre1997, De Cáceres et al. Reference De Cáceres, Legendre and Moretti2010, Legendre & Legendre Reference Legendre and Legendre2012) were performed to identify faunal and algal taxa significantly associated with substrate types. The indicator value index (IndVal) is only based on within-species abundance and occurrence comparisons and so would not be affected by the abundance values of other species (Legendre & Legendre Reference Legendre and Legendre2012). Species with a high fidelity (the degree to which a species is found only in a given group of sites) and high specificity (the degree to which a species is present at all sites of a group) within a habitat or a combination of habitats would have a high indicator value. According to Dufrêne & Legendre (Reference Dufrêne and Legendre1997), a species is considered as characteristic of a substrate type or habitat if the species IndVal is > 25% for a significant P-value of < 0.05.
All statistical analyses were performed using the R environment (version 4.2.0; R Core Team 2022). Rarefaction curves and diversity metrics were conducted using the packages iNEXT (Hsieh et al. Reference Hsieh, Ma and Chao2016) and vegan (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre and Mcglinn2020). PCA and RDA were realized using the package vegan (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre and Mcglinn2020). Indicator species analysis was conducted using the package indicspecies (De Cáceres & Legendre Reference De Cáceres and Legendre2011).
Results
Sampling effort and benthic communities diversity
The species lists for each transect conducted at Baie du Marin and Crique du Sphinx are presented in Table II (Table S1 for abundance and density data). A total of 51 faunal and 14 algal taxa were found at the two sites, corresponding to 43 faunal and 13 algal taxa at Baie du Marin and 35 faunal and 13 algal taxa at Crique du Sphinx, respectively. Among these taxa, 26 faunal and 11 algal taxa were shared between the two studied sites. Overall, rarefaction curves tend to reach a plateau (Fig. 3a,b), indicating that the total collection of analysed images gave a good representation of faunal and algal diversities at Baie du Marin and Crique du Sphinx.
Table II. Faunal and algal taxa identified along the transects conducted at Baie du Marin and Crique du Sphinx using underwater imagery. Black circles indicate the presence of this taxon.
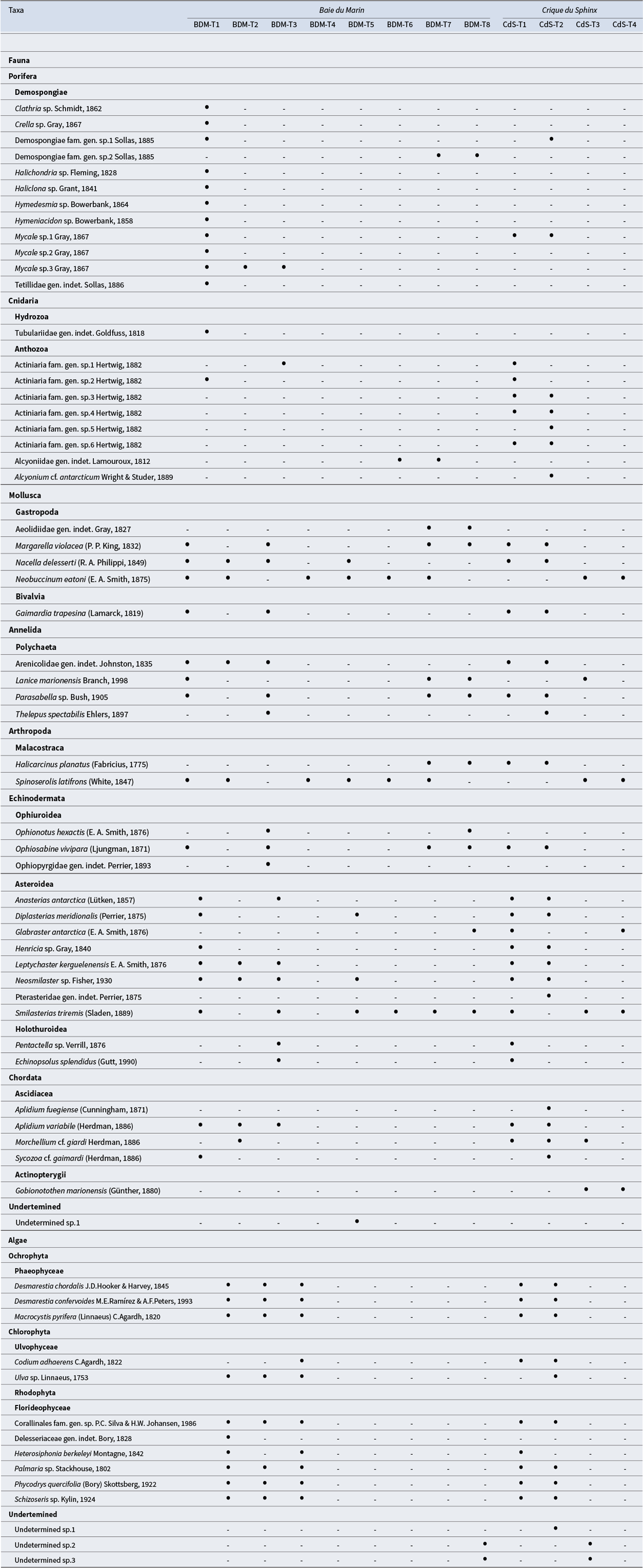
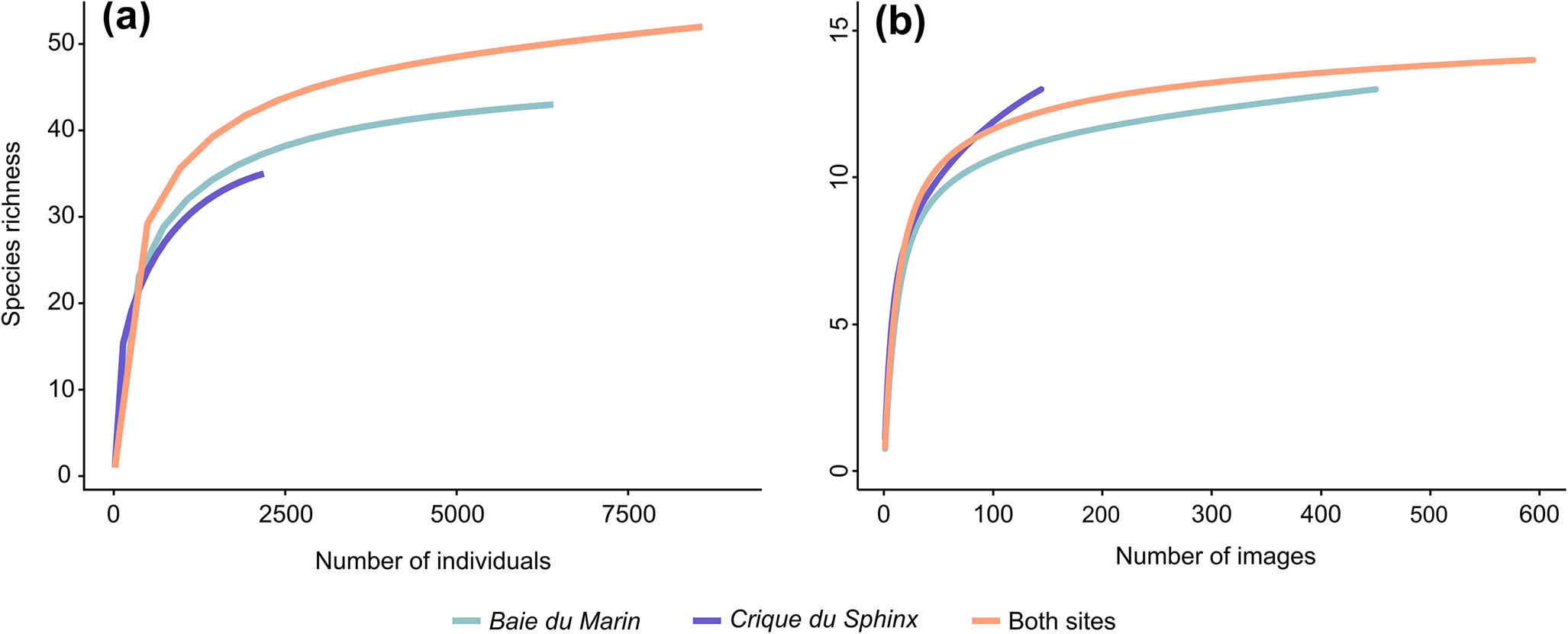
Figure 3. Rarefaction curves for a. faunal and b. algal communities at the Baie du Marin and Crique du Sphinx scale.
At Baie du Marin, BDM-T1 fauna was dominated by the ophiuroid Ophiosabine vivipara (2.4 individuals m-2; 26.6%), the polychaete Arenicolidae gen. indet. (1.4 individuals m-2; 15.6%) and the ascidian Aplidium variabile (1.3 individuals m-2; 14.8%). The algal component was dominated by Desmarestia confervoides (41.9%), followed by Macrocystis pyrifera (14.2%) and Phycodrys quercifolia (11.6%). Fauna of the BDM-T2 transect was dominated by the isopod Spinoserolis latifrons (3 individuals m-2; 64%) and to a lesser extent by the gastropod Nacella delesserti (1.2 individuals m-2; 26.3%); algae were also dominated by D. confervoides (35.7%), but also by Palmaria sp. (34%) and Ulva sp. (13.7%). BDM-T3 fauna was dominated by the holothurid Echinopsolus splendidus (124 individuals m-2; 51.1%), by the actiniid Actiniaria fam. gen. sp.1 (34.4 individuals m-2; 14.2%) and to a lesser extent by the polychaete Parasabella sp. (64.9 individuals m-2; 26.7%). Algal flora was dominated by Desmarestia chordalis (55.2%), followed by D. confervoides (20.4%) and Codium adhaerens (12.9%). Fauna of the BDM-T4 transect was largely dominated by the isopod S. latifrons (38.3 individuals m-2; 99.5%). BDM-T5 fauna was also dominated by the isopod S. latifrons (5.9 individuals m-2; 81.9%) and to a lesser extent by the gastropod Neobuccinum eatoni (0.7 individuals m-2; 10.4%). Fauna of the BDM-T6 transect was largely dominated by the isopod S. latifrons (34.3 individuals m-2; 95.6%). BDM-T7 fauna was dominated by the isopod S. latifrons (4 individuals m-2; 42%) and the polychaete Lanice marionensis (3.9 individuals m-2; 40.7%). No algal taxa were found along transects BDM-T4 to BDM-T7. A high density of polychaete L. marionensis (1032.6 individuals m-2; 85.9%) was found in BDM-T8, as well as the ophiuroid O. vivipara (44.2 individuals m-2; 2.3%), whereas algae were only represented by two undetermined taxa: Undetermined sp.2 (5.7%) and Undetermined sp.3 (94.3%).
At Crique du Sphinx, CdS-T1 fauna was dominated by the polychaete Parasabella sp. (44.2 individuals m-2; 29.1%), followed by the gastropod N. delesserti (11.9 individuals m-2; 40%) and the actiniid Actiniaria fam. gen. sp.3 (3.5 individuals m-2; 11.8%). Algal flora was dominated by C. adhaerens (39.1%), D. confervoides (16.1%) and Schizoseris sp. (15.1%). Similarly, transect CdS-T2 fauna was dominated by the polychaete Parasabella sp. (11.2 individuals m-2; 47.6%), by the actiniid Actiniaria fam. gen. sp.3 (3.2 individuals m-2; 13.4%) and to a lesser extent by the gastropod Margarella violacea (1.5 individuals m-2; 6.4%), whereas algae were dominated by D. chordalis (35.1%), followed by C. adhaerens (19.6%) and D. confervoides (17%). Fauna of the CdS-T3 transect was dominated by the polychaete L. marionensis (12.6 individuals m-2; 49.2%) and the isopod S. latifrons (10.9 individuals m-2; 42.4%). The algal component of CdS-T3 was dominated by Undetermined sp.2 (76.9%) and Undetermined sp.3 (23.1%). Finally, CdS-T4 fauna was largely dominated by the isopod S. latifrons (91.4 individuals m-2; 42.4%). No algae were found in CdS-T4.
Taxonomic richness and diversity indices
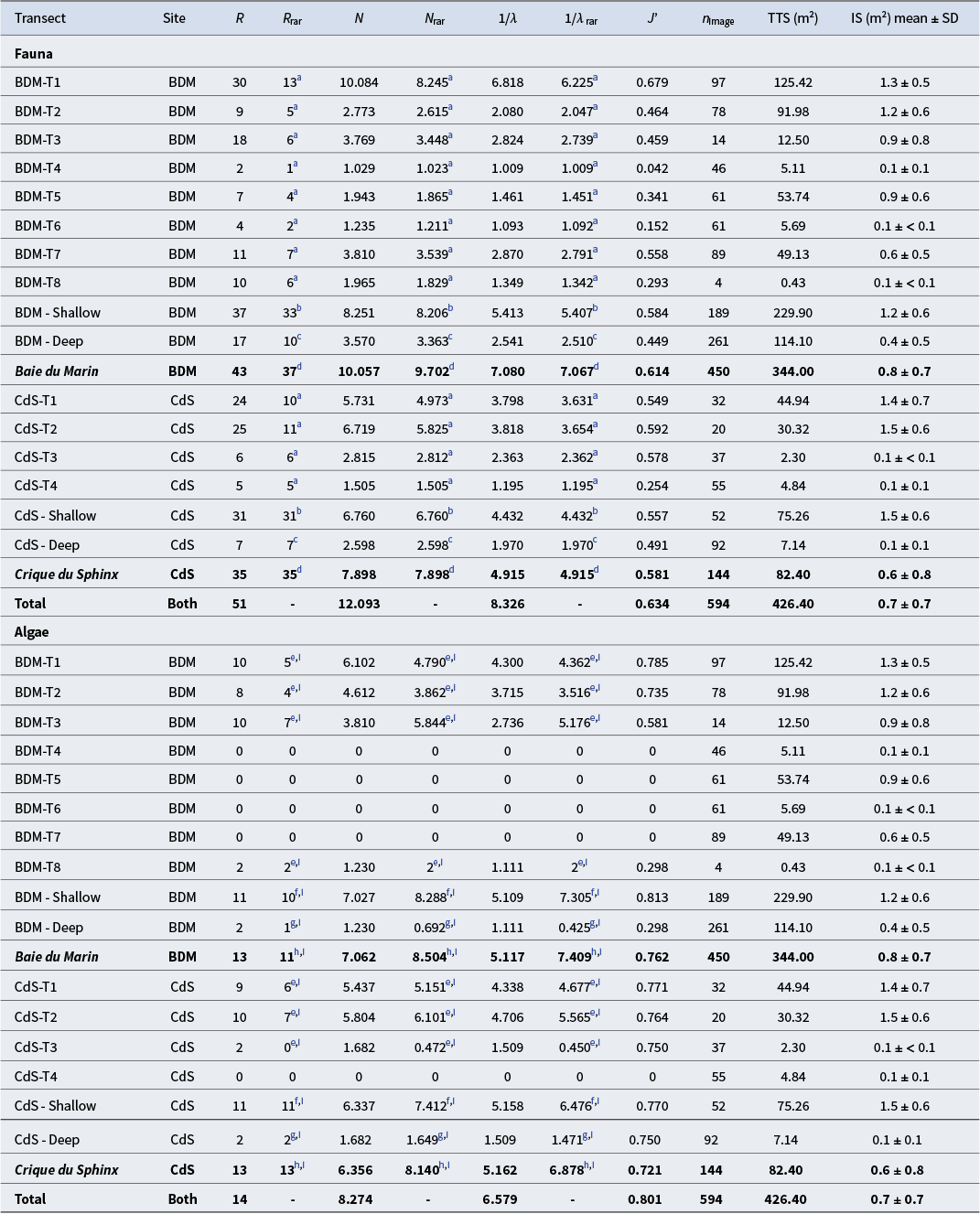
The shallow waters of Baie du Marin and Crique du Sphinx showed higher faunal richness and diversity values compared to deeper areas (Table III). Diversity metrics showed that BDM-T1, filmed along submarine cables, displayed the highest faunal species richness (R rar = 13) and diversity (N rar = 8.245; 1/λrar = 6.225) values, followed by the two transects conducted at Crique du Sphinx, CdS-T2 (R rar = 11; N rar = 5.825; 1/λrar = 3.654) and CdS-T1 (R rar = 10; N rar = 4.973; 1/λrar = 3.631), respectively (Table III). Faunal species richness showed no major differences between the respective shallow waters and deeper waters of both sites. The Shannon and Simpson diversity indices for shallow-water faunal communities were highest at Baie du Marin (N rar = 8.206; 1/λrar = 5.407) than at Crique du Sphinx (N rar = 6.760; 1/λrar = 4.432). A similar pattern was observed for deeper waters, where the highest values were recorded at Baie du Marin (N rar = 3.363; 1/λrar = 2.510) in contrast to Crique du Sphinx (N rar = 2.598; 1/λrar = 1.970). Finally, both sites showed a similar faunal species richness, with R rar = 32.802 for Baie du Marin and R rar = 31 for Crique du Sphinx, respectively. However, diversity values were higher at Baie du Marin (N rar = 9.702; 1/λrar = 7.067) than at Crique du Sphinx (N rar = 7.898; 1/λrar = 4.915; Table III).
Table III. Species richness and diversity indices of faunal and algal communities for each transect conducted at Baie du Marin (BDM) and Crique du Sphinx (CdS). Diversity indices are taxonomic richness (R), rarefied taxonomic richness (R rar), Shannon diversity (N), rarefied Shannon diversity (N rar), Simpson diversity (1/λ), rarefied Simpson diversity (1/λrar) and Pielou’s evenness (J’). The sampling effort is also provided with regards to the number of images analysed (n image), transect total surface (TTS) and the mean image surface (IS).
a Rarefied to 58 individuals.
b Rarefied to 433 individuals.
c Rarefied to 58 individuals.
d Rarefied to 2172 individuals.
e Rarefied to 4 images.
f Rarefied to 52 images.
g Rarefied to 92 images.
h Rarefied to 144 images.
i Rarefied univariate measures for algae were estimated from presence-absence data.
For algal communities, the species richness was predominantly located in shallow waters (R rar = 11 for both sites) compared to deeper waters (R rar = 2 for both sites). No major algal species richness differences were found between transects where algae were found. The highest algal diversity was found at CdS-T2 (N rar = 6.101; 1/λrar = 5.565), followed by BDM-T3 (N rar = 5.844; 1/λrar = 5.176) and CdS-T1 (N rar = 5.151; 1/λrar = 4.677). As reported for species richness, diversity indices were higher in shallow waters compared to deeper waters. Finally, algal diversity values were similar between Baie du Marin (N rar = 8.504; 1/λrar = 7.409) and Crique du Sphinx (N rar = 8.140; 1/λrar = 6.878; Table III).
Influence of habitat type on benthic communities
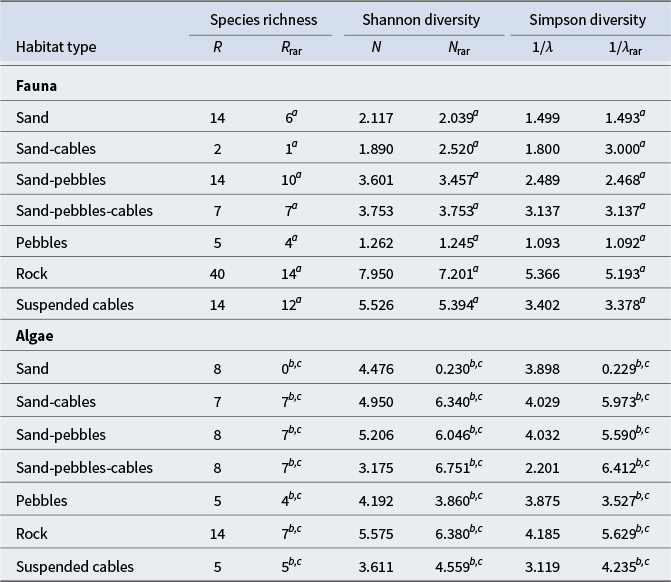
Diversity indices calculated on faunal communities showed that rocky habitats displayed the highest species richness (R rar = 14) and the highest diversity values (N rar = 7.201; 1/λrar = 5.193), followed by the suspended cables (N rar = 5.394; 1/λrar = 3.378) and sand-pebbles-cables (N rar = 3.753; 1/λrar = 3.137) habitats. In contrast, the lowest faunal diversity values were observed for the pebble habitat (N rar = 1.245; 1/λrar = 1.092), followed by sand (N rar = 2.039; 1/λrar = 1.493) and sand-cables (N rar = 2.520; 1/λrar = 3) habitats. For algal communities, with the exception of sand habitats, rarefied species richness and diversity values were similar between the various habitats (Table IV).
Table IV. Species richness and diversity indices of faunal and algal communities for each habitat type, including taxonomic richness (R), rarefied taxonomic richness (R rar), Shannon diversity (N), rarefied Shannon diversity (N rar), Simpson diversity (1/λ) and rarefied Simpson diversity (1/λrar).
a Rarefied to 128 individuals.
b Rarefied to 5 images.
c Rarefied univariate measures for algae were estimated from presence-absence data.
Faunal and algal composition variations between transects
PCAs were performed to investigate benthic community variations between transects and sites (Fig. 4). For faunal communities (Fig. 4a), the PCA showed that no major faunal composition variations were found between Baie du Marin and Crique du Sphinx. However, the PCA highlighted significant differences between shallow-water and deeper-water faunal communities. Transects are distributed into four groupings: 1) transects conducted by scuba divers between 0 and 20 m depth, including BDM-T1, BDM-T3, CdS-T1 and CdS-T2 - these transects were characterized by high densities of the polychaete Parasabella sp., the ophiuroid O. vivipara and the gastropod N. delesserti; 2) ROV transects conducted in the 20–60 m depth area, including BDM-T4, BDM-T5, BDM-T6, CdS-T4 and BDM-T2 - these transects were characterized by the presence of the isopod S. latifrons; 3) transect BDM-T8 at 60 m depth and characterized by a high density of the polychaete L. marionensis; and 4) transects BDM-T7 and CdS-T3, with an intermediate faunal composition between BDM-T8 and the transects of group 2 (Fig. 4a). For algal communities (Fig. 4b), the PCA showed a clear distinction between shallow waters and deeper waters. No algal composition variations were found between the BDM-T1 and BDM-T2 transects. These two transects were characterized by a dominance of Ulva sp., Palmaria sp. and D. confervoides. However, the BDM-T3 transect showed an algal composition that was similar to CdS-T1 and CdS-T2 transects, with a dominance of C. adhaerens, D. chordalis and several species of Corallinales (Fig. 4b).

Figure 4. Principal component analysis (PCA) on the Hellinger-transformed a. faunal and b. algal cover densities of the 12 imagery transects conducted at Ile de la Possession. The first two PCA axes captured 74.9% and 77.7% of the total variance of Hellinger-transformed faunal and algal composition, respectively. Circles and triangles correspond to transects conducted at Baie du Marin and Crique du Sphinx, respectively; light blue corresponds to shallow-water scuba diving surveys and dark blue to deeper remotely operated vehicle transects. Only species names that best fit the first two canonical axes are shown on the plot. BDM-T4 to BDM-T7 and CdS-T4 transects were not represented in b. due to the absence of algae.
Relationships between faunal communities, algal cover and habitat types
The RDA showed a high discrimination level between the different habitat types and the distribution of faunal and algal diversity (Fig. 5). The RDA highlighted three main benthic habitats: 1) sandy bottoms, 2) mixtures between soft and hard sediments (cables and pebbles) and 3) hard bottoms (rocky habitats and submarine cable suspended sections). Although no algae appeared to be associated with sandy habitats, the isopod S. latifrons and the polychaete Arenicolidae gen. indet. showed a close relationship with such environments. In environments characterized by a mixture between sand, pebbles and/or submarine cables, the algal diversity and surface cover are more important, with a high affinity degree of the gastropod N. delesserti and ascidian A. variabile. In contrast, rocky habitats showed a specific faunal and algal composition, dominated by the algae C. adhaerens and the polychaete Parasabella sp., the actiniids Actiniaria fam. gen. sp.3 and Actiniaria fam. gen. sp.4, as well as the gastropod M. violacea (Fig. 5). Interestingly, suspended cables and rocky habitats were closely associated.

Figure 5. Redundancy analysis (RDA) between the Hellinger-transformed faunal densities and algae surface cover across the habitat types identified within underwater transects conducted at the shallow waters of Baie du Marin and Crique du Sphinx. The first two canonical axes represented account together for 43.03% of the total variance.
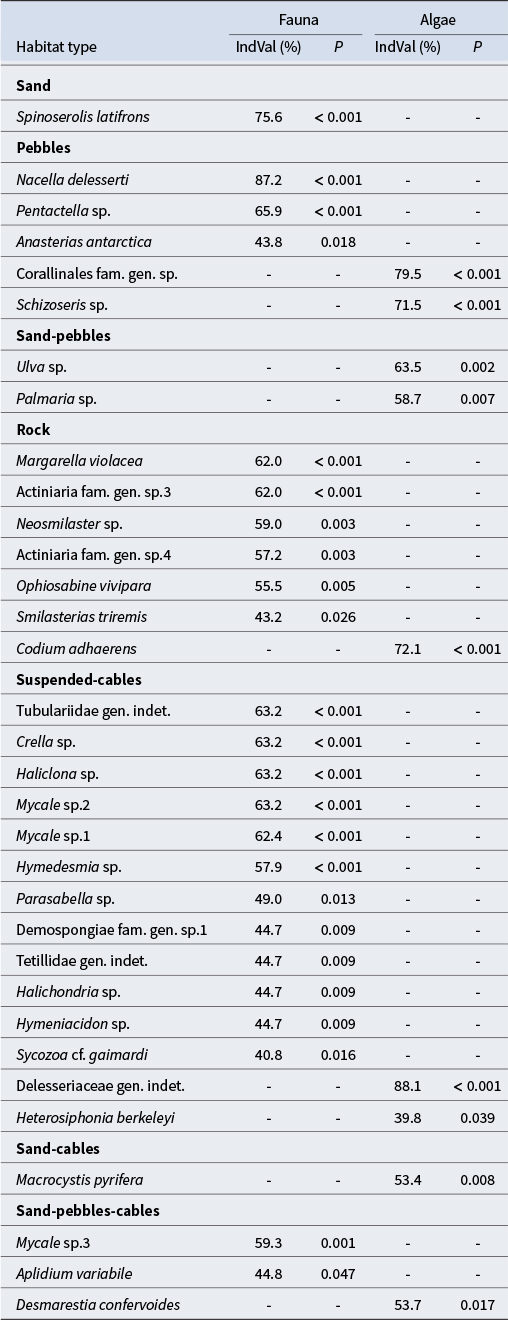
Indicator species
Among the 51 benthic faunal and 14 algal taxa identified at the two studied sites of Ile de la Possession, 24 faunal and 9 algal taxa were found to be indicator species of substrate types (Table V). The isopod serolid S. latifrons was the only significant indicator species of sandy habitats. Pebbles environments were characterized by three indicator faunal taxa, including the gastropod N. delesserti, the holothurid Pentactella sp. and the sea star Anasterias antarctica; and two indicator algal taxa, the crustose coralline Corallinales fam. gen. sp. and the Rhodophyta Schizoseris sp. No indicator faunal taxa were found to be specifically associated with the sand-pebbles habitats. However, the Chlorophyta Ulva sp. and Rhodophyta Palmaria sp. were recognized as indicator algal taxa. Seven faunal taxa, including the actiniids Actiniaria fam. gen. sp.3 and Actiniaria fam. gen. sp.4, the gastropod M. violacea, the sea stars Neosmilaster sp. and Smilasterias triremis, the brittle star O. vivipara and the Chlorophyta C. adhaerens algal taxa were identified as indicator species of rocky substrates. For the suspended cables habitat, a high diversity of Demospongiae were identified as indicator taxa, including Crella sp., Haliclona sp., Mycale sp.1, Mycale sp.2, Hymedesmia sp., Demospongiae fam. gen. sp.1, Tetillidae gen. indet., Halichondria sp. and Hymeniacidon sp. In addition, the tubulariid Tubulariidae gen. indet., the polychaete Parasabella sp. and the ascidian Sycozoa cf. gaimardi were also identified as indicator faunal taxa, and the Rhodophyta Delesseriaceae gen. indet. and Heterosiphonia berkeleyi were identified as indicator algal taxa. Whereas the alga M. pyrifera was identified as an indicator taxon of sand-cables substrate, no faunal taxa were specially associated at this habitat type. Finally, the sand-pebbles-cables habitat was characterized by two faunal indicator taxa: the sponge Mycale sp.3 and the ascidian A. variabile, as well as one algal taxa: D. confervoides.
Table V. List of the significant indicator faunal and algal taxa through the different habitat types identified at Baie du Marin and Crique du Sphinx, with their associated indicator value index (IndVal) percentages and P-values.
Discussion
Crozet coastal habitats and benthic communities
Whereas Lelièvre et al. (Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023) were focused on subtidal communities associated with the hard substrates of Baie du Marin, this study provides important ecological information on the structure of faunal and algal communities associated with a wide range of habitats at two sites located on the eastern coast of Ile de la Possession: Baie du Marin and Crique du Sphinx. As reported by Lelièvre et al. (Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023) at Baie du Marin and in numerous sub-Antarctic and Antarctic environments (Downey et al. Reference Downey, Griffiths, Linse and Janussen2012, Le Bourg et al. Reference Le Bourg, Saucède, Charpentier, Lepoint and Michel2022), Crozet faunal communities were dominated by a high diversity of Porifera (12 Demospongiae) and Echinodermata (3 Ophiuroidea, 8 Asteroidea and 2 Holothuroidea). Interestingly, some characteristic subtidal sub-Antarctic groups such as barnacles and mussels (Arnaud Reference Arnaud1974, Freeman et al. Reference Freeman, Cooper, Funnell and Neale2011) appear to be absent in Crozet studied areas. The algal diversity found in Crozet coastal waters was similar to the marine vegetation identified at the Kerguelen Islands, with a dominance of Rhodophyta taxa (Féral et al. Reference Féral, Verlaque, Rosenfeld, Poulin, Chenuil and Saucède2021). Whereas Kerguelen coastal waters are characterized by vast and dense M. pyrifera kelp forests (Féral et al. Reference Féral, Verlaque, Rosenfeld, Poulin, Chenuil and Saucède2021), the distribution of M. pyrifera in the investigated sites of Crozet is sporadic and in small patches. However, drawing comparisons between the benthic richness and diversity of Crozet and other sub-Antarctic islands remains difficult given the highly different collecting methods and depth ranges used and the large disparity in the sampling effort carried out off the different islands.
The coastal environments of Baie du Marin and Crique du Sphinx may be divided into two areas: 1) shallow waters (spanning from the coastline to 20 m depth) and 2) deeper waters (from 20 to 60 m depth). In both sites, shallow waters are inhabited by various invertebrate taxa and marine vegetation. The highest species richness and diversity values observed in shallow waters may be attributed to the high degree of habitat heterogeneity at the site scale. The habitat mosaic, formed by the alternation and combination of different habitats (e.g. sandy, pebbles and rocky substrates), enhances the coexistence of a wide range of benthic taxa by providing varied abiotic (e.g. environmental conditions) and biotic (e.g. resource availability, species interactions) conditions, thereby shaping the composition and diversity of benthic communities (Huston Reference Huston1994, Guégan et al. Reference Guégan, Lek and Oberdorff1998, Hewitt et al. Reference Hewitt, Thrush, Halliday and Duffy2005, Thrush et al. Reference Thrush, Gray, Hewitt and Ugland2006, Stein et al. Reference Stein, Gerstner and Kreft2014). In the shallow waters of the Prince Edward Islands, substrate heterogeneity is also linked to spatial variation in benthic communities, thereby supporting a rich benthic diversity (Branch et al. Reference Branch, Attwood, Gianakouras and Branch1993). Among the French sub-Antarctic territories, the Kerguelen archipelago is composed of numerous islands and islets favouring complex and diverse nearshore environments and promoting high diversity levels (Arnaud Reference Arnaud1974, Féral et al. Reference Féral, Poulin, González-Wevar, Améziane, Guillaumot, Develay and Saucède2019, Reference Féral, Verlaque, Rosenfeld, Poulin, Chenuil and Saucède2021). In addition, habitat-forming species such as marine vegetation also contribute to increasing habitat heterogeneity and complexity. Algal physical characteristics may alter local environmental conditions (e.g. hydrodynamic conditions, wave energy, deposition of sedimentary material, oxygen concentration) and generate a wide range of ecological niches that increase species richness and diversity (Gambi et al. Reference Gambi, Lorenti, Russo and Scipione1994, Delille et al. Reference Delille, Borges and Delille2009, Amsler et al. Reference Amsler, McClintock and Baker2014). With the exception of the BDM-T1 transect conducted along the cable, the shallow benthos at Crique du Sphinx was characterized by higher richness and diversity values than that of Baie du Marin. This difference may be explained by the predominance of rocky habitats at Crique du Sphinx, whereas the shallow bottom at Baie du Marin was characterized by large sandy stretches. In contrast to the shallow waters, deeper areas at Baie du Marin and Crique du Sphinx were dominated by vast sandy stretches, leading to a homogeneous habitat and low diversity. From 20 m depth, algae were nearly absent.
Apart from the BDM-T2 transect, no major faunal and algal composition differences were found between the two studied sites. The dominance of sandy stretches along the BDM-T2 transect and consequently the presence of a high abundance of the isopod S. latifrons explain the faunal similarity between the BDM-T2 and ROV transects. The BDM-T8 transect, conducted at 60 m depth, showed specific benthic faunal communities that could be explained by the presence of a long rocky area off Baie du Marin, colonized by a very dense colony of the polychaete L. marionensis. Finally, two ROV transects, BDM-T7 and CdS-T3, showed high diversity values compared to the other ROV transects, with an intermediate species composition between BDM-T8 and other ROV transects. This results from the presence of rocky patches in a seascape dominated by sandy sediments along these transects. The abundance and diversity is generally higher in structurally complex habitats compared to homogeneous environments (Hewitt et al. Reference Hewitt, Thrush and Dayton2008, Törnroos et al. Reference Törnroos, Nordström and Bonsdorff2013, Henseler et al. Reference Henseler, Nordström, Törnroos, Snickars, Pecuchet, Lindegren and Bonsdorff2019). The presence of hard substrates on the sandy sediment bottom increases habitat structural complexity and thereby enhances the development (diversity, abundance and biomass) of epibenthic communities by offering an additional space to colonize and to shelter from predation and unfavourable abiotic conditions, as well as offering food sources (Downes et al. Reference Downes, Lake, Schreiber and Glaister1998, Grzelak & Kuklinski Reference Grzelak and Kuklinski2010, Levin et al. Reference Levin, Sibuet, Gooday, Smith and Vanreusel2010, Lelièvre et al. Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023). Our results emphasize the important role of habitat heterogeneity as well as substrate type in the composition and structure of Crozet benthic communities.
Habitat types and benthic diversity
Baie du Marin and Crique du Sphinx sandy bottoms showed poorly diversified epifaunal communities and were largely dominated by the sand-dwelling isopod S. latifrons (up to 99.6% of species densities) and, to a lesser extent, by the gastropod Neobuccinum eatoni. The isopod S. latifrons was identified as a good indicator taxa of sandy bottoms and is widely distributed in Antarctic and sub-Antarctic waters (Castelló Reference Castelló2004, Xavier et al. Reference Xavier, Cherel, Boxshall, Brandt, Coffer and Forman2020). The presence of pebbles on the soft sediments provides a hard substrate for the settlement of a wide variety of seaweeds, promoting the abundance of the grazer gastropod N. delesserti that mainly feeds algal spores and sporelings (Blankley & Branch Reference Blankley and Branch1985). The genus Nacella is distributed across the Southern Ocean, including temperate and sub-Antarctic areas of South America and the Falkland/Malvinas Islands, Antarctica and the sub-Antarctic islands, such as Marion, Kerguelen, Heard, Macquarie and Campbell islands (González-Wevar et al. Reference González-Wevar, Hüne, Rosenfeld, Nakano, Saucède, Spencer and Poulin2019). However, the species N. delesserti is restricted to Marion and Crozet islands (Cantera & Arnaud Reference Cantera and Arnaud1985, González-Wevar et al. Reference González-Wevar, Hüne, Rosenfeld, Nakano, Saucède, Spencer and Poulin2019). This limpet is the most abundant gastropod on the shores of Marion Island and is the main component of intertidal and shallow-water benthic communities (Blankley & Branch Reference Blankley and Branch1985). In contrast to sandy and pebble habitats, Baie du Marin and Crique du Sphinx bedrocks are covered with more diverse and dense communities. In accordance with Lelièvre et al. (Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023), shallow rocky habitats were characterized by a wide diversity of invertebrate species, including the presence of dense colonies of the sessile suspension-feeding polychaete Parasabella sp. and the ophiuroid O. vivipara. The ophiuroid O. vivipara also occurs in numerous sub-Antarctic islands, including the Falkland/Malvinas Islands, South Georgia Island, Marion Island, the Prince Edwards Islands and the Kerguelen Islands (O’Hara & Thuy Reference O'Hara and Thuy2022). Deeper down (i.e. beyond 20 m), the polychaete Parasabella sp. was scarce on hard substrata and was replaced by dense colonies of the sessile deposit- and suspension-feeding tube-dwelling polychaete L. marionensis. High densities of the polychaete L. marionensis were also found at Baie du Marin on the rock located at the centre of the bay, at 19 m depth (Lelièvre et al. Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023). Similarly, a high abundance of the tube-dwelling polychaete L. marionensis was reported in the Prince Edward Islands and Marion Island (Sicinski & Gillet Reference Sicinski and Gillet2002, von der Meden et al. Reference von der Meden, Atkinson, Branch, Asdar, Ansorge and van den Berg2017), but the species was not reported from the Kerguelen Islands (Sicinski & Gillet Reference Sicinski and Gillet2002). The high abundance of the polychaete L. marionensis may be favoured on the one hand by the high flow rates off Baie du Marin, bringing a lot of suspended food, and on the other hand by its high trophic plasticity, as shown for the polychaete Lanice conchilega, which, in addition to suspension feeding, is able to actively switch to deposit feeding when suspended food is limited (Rabaut et al. Reference Rabaut, Guilini, Van Hoey, Vincx and Degraer2007, Allan Reference Allan2011).
Influence of submarine cables on benthic communities
At Baie du Marin, transect BDM-T1 performed along cables displayed the highest species richness and diversity values in contrast to transect BDM-T2 performed parallel to BDM-T1 and characterized by the lowest diversity values. The submarine cable suspended section was marked by high diversity values. Image analysis of the cables showed an important diversity of sponge taxa that were not observed along other transects, suggesting a potential influence of the cables on species diversity. Artificial structures such as submarine cables may have a reef effect on benthic communities depending on the surrounding environment and native species assemblages (Langhamer Reference Langhamer2012, Taormina et al. Reference Taormina, Bald, Want, Thouzeau, Lejart, Desroy and Carlier2018). Many studies showed that artificial structures do not host exactly the same species diversity and composition as natural hard substrata (Connell & Glasby Reference Connell and Glasby1999, Connell Reference Connell2001, Kogan et al. Reference Kogan, Paull, Kuhnz, Burton, Von Thun, Gary Greene and Barry2006). Sherwood et al. (Reference Sherwood, Chidgey, Crockett, Gwyther, Ho and Stewart2016) investigated the effects of the Basslink High Voltage Direct Current (HVDC) cable and its associated metallic return cable across Bass Strait in south-east Australia and reported that: 1) the ecological impacts of the cable on benthic communities have been transient and minor for soft sediments where the cable is buried; and 2) on hard substrata, the armoured cable provides a colonizable surface similar to the rocky substrata and is quickly utilized by reef species as new habitat with a species composition comparable to the surrounding reef (Sherwood et al. Reference Sherwood, Chidgey, Crockett, Gwyther, Ho and Stewart2016). These findings were consistent with other studies on the effects of submarine cables on benthic communities, which found no significant differences in communities between powered cables and natural hard bottoms (Andrulewicz et al. Reference Andrulewicz, Napierska and Otremba2003, Dunham et al. Reference Dunham, Pegg, Carolsfeld, Davies, Murfitt and Boutillier2015, Kuhnz et al. Reference Kuhnz, Buck, Lovera, Litvin, Whaling and Barry2020). In contrast, sections of unburied cables on soft substrata may have stronger effects on the composition and structure of benthic communities and host new species assemblages. Kogan et al. (Reference Kogan, Paull, Kuhnz, Burton, Von Thun, Gary Greene and Barry2006) reported more abundant actinarians (sea anemones) on unburied sections of the Acoustic Thermometry of Ocean Climate (ATOC)/Pioneer Seamount cable (Half Moon Bay, CA, USA) than in the surrounding habitats, probably due to the greater habitat complexity provided by the cable compared to soft bottom sediments (Kogan et al. Reference Kogan, Paull, Kuhnz, Burton, Von Thun, Gary Greene and Barry2006). At Baie du Marin, sections of unburied cables on sandy bottoms were characterized by crustose Corallinales fam. gen. sp. and other algae taxa that use it as a substrate for attachment.
The introduction of a new type of substrate at Baie du Marin increased habitat diversity and enhanced species recruitment, potentially including non-native species (Dumont et al. Reference Dumont, Harris and Gaymer2011, Macreadie et al. Reference Macreadie, Fowler and Booth2011, Adams et al. Reference Adams, Miller, Aleynik and Burrows2014). Several studies reported artificial habitats acting as stepping stones or even corridors for some exotic and/or invasive marine species to survive, settle and spread (Glasby et al. Reference Glasby, Connell, Holloway and Hewitt2007, Hulme Reference Hulme2009, Airoldi et al. Reference Airoldi, Turon, Perkol-Finkel and Rius2015). In well-established subtidal assemblages, the numbers of non-native species settled on new artificial substrata were 1.5–2.5 times higher than on natural substrata (Glasby et al. Reference Glasby, Connell, Holloway and Hewitt2007). At Crozet, greater knowledge of the potential impacts of the HA04 station cables on the benthic communities of Baie du Marin is needed to provide practical information and guidance for biodiversity conservation and management. Such knowledge will only be obtained through continuous environmental monitoring of community dynamics, which is one of the objectives of the current agreement between the TAAF and the CTBTO for the conservation of nearshore benthic environments of Crozet.
Monitoring strategies for enhanced benthic community conservation
Through a trait-based approach, Lelièvre et al. (Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023) have reported the high degree of vulnerability of Crozet marine communities to current and future environmental changes (e.g. global warming, biological invasions). Identifying sensitive or endangered species in the Crozet benthic communities is challenging due to the limited knowledge and only sporadic accessibility of these ecosystems. Monitoring indicator taxa associated with each habitat type during upcoming campaigns will be instrumental to evaluating the overall health and evolutionary trends of these ecosystems. By tracking changes in abundance and distribution, these indicator taxa serve as barometers in the detection of potential disturbances over time, providing valuable insights into ecosystem health (Carignan & Villard Reference Carignan and Villard2002).
In addition to indicator taxa, the conservation of habitat-forming species is a priority, as they are key organisms for the preservation of local biodiversity and its resilience to changing environments. Habitat-forming species are of high ecological importance and warrant special management measures (Braeckman et al. Reference Braeckman, Rabaut, Vanaverbeke, Degraer and Vincx2014). Their three-dimensional structure impacts the local biotic and abiotic conditions through a wide range of ecological mechanisms (e.g. water motion dampening, sediment deposition, refuge from predation, secondary substrate for sessile/vagile species or larval settlement, food source partitioning), therefore increasing the number of potential ecological niches and promoting species richness and local diversity (Jones et al. Reference Jones, Lawton, Shachak, Samson and Knopf1994). In this regard, previous studies have identified the polychaete Lanice as an important habitat-structuring and engineer genus (Rabaut et al. Reference Rabaut, Guilini, Van Hoey, Vincx and Degraer2007, Van Hoey et al. Reference Van Hoey, Guilini, Rabaut, Vincx and Degraer2008). These tube-building polychaetes introduce complexity and heterogeneity in habitats and exert a strong influence on ecosystem properties, including bottom sediment stabilization and bottom flow alteration (Rabaut et al. Reference Rabaut, Guilini, Van Hoey, Vincx and Degraer2007, Van Hoey et al. Reference Van Hoey, Guilini, Rabaut, Vincx and Degraer2008). In addition, the habitat provisioning and structural complexity provided by these tubeworms enhance the development of faunal communities, promoting species richness, abundance and diversity (Van Hoey et al. Reference Van Hoey, Guilini, Rabaut, Vincx and Degraer2008). Such a positive effect on the diversity and abundance of benthic ecosystems was already reported for the tube-building polychaete L. conchilega in the North Sea (Rabaut et al. Reference Rabaut, Guilini, Van Hoey, Vincx and Degraer2007, Van Hoey et al. Reference Van Hoey, Guilini, Rabaut, Vincx and Degraer2008). The preservation of biogenic habitats such as those provided by the tube-building polychaete L. marionensis, which is found in high density at Crozet, will also protect the range of species and communities that are associated with those habitats.
The value of image analysis as a conservation approach
Imaging techniques allowed us to assess an important benthic surface across two sites located on the eastern coast of Ile de la Possession, thereby providing an overview of coastal marine ecosystems in this region. However, despite many advantages, image analysis can only provide a partial and first estimate of the total benthic diversity and limit the taxonomic description of novel species, which rely on the deposition of type material according to the codes of nomenclature. Species identification is limited by image resolution and to organisms that can be seen on images, with small-sized taxa and endobenthic organisms remaining undetected. Additionally, diversity assessment of ill-known benthic communities from rarely explored regions requires a first biological sampling before further investigations and species identification can be performed from images (Solan et al. Reference Solan, Germano, Rhoads, Smith, Michaud and Parry2003, Thistle Reference Thistle2003, Hanafi-Portier et al. Reference Hanafi-Portier, Samadi, Corbari, Chan, Chen and Chen2021). Both approaches (biological and image sampling) are complementary to each other and are essential to improve our knowledge of the structure, composition and distribution of benthic communities. Whereas these results constitute a biodiversity baseline and are valuable to monitoring the potential impacts of climate change and anthropic stressors on Crozet benthic communities, additional imagery data and complementary biological sampling are therefore needed to complement this diversity assessment and to implement further monitoring protocols to be conducted in the French Southern Territories National Nature Reserve.
Conclusion
The present study investigated the composition, abundance and diversity of benthic communities along 12 imagery transects performed at two sites - Baie du Marin and Crique du Sphinx - located on the eastern part of Ile de la Possession, and it evaluated the role of the habitat on species distribution. Our results showed the benefits of imaging techniques for monitoring vulnerable and sensitive sub-Antarctic benthic communities such as those in Crozet (Lelièvre et al. Reference Lelièvre, Specq, Lamy, Boyé, Downey and Saucède2023). The imagery analysis results also contributed to improving our still-limited knowledge of the nearshore marine ecosystems of this sub-Antarctic archipelago and highlighted the significant influence of habitat heterogeneity and substrate complexity on species richness and diversity. Our study reveals that substrate diversity plays a crucial role in shaping the richness and composition of benthic communities in Crozet’s coastal habitats. We found that transects with diverse habitats - especially those where hard substrates are predominant - support the highest levels of species richness and diversity compared to those dominated by homogeneous substrates such as sandy stretches. These findings contribute to the refinement of the baseline knowledge of benthic biodiversity in Crozet, and the identification of indicator species provides essential tools for ongoing monitoring efforts, facilitating the assessment of ecosystem health and the detection of potential environmental disturbances. Particular attention must be paid to monitoring the sessile epibenthic community associated with submarine cables laid at the bottom of Baie du Marin (IMS-HA04 station) considering its original composition and structure. Monitoring the development and evolution of biological communities settled on submarine cables is therefore a need for conservation strategy regarding native and endemic species.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0954102024000543.
Ethical approval
Ethical approval was not required for the nature of this work.
Acknowledgements
The authors thank the captain and crew of the R/V Marion Dufresne II, as well as the staff and divers of the TAAF. We are particularly grateful to all taxonomists who have contributed to species identification from image data (R. Downey, S. Rosenfeld, C. Moreau, J-P. Féral, T. O’Hara, E. Rodríguez, S. Hourdez, P. Schuchert, G. Duhamel, N. Wilson and M. Tatián). We especially thank D. Champagnac (Biogéosciences, Université Bourgogne Europe) for the design conception of the image acquisition device and for his technical assistance. We thank also M. Zampolli, G. Haralabus and D. Metz from CTBTO for their helpful comments and productive discussions throughout the project. Philippe Dubois is a Research Director of the National Fund for Scientific Research (Belgium). Thanks are also extended to M. Zurowietz (Biodata Mining Group, Bielefeld University, Germany) for allowing the loading of image data on the BIIGLE 2.0 platform and for the technical support. This study is a contribution to the project with contract #2021-0882 ‘Nearshore Cable Inspection and Environmental Survey at IMS Hydroacoustic Station HA04 Crozet, France’ conducted by the French Southern Lands National Nature Reserve in response to the CTBTO solicitation and to the French Polar Institute project #1044 Proteker.
Author contributions
YL and TS conceived the research project and designed the study. LLG, PD and TS collected the imagery data. YL analysed the data and wrote the main manuscript text. TS supervised the research project. All authors contributed to the writing process and revised the manuscript.
Data availability
All data generated or analysed during this study are included in this published article. Video imagery data are available at the dat@UBFC repository (https://doi.org/10.25666/DATAUBFC-2024-03-15).
Financial support
YL was supported by TAAF-UB convention #2258.
Competing interests
The authors declare that they have no competing interests.













