Urinary incontinence (UI) is a complex disorder of multifactorial etiology that often has severe implications on daily function, sexuality, hygiene, and quality of life (Abrams et al., Reference Abrams, Cardozo, Khoury and Wein2009). Childbirth, obesity, age, and heritability are often implicated risk factors for female UI (Abrams et al., Reference Abrams, Cardozo, Khoury and Wein2009). A number of studies have reported a positive association between UI and depression (Melville et al., Reference Melville, Katon, Delaney and Newton2005; Moghaddas et al., Reference Moghaddas, Lidfeldt, Nerbrand, Jernstrom and Samsioe2005; Steers & Lee, Reference Steers and Lee2001; Zorn et al., Reference Zorn, Montgomery, Pieper, Gray and Steers1999). The prevalence of depression varies across different age groups but on average, one out of five women will experience depression during their lifetime (Kessler et al., Reference Kessler, McGonagle, Zhao, Nelson, Hughes, Eshleman and Kendler1994). Different theories have been proposed to explain this association, but the relationship between these disorders is incompletely understood. Two longitudinal studies on elderly women have found that major depression increases the risk of incident UI later in life but found no evidence of the opposite relationship (Melville et al., Reference Melville, Fan, Rau, Nygaard and Katon2009; Thom et al., Reference Thom, Haan and Van Den Eeden1997).
Neuroticism is one of the five personality traits used in contemporary psychology to describe human personality, and can be defined as the tendency to experience negative emotional states. Previous studies have shown that women with UI tend to score higher on neuroticism measurement scales compared to women without incontinence (Morrison et al., Reference Morrison, Eadie, McAlister, Glen, Taylor and Rowan1986; Yarnell et al., Reference Yarnell, Voyle, Sweetnam, Milbank, Richards and Stephenson1982).
Increasing evidence suggests that genetic factors play an important role in the occurrence of UI (Altman et al., Reference Altman, Forsman, Falconer and Lichtenstein2008; Hannestad et al., Reference Hannestad, Lie, Rortveit and Hunskaar2004; Rohr et al., Reference Rohr, Kragstrup, Gaist and Christensen2004; Wennberg et al., Reference Wennberg, Altman, Lundholm, Klint, Iliadou, Peeker and Milsom2011), as well as major depression (Sullivan et al., Reference Sullivan, Neale and Kendler2000), and neuroticism (Bouchard, Reference Bouchard2004; Loehlin, Reference Loehlin1992). However, the contribution of genetic and environmental factors to the comorbidity of incontinence and depression/neuroticism has not been investigated. The aims of the present study were to estimate the strength of the association between UI, depressive mood disorders (depressive symptoms and major depression), and neuroticism and to assess the genetic contribution to these associations using data from a cohort of premenopausal female twins.
Material and Methods
Study Population
The Study of Twin Adults: Genes and Environment (STAGE; Lichtenstein et al., Reference Lichtenstein, Sullivan, Cnattingius, Gatz, Johansson, Carlstrom and Pedersen2006) is a web-based survey performed in 2005 among all Swedish twins born between 1959 and 1985 (n = 42,852) that comprised approximately 1,300 questions about common complex diseases and common exposures. Methods for assigning zygosity used in the survey have been validated with DNA as having 98% or higher accuracy (Lichtenstein et al., Reference Lichtenstein, Faire, Floderus, Svartengren, Svedberg and Pedersen2002). As UI is more common among women, the current study was limited to female twins; the overall response rate to the survey among women was 66% (n = 14,098). No exclusions due to other urological or neurological disease have been made.
Assessment of Urinary Incontinence, Depression, and Neuroticism
Definitions of UI subtypes (overall, stress, urge, and mixed incontinence) were based on recommendations from the International Continence Society (Abrams et al., Reference Abrams, Cardozo, Fall, Griffiths, Rosier, Ulmsten and Wein2003). Information on the occurrence of UI in the 30-day period preceding the survey was collected, using questions that have been validated for implementation in epidemiological surveys (Hannestad et al., Reference Hannestad, Rortveit, Sandvik and Hunskaar2000). Women who answered yes to the question ‘Do you have present involuntary loss of urine?’ were classified as women with overall UI. Subjects with stress UI were identified using the question ‘Do you have involuntary loss of urine in connection with coughing, sneezing, laughing, lifting heavy items?’, and women with urge incontinence were identified using the question ‘Do you have involuntary loss of urine in connection with a sudden and strong urge to void?’ Participants who answered yes to both of the latter questions were classified as having mixed UI. Approximately 900 women did not provide any information about UI subtypes.
Current depressive symptoms were defined using the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, Reference Radloff1977). The 11-item Iowa form was used with a four-point response format (0 = never or almost never, 1 = seldom, 2 = often, and 3 = always or almost always; Kohout et al., Reference Kohout, Berkman, Evans and Cornoni-Huntley1993). Lifetime major depression was measured using the computerized Composite International Diagnostic Interview-Short Form (CIDI-SF), which was adapted from its original design for 12-month prevalence to assess lifetime prevalence of major depression (APA, 2000; Kessler et al., Reference Kessler, Andrews, Mroczek, Ustun and Wittchen1998). Neuroticism was measured using a short form of the Eysenck Personality Inventory (EPI-Q; Floderus, Reference Floderus1974), which includes nine yes/no questions. In logistic regression and co-twin control analysis, standardized log scores for both neuroticism and depressive symptoms have been used as continuous variables, while in the quantitative genetic analysis the standardized log scores have been categorized based on the number of standard deviations (SD) from the mean (i.e., −2 SD, −1 SD, 0 SD, +1 SD, and +2 SD). Almost 2,000 women (n = 1,963) did not respond to enough questions to compute a depressive symptoms score (CES-D score), while for approximately 3,000 women we do not have enough information to determine whether they had major depression (n = 3,115) or to determine their score on the neuroticism scale (n = 3,332). This study was approved by the Regional Ethics Board at Karolinska Institutet and conforms to the STROBE guidelines for reporting observational studies (www.strobe-statement.org).
Statistical Analyses
To evaluate the association between depressive mood disorders (major depression and depressive symptoms) and neuroticism with UI, we used a logistic regression model based on generalized estimating equations (GEE) to take into account the correlation within twin pairs. Odds ratios (ORs) with 95% confidence intervals were used as measure of association. Following previous studies, where a positive association between depression at baseline and incident incontinence has been reported (Melville et al., Reference Melville, Fan, Rau, Nygaard and Katon2009; Thom et al., Reference Thom, Haan and Van Den Eeden1997), we have modeled the odds of incontinence as a function of current depressive symptoms, lifetime major depression, and neuroticism using three different models. Analyses were adjusted for age, body mass index (BMI) as continuous variables, and also for parity (ever or never given childbirth) and use of antidepressant medications in the 30-day period preceding the survey (yes/no). Information about these variables was also obtained from the survey.
The statistically significant associations found in the logistic regression models were further assessed using a co-twin control analysis in order to determine whether these associations were confounded by familial factors. In the co-twin control analysis, only discordant twin pairs are taken into account; therefore, we considered only pairs where only one twin is affected by a specific type of UI while the other twin is not affected by that particular subtype of incontinence. Hence, the co-twin control method, resembling a matched case-control design, is used to control for genetic background and unmeasured environments shared by twins when studying the relationship between a presumed risk factor and a disease (Spector, Reference Spector2000). Because of its matched nature, it is possible to minimize confounding by factors shared within twin pairs such as intrauterine exposures, maternal factors, 50% dizygotic (DZ) or 100% monozygotic (MZ) of their segregating genes, and childhood and adolescent environment. If the results from the co-twin control analysis are similar to those observed using the entire cohort, then there is no familial confounding. If an attenuation of the association is observed, this could indicate familial confounding.
Structural equation modeling was used to study the genetic and environmental sources of the covariation of UI with depressive mood disorders and neuroticism. We used a trivariate Cholesky decomposition procedure, based on a liability threshold model, in which for each variable the variance is decomposed into genetic influences (A), shared environmental influences (C), and individual specific environmental influences (E), and A, C, and E influences shared by the measures (Neale & Cardon, Reference Neale and Cardon1992). In a trivariate Cholesky model, the first factor loads on all the three variables of interest, while the second factor loads only on the second and on the third, and the third factor affects only the third variable (Figure 1). The models included neuroticism, depression (or depressive symptoms), and the UI variable of interest, in that order. Subsequent models were tested to examine the worsening of the model fit caused by the removal of the various sources of variation. The goodness of fit of the reduced models was evaluated using the likelihood ratio test and the Akaike's Information Criteria (AIC). Once the best-fit model is determined, we can estimate the proportion of the phenotypic correlation that is attributable to genetic factors by first multiplying the square roots of the heritability of the two phenotypes with the genetic correlation (rg) and then dividing this product by the phenotypic correlation (rp), that is, (rg × hx × hy)/rp. The genetic correlation can be estimated using the standardized path coefficients of the genetic covariance matrix; for example, the path coefficient that refers to the loadings of the second genetic factor on the third variable (a32) is divided by the square root of the product of the loadings of the two genetic factors on the two variables (a22 and a33) (Figure 1). Standardized paths were obtained by fixing the variance of each variable to unity.

FIGURE 1 Trivariate Cholesky decomposition model for additive genetic and individual specific environmental influences on major depression and urge incontinence.
The GEE models and the co-twin control analysis were performed using SAS software (version 9.3; SAS Institute, Cary, NC, USA) and the structural equation modeling package Mx was used to model raw data for the quantitative genetic analysis (Neale et al., Reference Neale, Bocker, Xie and Maes1999). Since the prevalence of UI, depressive mood disorders and neuroticism changed substantially as a function of age, the quantitative genetic analyses were adjusted for age (used as a continuous variable).
Results
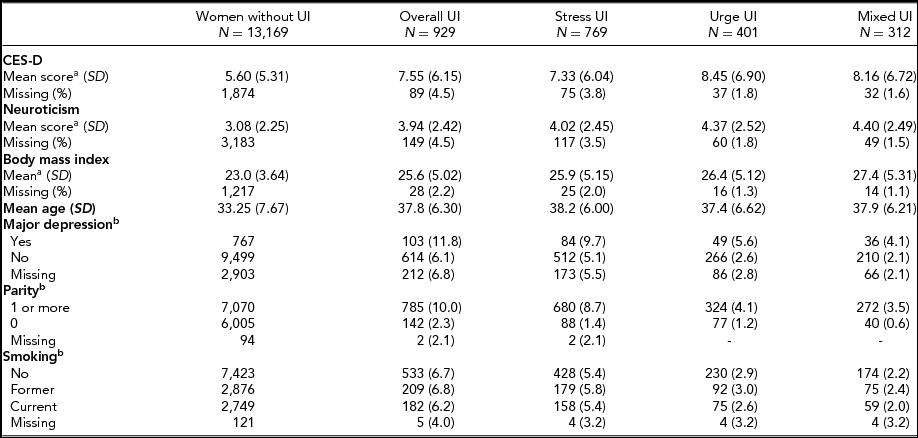
Cohort characteristics and distribution of UI subtypes (stress, urge, mixed, and overall incontinence) are shown in Table 1. The prevalence of depressive symptoms (defined as a CES-D score equal to or greater than 8) was 23.8%, the prevalence of major depression was 7.9%, and 25.9% of the women in the cohort scored high on the neuroticism scale (i.e., had a score of 5 or greater). Seven percent of the women enrolled in the study reported overall UI symptoms; the most common UI subtypes were stress UI (5.8%), followed by urge UI (3.0%), and mixed UI (2.4%). All UI subtypes were significantly more common among women with depression as compared to those without. Moreover, mean CES-D and neuroticism scores were higher among women affected by UI.
TABLE 1 Characteristics of Women Enrolled in the Study of Twin Adults: Genes and Environment (STAGE)

aAge-adjusted means; bValues are number of women with UI (% of women with UI).
UI = urinary incontinence; CES-D= Center for Epidemiological Studies Depression Scale.
Table 2 shows the age-adjusted logistic regression models, which affirmed that depressive mood disorders and neuroticism are positively associated with UI. After adjusting for BMI and parity, the strength of the associations remained on a similar level (data not shown). When the use of antidepressant medications was included in the analysis, we observed a small attenuation for the association between major depression and UI (Table 2); however, all the associations remained statistically significant. In a multivariable adjusted logistic regression model, in which major depression, depressive symptoms, and neuroticism were all included in the same model, we found that all the positive associations were further attenuated, the attenuation of the association was particularly pronounced for major depression. After adjusting for depressive symptoms and neuroticism, the association between depression and overall and stress UI was almost halved (OR = 1.45, 95% CI = 1.11–1.91 for overall UI; OR = 1.38, 95% CI = 1.03–1.86). Moreover, the association between major depression and urge, or mixed, UI was no longer statistically significant (OR = 1.20, 95% CI = 0.83–1.73; OR = 1.05, 95% CI = 0.68–1.61). A one unit increase in the standardized log score for depressive symptoms was associated with an approximately 20–30% higher odds of UI. A one unit increase in the standardized log score for neuroticism was associated with a 20% higher odds of overall and stress UI and with an approximately 60% higher odds of urge and mixed UI.
TABLE 2 Associations Between Major Depression, Depressive Symptoms, and Neuroticism With Urinary Incontinence

aAdjusted for age, parity, BMI, and antidepressant medication; bMajor depression, CES-D, and neuroticism were all included in the same model.
UI = urinary incontinence; CES-D= Center for Epidemiological Studies Depression Scale.
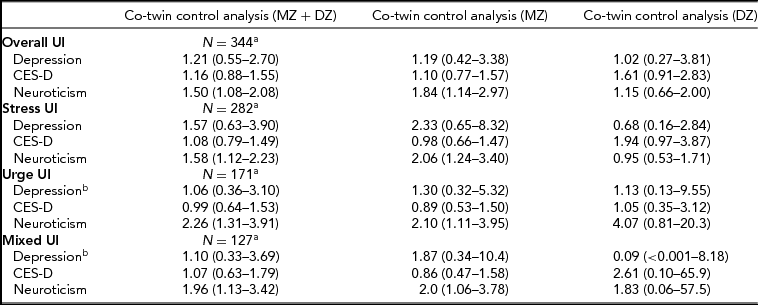
Subsequently, a co-twin control analysis was performed to determine whether the positive associations were confounded by familial factors. No evidence of familial confounding was observed for the association between neuroticism and all UI subtypes: the association among discordant twin pairs was similar to the one observed in the multi-adjusted logistic regression model, even when the analysis was stratified by zygosity (Table 3). The association between depressive symptoms and overall stress and mixed UI was attenuated only among MZ twin pairs and not among DZ twin pairs, suggesting that genetic factors may confound the association between depressive symptoms and these UI subtypes. The attenuation between depressive symptoms and urge UI was attenuated in both MZ and DZ twin pairs, suggesting that the association was probably confounded by familial factors. Regarding major depression, evidence of familial confounding was found for the association between depression and overall incontinence only.
TABLE 3 Multi-Adjusted Co-Twin Control Analysis

aNumber of discordant twin pairs; bThe association was not statistically significant in the logistic regression model. All models were adjusted for parity, BMI, and antidepressant medication. Major depression, CES-D, and neuroticism were all included in the same model.
UI = urinary incontinence; CES-D= Center for Epidemiological Studies Depression Scale.
We proceeded with the quantitative genetic analyses for the associations between depressive mood disorders, neuroticism, and UI. Among MZ twin pairs, both intraclass and cross-twin cross-trait correlations were approximately two times higher than the correlations observed among DZ twin pairs (Supplementary Table S1, available on the Cambridge Journals Online website). Initially, univariate quantitative genetic analyses were performed on all UI subtypes, depressive mood disorders, and neuroticism. We found that for all these phenotypes the AE model had the best goodness of fit (data not shown). The AE model was also the best-fit model in the trivariate analysis for overall and stress UI (Supplementary Table S2). However, for urge and mixed UI the best-fit model was the AE model, where the path from major depression (or depressive symptoms) to urge, or mixed UI, has been removed. This indicates that there were no genetic factors shared between depressive mood disorders and urge or mixed UI that were not shared with neuroticism. Even though the prevalence of overall and stress UI and the mean scores of CES-D and neuroticism were different among MZ and DZ twin pairs (Supplementary Table 3), the model with the best goodness of fit was the one in which both MZ and DZ twins had the same thresholds.
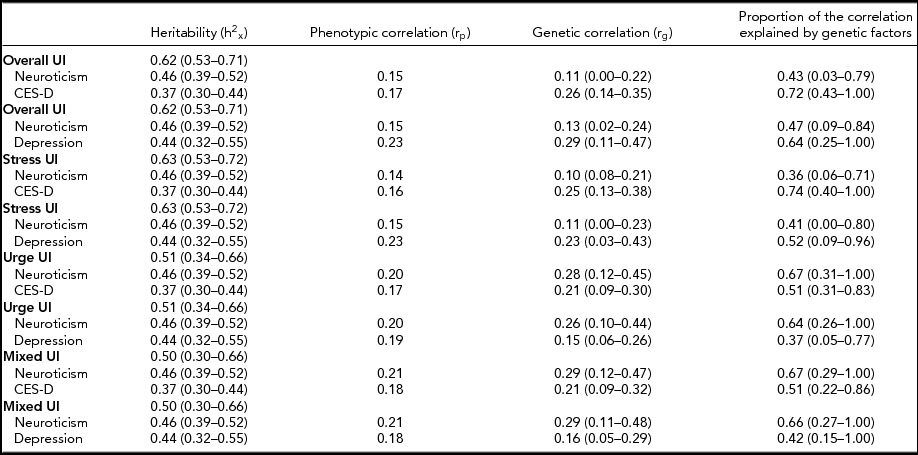
Results from the multivariate quantitative genetic analyses are shown in Table 4. The genetic correlations between depressive mood disorders and overall (or stress) UI suggested that the genetic covariation of these traits was modest while the genetic correlations between neuroticism and overall (and stress) UI were low. However, part of the variance that these traits shared due to genetic causes was also in common with neuroticism. If we consider as the phenotypes of interest neuroticism, major depression, and overall incontinence, we found that the genetic correlation between depression and overall UI was 0.29. Then, to estimate the proportion of the phenotypic correlation explained by genetic factors, we multiplied the genetic correlation by the square root of the heritability of the two phenotypes (hx = 0.66; hy = 0.79) and then divided by the phenotypic correlation (rp = 0.23). We found that approximately 65% of the phenotypic correlation between major depression and overall incontinence is explained by genetic factors. For depressive mood disorders and overall and stress UI, the proportion of phenotypic correlation explained by genetic factors ranged from 50% to 70%. As the path between depression (or depressive symptoms) and urge or mixed UI was not significant, the genetic correlations between depressive mood disorders and urge or mixed UI were entirely determined by neuroticism. All genetic factors shared between depressive mood disorders and urge or mixed UI were in common with neuroticism. For urge and mixed UI, the genetic correlations with neuroticism were modest and the proportion of phenotypic correlation explained by genetic factors ranged between 63% and 67%.
TABLE 4 Phenotypic and Genetic Correlations, Estimated Heritability, and Proportion of the Phenotypic Correlation Explained By Genetic Factors

Proportion of the phenotypic correlation explained by genetic factors estimated as: hx × hy × rg/rp.
In sensitivity analyses, women affected by medical conditions that may lead to UI (diabetes, neurological and rheumatic diseases, bowel, and ovarian cancer) or women who used medications with UI as a possible side effect (antihypertensive, neuroleptic, diuretic, anticholinergic, and anti-parkinsonism medications) were excluded: results from the sensitivity analyses were almost identical to the main analyses.
Discussion
The present study provides solid epidemiological evidence that among premenopausal female twins, depressive mood disorders (depressive symptoms and major depression) and neuroticism are positively associated with UI. However, the positive association between major depression and urge and mixed UI was explained by neuroticism. Furthermore, the twin study design allowed for an estimation of the genetic contribution to the association. Results from co-twin control analysis suggested that the associations between depressive symptoms and UI subtypes could be confounded by familial factors. In quantitative genetic analysis, we found a modest genetic correlation between depressive mood disorders and overall (or stress) UI. Our study suggests that the relatively small phenotypic correlation was largely explained by genetic factors.
The association between UI and depression is well known (Melville et al., Reference Melville, Katon, Delaney and Newton2005; Moghaddas et al., Reference Moghaddas, Lidfeldt, Nerbrand, Jernstrom and Samsioe2005; Morrison et al., Reference Morrison, Eadie, McAlister, Glen, Taylor and Rowan1986; Steers & Lee, Reference Steers and Lee2001; Yarnell et al., Reference Yarnell, Voyle, Sweetnam, Milbank, Richards and Stephenson1982; Zorn et al., Reference Zorn, Montgomery, Pieper, Gray and Steers1999). However, the temporality of the association has been more difficult to establish because of the cross-sectional design of most studies. Two longitudinal studies supported the notion that depression increases the risk for incontinence later in life (Melville et al., Reference Melville, Fan, Rau, Nygaard and Katon2009; Thom et al., Reference Thom, Haan and Van Den Eeden1997); moreover, both studies showed that UI at baseline did not predict incident depression. Based on these two longitudinal studies, we decided to model the odds of having incontinence as a function of depression and neuroticism rather than the opposite.
The importance of genetic factors for liability of UI (Altman et al., Reference Altman, Forsman, Falconer and Lichtenstein2008; Hannestad et al., Reference Hannestad, Lie, Rortveit and Hunskaar2004; Rohr et al., Reference Rohr, Kragstrup, Gaist and Christensen2004; Wennberg et al., Reference Wennberg, Altman, Lundholm, Klint, Iliadou, Peeker and Milsom2011), major depression (Sullivan et al., Reference Sullivan, Neale and Kendler2000), and neuroticism (Bouchard, Reference Bouchard2004; Loehlin, Reference Loehlin1992) has been shown in previous studies. However, to the best of our knowledge, this is the first study that has evaluated the contribution of genetic and environmental factors to the comorbidity of incontinence with depression and neuroticism. We found that the association between depression and urge, as well as mixed UI, was confounded by neuroticism. Moreover, a large proportion of the correlation between depressive mood disorders, as well as stress, and overall UI was explained by genetic factors; however, the genetic correlations between these traits were modest. This modest genetic overlap was partially determined by neuroticism. In contrast, the genetic factors that were in common between depressive mood disorders and urge or mixed UI were entirely shared with neuroticism. These findings suggest that genetic variants for neuroticism are largely driving the association between depressive mood disorders and incontinence.
One possible explanation for the association between depressive mood disorders and UI is that a decreased serotonin activity can lead to depression (Nemeroff, Reference Nemeroff1998) and also affect bladder function (Steers & Lee, Reference Steers and Lee2001). An alternative explanation would be that the increased activity of the hypothalamic–pituitary axis seen in depressed individuals may cause physiological changes in the bladder, leading to incontinence. Pharmacological studies have shown that anti-depressant drugs such as serotonin–norepinephrine reuptake inhibitors (SSRI/SNRI), 5-HT agonists, and norepinephrine receptor antagonists influence central control of lower urinary tract function (Steers & Lee, Reference Steers and Lee2001). We were able to adjust for antidepressant medications, which could influence both the self-reported occurrence and severity of both depressive and incontinence symptoms. Thus, after adjusting for use of antidepressant medications we observed a decrease in the point estimates for the effect of major depression on UI subtypes, but all the associations remained statistically significant. One of the strengths of our study is that we used a validated questionnaire developed for epidemiological surveys to identify women with UI subtypes (Hannestad et al., Reference Hannestad, Rortveit, Sandvik and Hunskaar2000). The nationwide study design, large study population, and use of uniform classifications of both exposures and outcomes further strengthen our results and generalizability. Moreover, the heritability estimates for UI, depression, and neuroticism that we reported in our study are similar to the estimates reported in previous studies (Altman et al., Reference Altman, Forsman, Falconer and Lichtenstein2008; Bouchard, Reference Bouchard2004; Sullivan et al., Reference Sullivan, Neale and Kendler2000).
Due to the low number of twin pairs that were discordant for UI, confidence intervals in the co-twin control analysis were wide, making the interpretation of this analysis difficult for certain associations. In this study, we have analyzed data from a relatively young cohort of women. Some of the continent women might develop UI in the future; therefore, the nature of the association between UI and depressive mood disorders (and neuroticism) might change. Another limitation is that the diagnosis of depression used in this study could not differentiate between a single episode and recurrent major depressive disorder as items addressed ‘at least one’ major depressive episode. Moreover, the diagnosis of depression was a lifetime assessment, while the CES-D inventory was used to assess current depressive symptoms. This could explain why the association between current depressive symptoms and urge, as well as mixed, UI was statistically significant after adjusting for neuroticism, while the effect of major depression was confounded by neuroticism.
It has been suggested that the negative impact on quality of life from having UI may precipitate depression. Although this may to some extent be true, we postulated an inverse association based on evidence from longitudinal studies (Melville et al., Reference Melville, Fan, Rau, Nygaard and Katon2009; Thom et al., Reference Thom, Haan and Van Den Eeden1997). This is a relatively young cohort of women, and while the most common age of onset of depression is between the ages of 20 and 30, the age of onset of incontinence is expected to occur later; therefore, we believe that this association is more reasonable rather than the opposite.
In conclusion, our nationwide, population-based study showed that depressive mood disorders and neuroticism are associated with UI; the association was partially explained by genetic factors in common to the disorders. While the genetic factors shared between depressive mood disorders and urge or mixed UI were completely shared with neuroticism, the majority of the genetic factors in common between depressive mood disorders and overall and stress UI were independent from neuroticism.
Acknowledgments
This study was based on data from the Swedish Twin Register. The study was supported by grants from the National Institute of Digestive Disorders and Kidney Diseases (U01 DK066134 to N.L.P.), the Swedish Research Council (K2010-21574-01-4 to D.A.), and the OAB-LUTS Competitive Grants Program, Pfizer Inc. (to D.A.).
Declaration of Interest
D. Altman is a consultant for Gynecare Scandinavia, Astellas Nordics, Contura A/S. G. Tettamanti, N. L. Pedersen, R. Bellocco, and A. N. Iliadou declare no conflicts of interest.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/thg.2013.60.