Diets rich in plant-based foods such as vegetables, fruits and tea are found to reduce the risk of developing colorectal cancer( Reference van Duijnhoven, Bueno-De-Mesquita and Ferrari 1 , Reference Green, de Dauwe and Boyle 2 ). The mechanism by which these foods exert a protective effect is unclear, but one hypothesis is the presence of potentially anti-carcinogenic phytochemicals( Reference Bradbury, Appleby and Key 3 ). Flavonoids are a large group of phytochemicals widely distributed in vegetables, fruits and beverages of plant origin( Reference Aherne and O’Brien 4 ). More than 5000 plant flavonoids have been identified thus far, and studies have usually focused on six principal subclasses: anthocyanidins, flavan-3-ols, flavanones, flavones, flavonols and isoflavones. These flavonoid compounds have been demonstrated in vitro to inhibit colon cancer cell proliferation, minimise mutation, inhibit DNA oxidation, induct phase I and II metabolic enzymes, modulate cell growth signalling pathways and mediate inflammatory response( Reference Pierini, Gee and Belshaw 5 , Reference Nichenametla, Taruscio and Barney 6 ). Therefore, we assumed that flavonoids present in vegetables, fruits or tea may play an important role in the apparent protection against colorectal cancer.
Epidemiological studies about the relationship between dietary flavonoid intake and colorectal cancer are inconsistent; two reviews published in 2013( Reference Kocic, Kitic and Brankovic 7 , Reference Woo and Kim 8 ) and a Cochrane review published in 2012( Reference Jin, Leng and Li 9 ) concluded that no clear evidence was found to support the notion that high intake of flavonoids is associated with colorectal cancer prevention. Although some studies failed to detect an association( Reference Lin, Zhang and Wu 10 – Reference Nimptsch, Zhang and Cassidy 15 ), several case–control studies( Reference Zamora-Ros, Not and Guino 16 – Reference Rossi, Negri and Talamini 21 ) and the Iowa Women’s Health prospective cohort study( Reference Arts, Jacobs and Gross 22 ) suggested an inverse association between high flavonoid intake, particularly flavonols, flavones, catechin (flavan-3-ols) and anthocyanidins, and colorectal cancer risk.
Vegetables, fruits, tea and red wine are the main dietary sources of flavonoids, with different combination of flavonoid subclasses( Reference Zamora-Ros, Knaze and Lujan-Barroso 23 ). Vegetables and fruits almost cover all subclasses of flavonoids. Tea mainly contains flavan-3-ols, flavonols, theaflavins, thearubigins and proanthocyanidins, whereas red wine mainly contains anthocyanidins, flavan-3-ols, flavonols and proanthocyanidins. The bioavailability of flavonoids also varies in different dietary sources, depending on the exact compounds of glycoside, suggesting that the protective effect of flavonoids from different dietary sources in colorectal cancer may be different( Reference Arts, Hollman and Bueno 24 ). Therefore, it is necessary to examine the disparate effects on colorectal cancer risk depending on different dietary sources. However, so far, only two studies have evaluated the independent associations of different dietary sources of flavonoids with colon cancer risk. One study found that the protective effects of quercetin (a subclass of flavonols) on the risk of proximal colon cancer were significant only when fruit intake or the Healthy Eating Index score was high or when tea intake was low. In addition, increased quercetin intake had no protective effects when tea intake was high( Reference Djuric, Severson and Kato 17 ). Another study found that non-tea flavonol intakes may be linked with the reduced risk of developing colorectal cancer, with an OR of 0·6 (95 % CI 0·4, 1·0) in the highest quartile of non-tea flavonols intake compared with the lowest quartile( Reference Kyle, Sharp and Little 18 ), suggesting that flavonoids from dietary sources other than tea may decrease colorectal cancer risk.
In addition, epidemiological studies investigating the effects of dietary flavonoids intake on colorectal cancer risk are scarce in China. It is likely that the distribution of flavonoid subclasses differs in different populations depending on the relative intakes of vegetables, fruits or tea. The typical Chinese diet is known to be high in plant-origin foods( Reference Hall, Moore and Harper 25 ). Therefore, further studies in different regions will help elicit the role of flavonoids in colorectal cancer risk.
The aim of this study was to evaluate the associations between flavonoid intake from different dietary sources and colorectal cancer risk in a Chinese population. We hypothesised that flavonoids from different dietary sources may play different roles in colorectal cancer risk.
Methods
Study subjects
Details of this ongoing, hospital-based, case–control study, which began in July 2010 in Guangdong province of China for the purpose of examining the relationship between lifestyle factors and colorectal cancer risk, have been reported previously( Reference Luo, Fang and Lu 26 – Reference Zhong, Fang and Pan 28 ). In brief, between July 2010 and December 2015, a total of 1822 eligible histologically confirmed incident colorectal cancer patients were identified, and 1635 were successfully interviewed from Sun Yat-sen University Cancer Center, Guangzhou, China, with a participation rate of 89·74 %. Among the cancer cases who completed the FFQ, we excluded those who reported very high or very low energy intakes (<2510 or >14 644 kJ/d (<600 or >3500 kcal/d) for women, <3347 or >17 573 kJ/d (<800 or >4200 kcal/d) for men); finally, 1632 cases were included in our analysis.
Controls were frequency matched to cases by 5-year age group and sex. Eligibility criteria for controls were the same as that described for the cases, except that they had no history of colorectal cancer or other cancers. Control subjects were recruited from inpatients of three affiliated hospitals of Sun Yat-sen University during the same period as the case subjects. The control subjects were selected from the Department of Ophthalmology, Ear Nose Throat, Plastic and Reconstructive Surgery, and Vascular Surgery with eye disorders, ear nose throat diseases, trigeminal neuralgia, varicose veins, osteoarthritis, degenerate joint disease, orthopaedic diseases, facial paralysis and acute appendicitis. A total of 937 hospital-derived controls were identified and 815 were successfully interviewed, yielding a participation rate of 86·98 %. A total of 817 control subjects were recruited from apparently healthy community residents in the same cities, invited through a number of strategies such as written invitations, flyers or referrals.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures involving human subjects were approved by The Ethical Committee of School of Public Health, Sun Yat-sen University. All participants signed informed consent forms before the interview.
Data collection
All study participants completed a face-to-face interview by trained interviewers using a structured questionnaire to collect information on dietary habits and potential confounding factors. The core questionnaire collected data on social-demographic characteristics, body height and weight, active and passive smoking, alcohol and tea intakes, physical activity, family history of cancer in first-degree relatives and previous disease history for all subjects. Data on menstrual and reproductive factors were also obtained for female subjects. Relevant medical information, medical diagnosis and histological findings were abstracted from medical records. BMI was calculated by dividing weight (kg) by height squared (m2).
In the present study, a current smoker was defined as someone smoking at least one cigarette a day for no less than 6 consecutive months at present( 29 ). Subjects were categorised as having been exposed to passive smoke if they reported ever being exposed to tobacco smoke at home or at the workplace. Regular drinking was defined as alcohol consumption at least once per week for no less than 6 consecutive months during the past year; we also asked for the type of wine consumed and the frequency and amount (Liang) of wine intake per month during the past year. Regular tea drinking was defined as tea consumption at least twice per week for no less than 3 consecutive months. Data on the type (green tea, black tea, oolong tea or others) and amount (dry weight) of tea (tea leaves) consumed during the year preceding the interview as well as the current drinking status such as frequency and how many cups (1 cup=200 ml) of tea intake per day were collected. Postmenopausal status was defined as at least 12 months since the last menstrual cycle. In addition, physical activity was assessed on the basis of self-reported occupational, household and leisure-time activities. The participants were asked to describe their occupational activities during work in the past year as (1) not working or being retired, (2) mainly sitting, (3) light intensity, (4) moderate intensity or (5) vigorous intensity. Household and leisure-time activities were also categorised into light (e.g. walking), moderate (e.g. jogging, mountaineering and playing table tennis) and vigorous (e.g. running, swimming and playing football/basketball) physical activities, and data were collected on their frequency (d/week) and typical duration (h/d). The mean metabolic equivalent task (MET) hour value of each activity was obtained by estimating the average of all comparable activities in the Compendium of Physical Activities( Reference Ainsworth, Haskell and Herrmann 30 , Reference Ainsworth, Haskell and Whitt 31 ). MET-h/week were computed using the following equation: (how many days/week×how many hours/day×MET for a specific type of activity=MET-hours/week).
Food consumption data were collected using an 81-item FFQ, including eighteen questions on fresh vegetables, twelve on fruits and seven on soya products; the questionnaire also covered fruit juice, nuts and beverages with milk and chocolate. Participants were asked to provide information on the frequency of intake and portion size during the preceding 12 months before diagnosis for cases or interview for controls, which was used to calculate the average intake of each food item in grams per day. We also provided food photographs with usual intake portion sizes to help participants estimate and record the amounts of food consumed. Energy and other nutrient intakes were computed on the basis of the 2002 Chinese Food Composition Table( Reference Yang, Wang and Pan 32 ).
In this analysis, we obtained data on total food (including grains, vegetables, fruit, tea and wine) and beverage content in terms of seven subclasses of flavonoids (anthocyanidins, flavan-3-ols, flavanones, flavones, flavonols, theaflavins and thearubigins, and proanthocyanidins) according to the updated flavonoid food composition database published by the US Department of Agriculture( Reference Bhagwat, Haytowitz and Holden 33 ). Isoflavones intake was computed on the basis of the 2002 Chinese Food Composition Table( Reference Yang, Wang and Pan 32 ). The measure ‘total flavonoids’ represents the sum of these eight compounds. As the flavonoids intake from grains and wine was low in this population, the analyses of dietary sources were focused on flavonoids intake from vegetables and fruits and flavonoids intake from tea. In this database, each subclass is a summary of individual flavonoid compounds as outlined: anthocyanidins (cyanidin, delphinidin, malvidin, pelargonidin, peonidin and petunidin); flavon-3-ols ((+)-catechin, (−)-epicatechin, (−)-epigallocatechin, (−)-epigallocatechin 3-gallate, (−)-epicatechin 3-gallate and (+)-gallocatechin); flavones (apigenin and luteolin); flavonols (isorhamnetin, quercetin, kaempferol and myricetin); flavanones (eriodictyol, hesperidin and naringenin); theaflavins and thearubigins (theaflavin, theaflavin 3-gallate, theaflavin 3'-gallate, theaflavin 3,3'-digallate, thearubigins); isoflavones (daidzein, genistein, glycitein); and proanthocyanidins. Development and validation of the FFQ have been described elsewhere, and this FFQ has been used in a previous study( Reference Zhang and Ho 34 ). The energy-adjusted correlation coefficients comparing the FFQ and the six 3-d dietary records were 0·34 for anthocyanidins, 0·61 for flavan-3-ols, 0·20 for flavanones, 0·34 for flavones, 0·27 for flavonols, 0·48 for isoflavones, 0·37 for proanthocyanidins and 0·54 for total flavonoids. The correlation coefficients between the two FFQ administered 1 year apart were 0·12 for anthocyanidins, 0·51 for flavan-3-ols, 0·66 for flavanones, 0·32 for flavones, 0·54 for flavonols, 0·39 for isoflavones, 0·36 for proanthocyanidins and 0·44 for total flavonoids.
Statistical analysis
SPSS software (version 21.0) was used to conduct the data analysis. Flavonoids intakes and food groups were adjusted for total energy intake by the regression residual method( Reference Willett, Howe and Kushi 35 ). Subjects were divided into quartiles (Q1–Q4) based on the distribution of flavonoids intakes among the control group. Continuous variables (such as age, subclasses of flavonoids intake) were compared between cases and controls using Student’s t test, whereas categorical variables (such as income, education level, smoking status) were analysed using χ 2 tests. Unconditional logistic regression models were used to estimate the OR and 95 % CI for the associations of flavonoids intakes with the risk of colorectal cancer, using the lowest quartile as the reference group. The OR and 95 % CI were further adjusted for potential confounding by the following variables: age, sex, marital status, education, income, occupation, family history of cancer, smoking status, passive smoking, alcohol drinking, occupational activity, household and leisure-time activities, and BMI. Intakes of red and processed meat, poultry and fish, total dairy products and eggs were statistically significantly different between cases and controls. As these foods were not sources of flavonoids, they were treated as confounders and added into the logistic regression models. Tests for trend were performed by entering categorical variables as continuous variables in the multiple regression models.
Stratified analyses by sex were conducted for the associations between flavonoids intake and colorectal cancer risk. The interaction between sex and intake of flavonoids in relation to the risk of colorectal cancer was evaluated in multiplicative models by including the product term in it. Stratified analysis by socio-economic status (according to education level and income level) was also conducted, which was not well balanced between cases and controls in the present study. Education levels were classified into four stages (primary school or below, secondary school, high school and college or above). Income levels were also classified into four stages (<2000, 2001–5000, 5001–8000 >8001 yuan/month). Subjects with education stage plus income stage values less than 5 were defined as individuals with low socio-economic status, and otherwise defined as individuals with high socio-economic status. In addition, we conducted subgroup analyses by cancer site (colon or rectal cancer) and by sources of controls (community-derived controls and hospital-derived controls). Sensitivity analysis using only community-derived controls or hospital-derived controls was also conducted. All P values were two-sided and statistical significance was determined at the P<0·05 level in the present study.
Results
Table 1 shows the socio-demographic characteristics of the study subjects and the distribution of selected colorectal cancer risk factors. Of the 1632 cases, 910 were males and 722 were females; 983 (567 males and 416 females) subjects were diagnosed with colon cancer and 649 (343 males and 306 females) with rectal cancer. Compared with controls, case subjects had lower education levels, greater household income, heavier occupational activity, less household and leisure-time activity and were more likely to have a family history of cancer, to be drinkers and current smokers and have passive smoking experience. All of the above variables were considered potential confounders and adjusted for in the subsequent analysis. No significant differences were found between cases and controls in other factors including marital status, occupation status, BMI and menopausal status (in female subgroup).
Table 1 Demographic and selected risk factors of colorectal cancer cases and controls in a Chinese populationFootnote * (Numbers and percentages; mean values and standard deviations; medians and 25th, 75th percentiles)
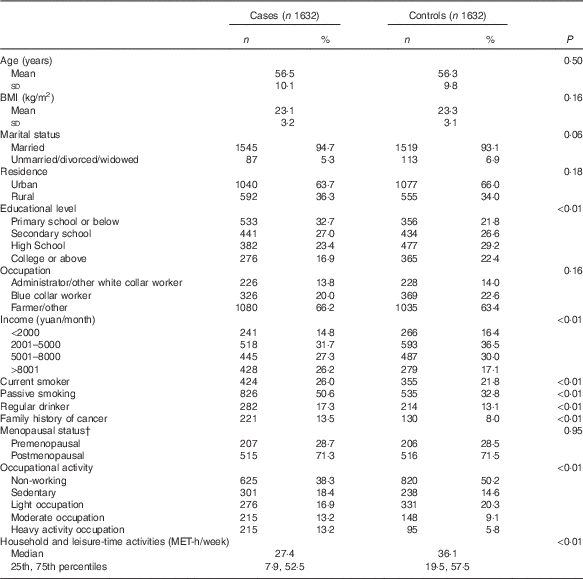
MET, metabolic equivalent task.
* Continuous variables were evaluated using t tests or Wilcoxon’s rank-sum tests. Categorical variables were evaluated using χ 2 tests.
† Among female subgroups.
The comparison of intakes of flavonoid subclasses from different dietary sources between cases and controls and selected dietary confounders are shown in Table 2. The energy-adjusted median intake of total flavonoids showed no difference between cases (241·0 mg/d) and controls (256·5 mg/d). Compared with controls, anthocyanidins, flavan-3-ols, flavanones, flavones, flavonols, proanthocyanidins and total flavonoids intakes from vegetables and fruits were significantly lower in cases. The energy-adjusted median intakes of flavan-3-ols, flavonols, proanthocyanidins and total flavonoids form tea were significantly higher among cases than among controls. Moreover, cases were reported to have more red and processed meat, less poultry and fish, total dairy products and eggs compared with controls.
Table 2 Intakes of energy, flavonoids and food groups among case and control subjects in Guangdong, ChinaFootnote * (Mean values and standard deviations; medians and 25th, 75th percentiles)
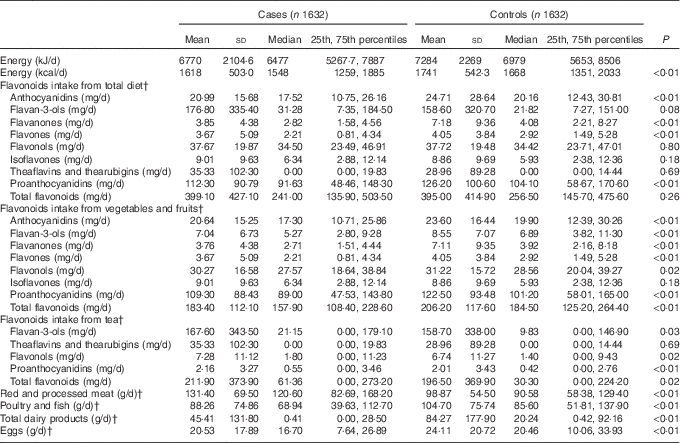
* Wilcoxon’s rank-sum test comparing the median intake levels between cases and controls.
† Adjusted for total energy intake by the regression residual method.
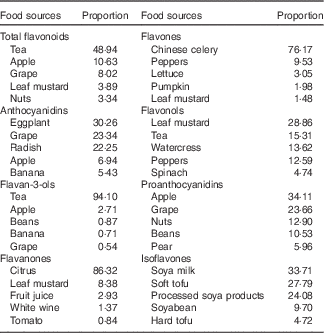
The main food sources of subclasses and total flavonoids among control subjects are listed in Table 3. The richest source of total flavonoids was tea (48·94 %), followed by apple (10·63 %) and grapes (8·02 %). Anthocyanidins were rich in vegetables (such as eggplant, radish), grapes, apple and banana. Most of the flavan-3-ols were contained in tea and apple. Flavanones were rich in fruits, especially in citrus fruits or citrus juice. Flavones were also rich in vegetables such as Chinese celery, peppers and lettuce. Flavonols were rich in leaf mustard and tea. The top five sources of proanthocyanidins were apple, grapes, nuts, beans and pear. Isoflavones were mainly contained in soya products.
Table 3 Top five food sources of subclasses of flavonoids among control subjects
As shown in Table 4, significant inverse associations were observed between dietary flavanones, flavones and the risk of colorectal cancer. The multivariate OR for the highest quartile compared with the lowest quartile were 0·28 (95 % CI 0·22, 0·36) for flavanones and 0·54 (95 % CI 0·43, 0·67) for flavones. There was borderline significant inverse association between total anthocyanidins intake and colorectal cancer risk, with OR for the highest quartile compared with the lowest quartile of 0·80 (95 % CI 0·64, 1·00) (P trend=0·08). No significant association was found for flavan-3-ols, flavonols and total flavonoids from total diet.
Table 4 Colorectal cancer according to quartiles (Q) of flavonoids intake from total diet (Odds ratios and 95 % confidence intervals)
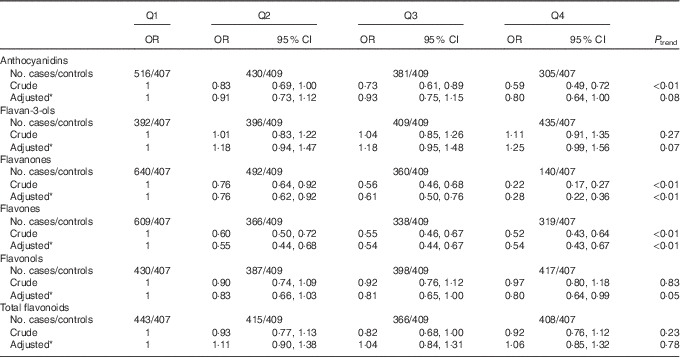
* OR was adjusted for age, sex, marital status, education, income, occupation, family history of cancer, smoking status, passive smoking, alcohol drinking, occupational activity, household and leisure-time activities, BMI, and intakes of red and processed meat, poultry and fish, total dairy products and eggs.
The association between flavonoid intake from different dietary sources and colorectal cancer risk is shown in Table 5. Significant inverse associations were observed between all subclasses of flavonoid intake from vegetables and fruits and colorectal cancer risk. The multivariate OR for the highest quartile of flavonoids from vegetables and fruits compared with the lowest quartile were 0·74 (95 % CI 0·59, 0·92) for flavan-3-ols, 0·28 (95 % CI 0·22, 0·36) for flavanones, 0·54 (95 % CI 0·43, 0·67) for flavones, 0·74 (95 % CI 0·59, 0·92) for flavonols and 0·70 (95 % CI 0·55, 0·87) for total flavonoids. Borderline significant inverse association was also found between anthocyanidins intake from vegetables and fruits and risk of colorectal cancer (OR 0·79; 95 % CI 0·63, 0·98, P trend=0·06). However, no significant association was found between flavonoids intake from tea and colorectal cancer (OR 0·94; 95 % CI 0·75, 1·17).
Table 5 Colorectal cancer according to quartiles (Q) of flavonoids intake from different dietary sources (Odds ratios and 95 % confidence intervals)
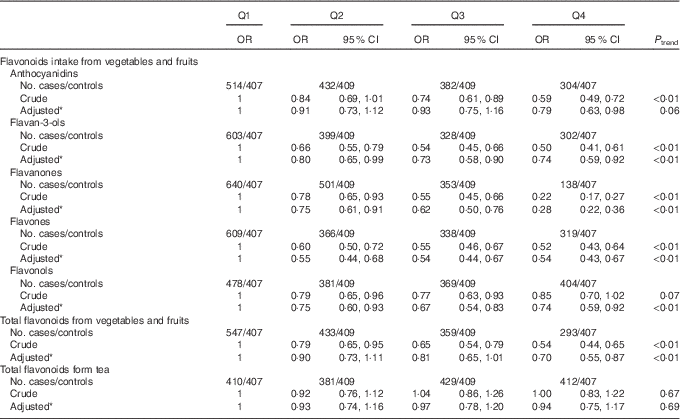
* OR was adjusted for age, sex, marital status, education, income, occupation, family history of cancer, smoking status, passive smoking, alcohol drinking, occupational activity, household and leisure-time activities, BMI, and intakes of red and processed meat, poultry and fish, total dairy products and eggs.
Consistent with the total participants, stratified analysis by sex showed that the inverse associations between intakes of flavanones and flavones and colorectal cancer risk were observed in both sexes (Table 6). No significant associations were found between intakes of flavan-3-ols and total flavonoids and risk of colorectal cancer among men and women. Although inverse associations between anthocyanidins and flavonols intakes and colorectal cancer risk were observed only among men and not women, no significant interaction was found between anthocyanidins and flavanols intakes and sex on the risk of colorectal cancer (P interaction=0·06 for anthocyanidins, P interaction=0·12 for flavonols). The results of subgroup analysis by cancer site were consistent with the main results (Table 7). The associations of flavonoids intake with colorectal cancer risk did not differ significantly when stratified by socio-economic status (low socio-economic status and high socio-economic status) (Table 8). Subgroup analysis by community-derived controls and hospital-derived controls showed no significant difference when using either group. Sensitivity analysis by using only community-derived controls or hospital-derived controls also showed that the results were relatively stable (data not shown).
Table 6 Colorectal cancer according to quartiles (Q) of flavonoids intake from total diet by sex (Odds ratios and 95 % confidence intervals)
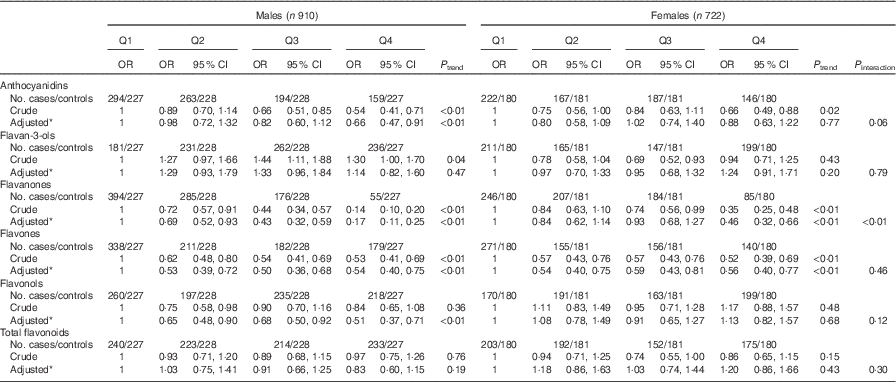
* OR was adjusted for age, sex, marital status, education, income, occupation, family history of cancer, smoking status, passive smoking, alcohol drinking, occupational activity, household and leisure-time activities, BMI, and intakes of red and processed meat, poultry and fish, total dairy products and eggs.
Table 7 Colorectal cancer according to quartiles (Q) of flavonoids intake from total diet by cancer site (Odds ratios and 95 % confidence intervals)
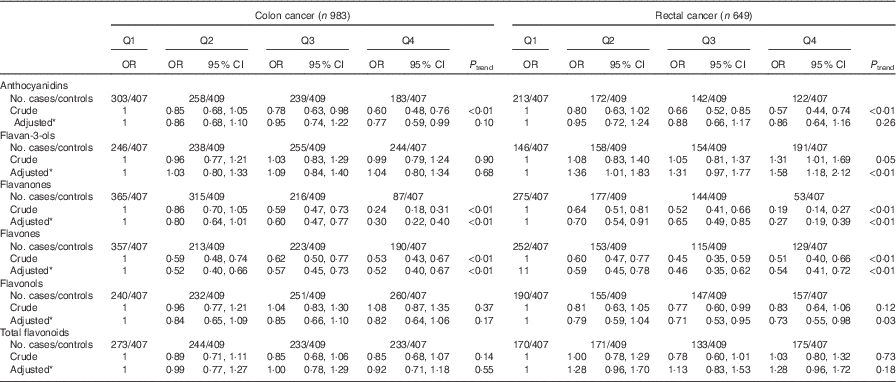
* OR was adjusted for age, sex, marital status, education, income, occupation, family history of cancer, smoking status, passive smoking, alcohol drinking, occupational activity, household and leisure-time activities, BMI, and intakes of red and processed meat, poultry and fish, total dairy products and eggs.
Table 8 Colorectal cancer according to quartiles (Q) of flavonoids intake from total diet by socio-economic status (Odds ratios and 95 % confidence intervals)
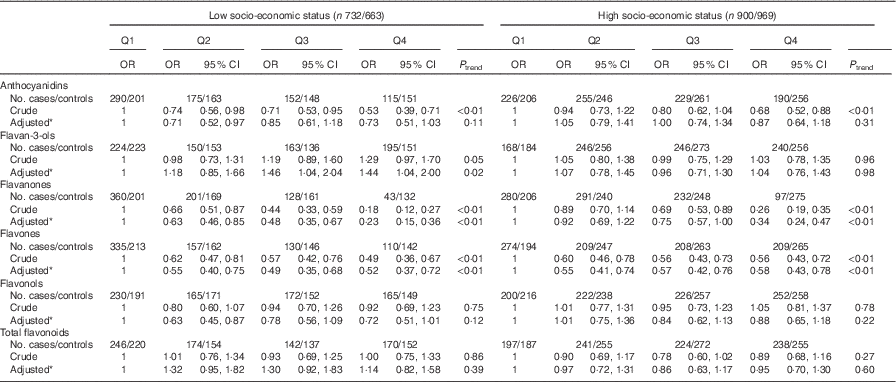
* OR was adjusted for age, sex, marital status, education, income, occupation, family history of cancer, smoking status, passive smoking, alcohol drinking, occupational activity, household and leisure-time activities, BMI, and intakes of red and processed meat, poultry and fish, total dairy products and eggs.
Discussion
This case–control study, with 1632 cases and 1632 controls, revealed no significant association between total flavonoids intake and colorectal cancer risk. However, higher intake of anthocyanidins, flavanones and flavones from total diet was inversely associated with colorectal cancer risk. All subclasses of flavonoids from vegetables and fruits were inversely associated with colorectal cancer risk. However, tea flavonoids were not significantly associated with colorectal cancer risk.
Some previous studies have examined the association between total flavonoids intake and colorectal cancer risk, and the results are consistent with the present study( Reference Lin, Zhang and Wu 10 , Reference Mursu, Nurmi and Tuomainen 13 , Reference Rossi, Negri and Talamini 21 ). A US cohort of 107 401 subjects (71 976 from the Nurses’ Health Study and 35 425 from the Health Professionals Follow-up Study) found that the intake of total flavonoids was not associated with colorectal cancer risk, with the relative risk (RR) for the highest quintile of total flavonoids intake being 1·19 (95 % CI 0·94, 1·49) compared with the lowest( Reference Lin, Zhang and Wu 10 ). No significant association was found between total flavonoids intake and risk of colorectal cancer in Finnish men. The hazard risk for the highest quartile compared with the lowest was 1·16 (95 % CI 0·58, 2·34)( Reference Mursu, Nurmi and Tuomainen 13 ). The multicentric, Italian case–control study with 1953 cases and 4154 controls also found no association between total flavonoids and colorectal cancer risk; the adjusted OR for the highest quintile compared with lowest was 0·97 (95 % CI 0·81, 1·16)( Reference Rossi, Negri and Talamini 21 ). However, the Spanish case–control study with 424 cases and 401 controls found an inverse association between total flavonoids intake and colorectal cancer risk (OR 0·59; 95 % CI 0·35, 0·99 for the highest quintile v. the lowest)( Reference Zamora-Ros, Not and Guino 16 ). Although it was reported that total flavonoid intake may be greatly influenced by thearubigins, particularly in high black tea-consuming countries( Reference Zamora-Ros, Knaze and Romieu 36 ), the present study found no significant difference between total flavonoids intake and colorectal cancer risk before and after thearubigins were excluded from total flavonoid intake. This result is consistent with two other studies that did not include thearubigins in total flavonoid intake( Reference Zamora-Ros, Not and Guino 16 , Reference Rossi, Negri and Talamini 21 ). Different results between total flavonoids intake and colorectal cancer risk in different studies could be partly explained by the variability in flavonoid sources. The participants of the Spanish case–control study were reported to consume relatively large amounts of flavonoids from vegetables and fruits and the flavonoids from tea was <10 %( Reference Zamora-Ros, Not and Guino 16 ), whereas tea was a major single food source of flavonoids (35 % contribution) in the US cohort( Reference Lin, Zhang and Wu 10 ). It is likely that the distribution of flavonoid subclasses differs in different dietary resources. Examining flavonoids from total diet and colorectal cancer without taking into account the different sources may have contributed to the null result. If the effect of flavonoids from vegetables and fruits is contrary to that from tea, then the effect of the combined flavonoids from vegetables, fruit and tea together will be weakened.
Intake of anthocyanidins, flavanones and flavones from total diet was inversely associated with colorectal cancer risk in the present study. The Spanish case–control study observed a 41 % reduction in colorectal cancer risk in the highest quintile of flavones intake( Reference Zamora-Ros, Not and Guino 16 ). A reduced risk of colorectal cancer was found for high intake of anthocyanidins (OR 0·67, the highest v. the lowest quintile), flavonols (OR 0·64, the highest v. the lowest quintile), flavones (OR 0·78, the highest v. the lowest quintile) in a network of case–control studies from Italy( Reference Rossi, Bosetti and Negri 19 ). Another Scottish case–control study with 1456 cases and 1456 controls found reductions in colorectal cancer risk associated with intake of flavonols and its main compound quercetin( Reference Theodoratou, Kyle and Cetnarskyj 20 ). The multicentric, Italian, case–control study also found a reduced risk of developing colorectal cancer with increasing intake of anthocyanidins, flavones and flavonols( Reference Rossi, Negri and Talamini 21 ). Although the protective effect of flavanones on colorectal cancer risk has not been observed in previous studies, in vitro studies have shown that flavanones can induce apoptosis, inhibit cancer growth and have anti-proliferative effects on colon carcinogenesis( Reference Saiprasad, Chitra and Manikandan 37 , Reference Song, Park and Eo 38 ). Moreover, a recently published review summarised the molecular mechanisms behind the biological effects of flavanones, and concluded that flavanones may even have a broader range of biological applications in cancer prevention( Reference Roohbakhsh, Parhiz and Soltani 39 ). However, intakes of subclasses of flavonoids were not associated with the risk of colorectal cancer in a recently published cohort study by Nimptsch et al.( Reference Nimptsch, Zhang and Cassidy 15 ). Multivariable adjusted RR comparing the highest with the lowest quintiles were 0·98 (95 % CI 0·81, 1·19) for anthocyanidins, 1·07 (95 % CI 0·95, 1·21) for flavan-3-ols, 0·96 (95 % CI 0·84, 1·10) for flavanones, 1·01 (95 % CI 0·89, 1·15) for flavones and 1·04 (95 % CI 0·91, 1·18) for flavonols. The difference could be partly explained by the variability in subclasses of flavonoid intakes among different populations. For example, the median anthocyanidins intake was about 14·6 mg/d in the US cohort( Reference Nimptsch, Zhang and Cassidy 15 , Reference Cassidy, O’Reilly and Kay 40 ) and 20·16 mg/d in the present study. The food sources of anthocyanidins in each study were also very different. The top three food sources of anthocyanidins in the US cohort were blueberries, bananas and strawberries( Reference Nimptsch, Zhang and Cassidy 15 , Reference Cassidy, O’Reilly and Kay 40 ), whereas anthocyanidins in the present study mainly came from eggplant, grapes and radish.
A few studies have examined the relationships between different dietary sources of flavonoid intake and colorectal cancer risk. As most of anthocyanidins, flavanones and flavones came from vegetables and fruits, the relationship between subclasses of flavonoids from vegetables and fruits was consistent with those of the total diet. However, inverse associations were found between flavan-3-ols, flavonols and total flavonoids from vegetables and fruits and colorectal cancer risk, which were not found in total diet. Consistent with the present study, a case–control study with 1163 cases and 1501 controls in the USA showed a significant protective effect of quercetin in the proximal colon when fruit intake was high or tea intake was low( Reference Djuric, Severson and Kato 17 ). Another population-based, case–control study in the UK showed that flavonols and quercetin intakes from non-tea dietary sources, mainly from vegetables and fruits, were inversely associated with colorectal cancer, with OR of 0·6 (95 % CI 0·4, 1·0) for flavonols and 0·6 (95 % CI 0·4, 0·9) for quercetin( Reference Kyle, Sharp and Little 18 ). Moreover, the Iowa Women’s Health Study also found that the intake of (+)-catechin and (−)-epicatechin, a subgroup of flavan-3-ols derived primarily from fruits, tended to be inversely associated with digestive tract cancer incidence( Reference Arts, Jacobs and Gross 22 ).
To the best of our knowledge, no previous studies have reported the relationship between tea flavonoids and colorectal cancer risk. Our study found no significant association between tea flavonoids intake and colorectal cancer risk. The percentage of tea drinkers among cases was 62·81 %, whereas among controls it was 54·41 %. This result is consistent with some previous studies( Reference Green, de Dauwe and Boyle 2 ). However, no association was found between tea intake and colorectal cancer risk in the present study (OR 0·96; 95 % CI 0·77, 1·19, for the highest quartile v. the lowest). Recent findings about the association between tea intake and colorectal cancer risk may also contribute to non-detection of the significant association of tea flavonoids with colorectal cancer risk. After a median follow-up of 11·6 years, the European Investigation into Cancer and Nutrition cohort found no significant associations between tea consumption and colorectal cancer, and the RR of the highest quintile of tea intake compared with the lowest was 0·97 (95 % CI 0·86, 1·09)( Reference Dik, Bueno-de-Mesquita and Van Oijen 41 ). The National Cancer Institute–Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial found that greater tea intake was not associated with colorectal cancer risk (RR 0·77; 95 % CI 0·55, 1·09 for the highest quartile v. the lowest)( Reference Dominianni, Huang and Berndt 42 ). Two meta-analyses summarised the results of case–control studies and prospective studies and found that data were insufficient to conclude that green tea may protect against colorectal cancer( Reference Wang, Zeng and Duan 43 , Reference Wang, Gao and Fang 44 ).
The present study showed an inverse association between colorectal cancer risk and flavonoids intake from vegetables and fruits but not from tea. One potential reason might be that the flavonoid glycosides vary among different dietary sources and different compounds of glycosides have different bioavailability. Previous studies have shown that onions contain glucose conjugates of quercetin, apples contained both glucose- and non-glucose quercetin glycosides and the major quercetin glycoside in tea is pure quercetin-3-rutinoside( Reference Hollman and Katan 45 ). Comparing the bioavailability of flavonol quercetin from major dietary sources in human subjects found that quercetin glucosides from onions are absorbed rapidly, and the rutinoside in tea is absorbed slowly, whereas the overall absorption rate of various glycosides from apples is intermediate( Reference Hollman and Katan 45 ). In addition, other compounds such as dietary fibre, carotenoids and vitamins present in vegetables and fruits might be responsible for the beneficial effects. Some studies have shown that intakes of dietary fibre, carotenoids and vitamins from vegetables and fruits are inversely associated with colorectal cancer risk( Reference Lu, Fang and Chen 46 – Reference Byers and Guerrero 48 ).
Flavonoids, especially from vegetables and fruits, have several important biological functions, which might be related to cancer risk( Reference Pierini, Gee and Belshaw 5 ). Isorhamnetin diglucoside, a major flavonoid present in mustard leaf, was found to be metabolised to isorhamnetin in vivo by intestinal bacteria and plays an important role as an antioxidant( Reference Yokozawa, Kim and Cho 49 ). It has been shown that flavan-3-ols from apples can reactivate silenced tumour suppressor genes in colorectal cancer cells( Reference Fini, Selgrad and Fogliano 50 ). Another study demonstrated that a standardised berry anthocyanin-rich extract inhibited the proliferation of the Caco-2 human colorectal cancer cell line by promoting reactive oxygen species accumulation, inducing caspase-3 activation, and by up-regulating the expression of p21Waf/Cif1 ( Reference Anwar, Fratantonio and Ferrari 51 ). Moreover, in vitro and in vivo studies have also shown that flavonoids can influence signal transduction pathways, stimulate apoptosis, and inhibit inflammation and proliferation in human cancer cell lines( Reference Pierini, Gee and Belshaw 5 , Reference Nichenametla, Taruscio and Barney 6 ). In addition, substantial amounts reach the colon, where they are further metabolised into metabolites that may also mediate some biological activity( Reference Williamson and Clifford 52 ).
The present study showed no interaction between flavonoids intakes and sex on the risk of colorectal cancer. The inverse association between intakes of anthocyanidins and flavonols and colorectal cancer risk observed among men but not among women in the present study might be a chance finding. Moreover, no previous studies have reported that different hormone status between men and women could affect the bioavailability of flavonoids( Reference Lin, Zhang and Wu 10 , Reference Nimptsch, Zhang and Cassidy 15 ). Subgroup analysis by cancer site showed that the protective effect exerted by total anthocyanidins, flavanones and flavones intakes on colon, rectal and combined cancers was similar, in agreement with other studies( Reference Zamora-Ros, Not and Guino 16 , Reference Theodoratou, Kyle and Cetnarskyj 20 , Reference Rossi, Negri and Talamini 21 ).
At least two strengths were present in this study. First, a few studies have examined the associations between flavonoids from different dietary sources and the risk of colorectal cancer. Flavonoids intake from vegetables and fruits may exert more protective effect on colorectal cancer risk than tea flavonoids. Therefore, it is necessary to detect the association between flavonoids from different dietary sources and colorectal cancer in future studies. Second, compared with previous case–control studies, the present study had a relatively large sample size. We have enough power to detect small associations in risk of colorectal cancer.
There are several limitations that should be taken into account when interpreting the results. First, selection bias is a potential limitation in case–control studies. To minimise selection bias, we tried to recruit controls from different departments of three general hospitals with ailments that had no apparent association with dietary habits. Moreover, the relatively high participation rate (89·57 % for cases and 86·98 % for hospital-derived controls, respectively) in the present study also reduced the selection bias. Furthermore, although cases from Sun Yat-sen University Cancer Center had greater household income and lower education levels than controls recruited from the community and other hospitals, no interaction effect was found between socio-economic status and flavonoids intake. Therefore, the possibility of selection bias should be reduced in our study. Second, dietary habits can be influenced by a recent diagnosis of cancer or by disease progress, so we tried to interview the cases as soon as the diagnosis was made. The average interval between diagnosis and interview for cases was 11 d. Third, the potential for recall bias exists in our study, as in any case–control study. To minimise this bias, a standardised questionnaire interview method was used to improve the comparability of recall between cases and controls. We also provided photographs of foods with usual portion size to help participants accurately estimate food intake. Fourth, although food intake was assessed only once during the past 12-month period, dietary assessment using the validated FFQ can be representative of long-term dietary habits( Reference Zhang and Ho 34 ). In addition, random measurement error in the estimation of dietary flavonoids intake using FFQ is also of concern. However, usually, this misclassification is non-differential among cases and controls( Reference Kipnis, Midthune and Freedman 53 ) and would attenuate the true association between flavonoids intake and colorectal cancer risk. Finally, despite the fact that we adjusted for a number of potential confounders, the possibility of residual confounding may have affected the results.
In conclusion, the results from this relatively large case–control study suggest that intakes of total anthocyanidins, flavanones and flavones may decrease the risk of colorectal cancer. In addition, the inverse association between colorectal cancer risk and flavonoids from vegetables and fruits, but not tea flavonoids, suggest the importance of assessing independent associations between different dietary sources of flavonoids and disease risk.
Acknowledgements
The authors gratefully acknowledge the cooperation of the study participants.
This study was supported by Guangdong Natural Science Foundation (No.: 2014A030313188, 2016A030313225). The funders had no role in the design, analysis or writing of this article.
The authors’ responsibilities were as follows: M. X. collected the data, analysed the data and wrote the paper. Y.-M. C. provided significant advice regarding the analyses and interpretation of the data. J. H., W.-Q. H., B. Y. and M.-S. L. participated in data collection. Y.-J. F. and Z.-Z. P. were responsible for connecting and coordinating the field work. C.-X. Z. constructed the project design, supervised the study and contributed to manuscript writing.
None of the authors has any conflicts of interest to declare.











