INTRODUCTION
Many infections with rubella virus are subclinical and disease is most often self-limiting. Clinical and public health relevance results from the potential serious consequences of congenital rubella infection, namely spontaneous abortion and infants born with congenital rubella syndrome (CRS). Very effective vaccines against rubella have been developed and used [Reference Best and Reef1]. In order to prevent CRS, rubella control and elimination programmes have been implemented in countries in which routine infant vaccine coverage is high and sustained, in order to interrupt transmission [Reference Cutts, Lessler and Metcal2]. Moreover, studies have shown the economic benefit of including rubella vaccine in vaccination programmes [Reference Cutts, Lessler and Metcal2, Reference Hinman3].
Mumps is an acute viral illness with usual airborne transmission. The clinical presentation ranges from asymptomatic infection to complications with or without parotitis. Severe complications, including deaths, are rare [Reference Mclean, Hickman and Seward4]. The WHO recommends mumps vaccination in countries with a well-established, effective childhood vaccination programme and the capacity to maintain high-level vaccination coverage [5]. Mumps vaccination is highly cost-effective [Reference Koplan and Preblud6].
Live attenuated rubella and mumps vaccines are available in monovalent formulations [Reference Best and Reef1, Reference Mclean, Hickman and Seward4]. However, the trivalent measles, mumps, and rubella vaccine (MMR) formulation is most commonly used [Reference Cutts, Lessler and Metcal2]. The economical [Reference White, Koplan and Oreinstein7] and programming [Reference Bart, Orenstein and Hinman8] benefits of using the combined trivalent formulation instead of the monovalent vaccines, have been well established and, at least in developed countries, the WHO has recommended the use of MMR to control and eventually eliminate CRS [Reference Hinman3] and mumps [Reference Koplan and Preblud6].
Taking into account mortality and disease burden, the WHO considers the control of measles and the prevention of CRS higher priorities than the control of mumps [Reference Cutts, Lessler and Metcal2] although this disease is a cause of relevant morbidity [Reference Mclean, Hickman and Seward4, Reference LeBaron9]. To achieve elimination, two doses of measles vaccine are recommended [Reference Cutts, Lessler and Metcal2]. Countries using MMR in two-dose schedules recommend different ages at which to give the first (MMR1) and the second (MMR2) dose [10] in order to optimize the use of measles vaccine [Reference Moss and Scott11]. Thus, vaccination against rubella and mumps has ‘followed’ the strategy of vaccination against measles in the number of doses (two) and schedule of administration.
High response to a single dose of vaccine against rubella (>95% seroconversion), associated with long-term persistence of protection, might not support the need for a second dose of vaccine [Reference Best and Reef1]. Nevertheless, two-dose schedules have been recommended to ‘help to boost low rubella antibody concentrations’ [Reference Best and Reef1] and reported cases of rubella seem not to occur in those who have received two doses of MMR [Reference LeBaron12]. Some authors have reported that response to MMR2 was ‘vigorous’ but declined to pre-MMR2 titres over time [Reference LeBaron12], while in another study no such antibody decline was observed [Reference Kakoulidou13]. Some studies have reported reductions in seropositivity (protective level of rubella antibodies) over time since MMR2 [Reference Poethko-Müller and Mankertz14], while others have reported that none of the subjects were seronegative several years after having received MMR2 [Reference Davidkin15, Reference Vandermeunlen16]. Antibody levels several years after MMR2 were reported to be higher in those vaccinated who had received vaccination in kindergarten (~5 years) than in those vaccinated at ~10 years. However, the authors noted that ‘the finding must be regarded with caution’. In the same study, males were more likely to be seronegative to rubella, some years after MMR2 [Reference LeBaron12].
Good serological response to mumps after MMR2 seems to be the rule [Reference Mclean, Hickman and Seward4, Reference LeBaron9]. It was observed that mumps antibody concentrations were higher in those who had received two doses of MMR instead of only one [Reference Vandermeunlen16]. Other studies have reported that, 17 years after MMR2, the proportion of vaccinees with low titres was not significantly different from that before revaccination [Reference LeBaron9]. A decline in the serum concentrations of mumps antibodies over time after MMR2 (waning immunity) has been reported [Reference Mclean, Hickman and Seward4, Reference LeBaron9, Reference Poethko-Müller and Mankertz14–Reference Vandermeunlen16]. Giving MMR2 at ages 5 or 10 years seems not to result in differences in antibody levels after some years [Reference LeBaron9].
In Portugal, selective vaccination of girls aged 11–13 with a monovalent rubella vaccine began in 1984 and in 1987 it was substituted by the vaccination of both sexes with MMR [Reference Gonçalves17]. The Wistar RA 27/3 vaccine strain was used in both vaccines. A decrease in CRS incidence was reported after vaccination began [Reference Gonçalves17, Reference Saldanha and Azevedo18].
Vaccination against mumps in Portugal began in 1987 (with the Urabe strain in MMR) and the number of reported cases decreased sharply until 1993. Thereafter numbers increased each year until 30 000 cases of mumps were reported in a national epidemic between 1996 and 1997. This epidemic, in the presence of high vaccine coverage, was due to vaccine failure associated with the Rubini strain used between October 1992 and June 1997. Since then the vaccine strain in use has been Jeryl Lynn [Reference Gonçalves, Araújo and Cardoso19]. In the period 2009–2012, only 35 cases of mumps were reported as ‘confirmed’ [20]. In Portugal, coverage with both MMR1 and MMR2 is high (⩾95%) [21, Reference Gonçalves22] and has been sustained at that level at least from 2006 [21].
In 2000, the recommended age for the second dose of MMR was changed from 10–13 years to 5–6 years for those born in 1994 and afterwards [Reference Gonçalves17]. Thus, those born in 1993 were supposed to be the last Portuguese to receive MMR2 at age 10–13 years and this provided a unique opportunity to compare birth cohorts that had followed different schedules and to assess the level of immunity against mumps and rubella of this two-dose vaccination programme.
The main objectives of this study were to asses if the concentration of mumps- and rubella-specific IgG antibodies depended on the age of administration of MMR2 and if immunity against both diseases waned with time since MMR2. Although not a main objective, other variables associated with the vaccination schedule were also assessed.
METHODS
In this study we used 166 of the 167 sera tested in a previous study on measles [Reference Gonçalves23]. This study required laboratory procedures concerning rubella and mumps and the consequent data analysis. General selection criteria and procedures have been described elsewhere [Reference Gonçalves23]. The study was conducted in convenience samples of three Portuguese birth cohorts which were selected taking into account the recommended age of receiving MMR2. Recruiting, collection of blood samples and consultation of vaccination records were done between May 2012 and August 2013.
Individuals born during 1990–1995 were recruited from students of a school of health sciences (ESS/IPL, Leiria). Year of birth was chosen deliberately to include the last cohorts to receive MMR2 at 10–13 years (those born 1990–1993) and the first cohort to receive MMR2 at the new recommended age of 5–6 years (those born 1994–1995). During lectures 312 students were invited to participate. The objectives and procedures of the study were explained. Students were asked to attend the school laboratory on specified dates and bring their written vaccination records. Participants signed a written consent, were interviewed and a blood sample was collected. Dates of vaccination with MMR were recorded from individual written records. Where those records were not available, the vaccination history was checked in the computerized vaccination records of the Portuguese National Health Service health centre (NHS-HC) where individuals had been vaccinated.
Individuals in the younger cohort, born between 2001 and 2003 (MMR2 recommended at 5–6 years) were recruited at a rural NHS-HC. Following a routine procedure 131 individuals identified in the vaccination files were sent a letter inviting them to attend the health centre for vaccination, thereby maintaining the vaccination schedule (tetanus-diphtheria vaccine in this case). For the purpose of this study, they received additional written information and an informed consent form. When the children arrived for vaccination with their parent/s they were invited to participate. Informed consent was signed by the responsible parent, a brief interview (using a standard questionnaire) was conducted and a venous blood sample was collected. MMR vaccination dates were collected from the health centre computer files.
Laboratory study
Specific IgG antibodies to rubella virus (anti-rubella IgG) and mumps virus (anti-mumps IgG) were measured in the sera, using the commercial immunoassays Euroimmun® anti-rubella Virus AT ELISA and anti-mumps Virus AT ELISA (Euroimmun AG, Germany). Antibody levels were calculated by correlation to standard curves, accordaning to the manufacturer's instructions, and expressed as international units per millilitre (IU/ml) for rubella and relative units per millilitre (RU/ml) for mumps. Participants were considered ‘seronegative’ for rubella if the concentration was <8 IU/ml and ‘seronegative’ for mumps if the concentration was <16 RU/ml.
Strategy of analysis and statistical methods
The dependent variables used in this study were the concentrations of mumps- and rubella-specific IgG antibodies, and corresponding seronegative status. The main predictive variables (independent variables) were age at MMR2 and time elapsed since MMR2. However, age when enrolled in this study and other variables concerning the vaccination schedule were also evaluated (age at MMR1, and time elapsed between MMR1 and MMR2). To compare the cohorts and the two MMR2 schedules, ANOVA and χ 2 tests were used. Data from all participants of the three birth cohorts were pooled together and multiple regression models were used for each specific antibody concentration. Associations were considered statistically significant at the 0·05 level.
Mumps IgG and rubella IgG concentration values were log-transformed (natural logs = ln) for analysis. Concentrations reported in tables and text are back transformations of ln values. In the case of mumps antibodies, specific analysis was done using the vaccine strain (Urabe, Rubini and Jeryl Lynn) in MMR1 as predictive variable. All participants had received the Jeryl Lynn strain with MMR2.
Ethical approval
This study was approved by the Ethics Committee of a local NHS unit (Unidade Local de Saúde da Guarda), the Board of a school of health sciences (Escola Superior de Saúde do Instituto Politécnico de Leiria) and the Board of a local public health unit (ACES Pinhal Litoral).
RESULTS
Two hundred and four participants were enrolled in the study, and signed the informed consent form. The criteria to reject several potential participants were described in detail elsewhere [Reference Gonçalves23]. Finally, 166 participants were selected for analysis, distributed across three birth cohorts as follows:
-
• 66 participants born in 1990–1993, who received MMR2 at 10–13 years;
-
• 59 participants born in 1994–1995, who also received MMR2 at 5–6 years;
-
• 41 participants born in 2001–2003, who received MMR2 at 5–6 years.
The female to male ratio (F/M) was close to 1 (F/M = 21/20) in the youngest cohort (born in 2001–2003), selected at the health centre. A higher proportion of females (F/M = 107/19) was selected in the two other cohorts, recruited in a professional health school with a very high F/M ratio.
Summary parameters of the attributes (independent variables) of participants, chosen as potentially predictable of antibody levels, are displayed in Table 1. When comparing the three birth cohorts, similarities and differences in the values of those variables result clearly from the combination of age at recruiting and the ages of vaccination with MMR1 and MMR2, imposed by the selection criteria.
Table 1. Attributes of participants of the three birth cohorts selected for the study

a Recommended age for MMR2 was 10–13 years.
b Recommended age for MMR2 was 5–6 years.
Mean ages to receive MMR1 were very similar between the groups (Table 1) since we have selected only those who received MMR1 in the second year of life. About 62% participants received MMR1 in the 16th month of life and 80% were vaccinated between 15 and 17 months.
For programming reasons, the age distribution at MMR2 was similar between cohorts born in 1994–1995 and 2001–2003 (Table 1).
The criteria used to select the three birth cohorts, with different ages at MMR2, resulted in a wide range of values for the variable ‘time since the second dose of MMR’ (from 4·68 to 14·93 years), which almost do not overlap between the cohorts (Table 1).
The distributions of antibody (IgG) concentrations against mumps and rubella, and corresponding proportions of seronegative participants, in the three selected birth cohorts are displayed in Table 2. Neither the concentrations of rubella antibodies nor the concentrations of mumps antibodies were significantly different between the three selected cohorts. The same was true for seronegative proportions. If analysis was done comparing those receiving MMR2 at 5–6 or 10–13 years, no significant differences were observed.
Table 2. Levels of specific IgG antibodies against mumps and rubella, and seronegative proportions
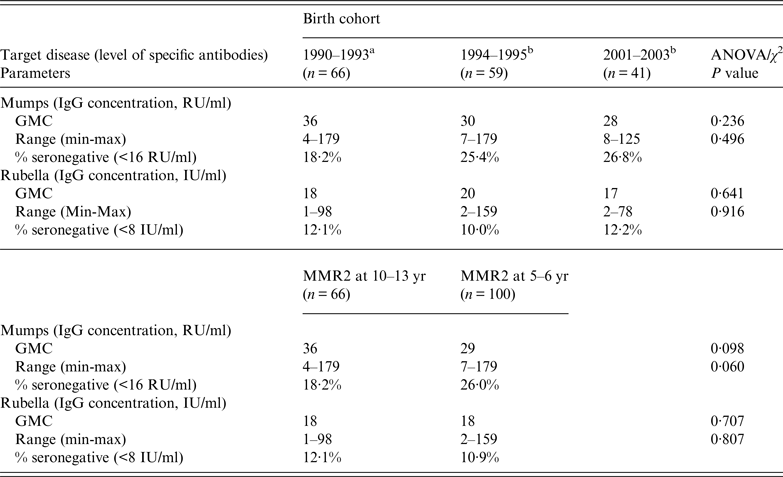
GMC, Geometric mean concentration.
a For those born in 1990–1993, the recommended age for MMR2 was 10–13 years.
b For those born in 1994–1995 and 2001–2003, the recommended age for MMR2 was 5–6 years.
Determinants of the concentration of anti-rubella IgG
Antibody levels were similar in the three birth cohorts and no differences were observed over time since MMR2 or between those receiving MMR2 at ages 5–6 or 10–13 years. Rubella IgG levels were lower (P = 0·029) in males [geometric mean concentration (GMC) = 27 RU/ml, range 9–98] than females (GMC = 33 RU/ml, range 4–179).
Determinants of the concentration of anti-mumps IgG
Using either a univariate analysis or a saturated model (multiple linear regression) gave the same result: no single variable was predictive of anti-mumps IgG concentration. Antibody levels were similar in the three birth cohorts and no differences were observed time since MMR2. No difference was observed between those receiving MMR2 at ages 5–6 or 10–13 years.
All participants had received the Jeryl Lynn vaccine strain with MMR2. The mumps vaccine strains used with MMR1 were Urabe (n = 10), Rubini (n = 115) and Jeryl Lynn (n = 41). The GMC of mumps IgG was slightly higher in those who received the Urabe strain with the first dose but differences were not statistically significant (P = 0·262).
Proportions seronegative for rubella and mumps
There were no significant differences between the three cohorts either for antibody concentrations against mumps and rubella or for seronegative proportions (Table 2).
When the analysis (χ 2 test) was performed separately within each birth cohort, the proportion of seronegatives was lower for rubella than for mumps in all three cohorts, but the difference was only significant for the 1994–1995 birth cohort (P = 0·03), which was the cohort that received MMR longer ago (see Table 1). Proportions seronegative (for rubella antibodies) in males and females (15·4% and 10·2%, respectively) were not significantly statistically different (P = 0·368).
DISCUSSION
The main findings of this study were that antibody concentrations against mumps and rubella were not associated with age of administration of MMR2 or with time elapsed since that dose. These results are consistent with those from some studies while different from others, which is explored further below.
Data collected on vaccination were reliable and precise. The use of a convenience sample limits the external validity: because the sample is not representative of the Portuguese population (even in the same age group) extrapolations should be made with caution. However, the use of convenience sample is not likely to have affected the internal validity: the analysis of association between the potential predictive variables and antibody concentrations was valid. It is known that immunological response to each component to a second dose of MMR depends on the age of administration and the previous serological response to the first dose [Reference Best and Reef1, Reference Mclean, Hickman and Seward4]. Having no data on the antibody levels after MMR1 or immediately before MMR2 is a limitation of this study. On the other hand, the antibody concentration of specific individuals may have been influenced by previous exposure to wild mumps and/or rubella viruses, but we had no clinical or serological information on those events to confirm this.
Anti-rubella IgG
All other studies reporting concentrations of anti-rubella IgG (Table 3) observed higher concentrations than those in any of the three cohorts in this study. GMC values in those studies were particularly high in older cohorts [Reference Kakoulidou13, Reference Davidkin15, Reference Vandermeunlen16], which may be compatible with a ‘cohort effect’. In other words it may have resulted from a considerable proportion of individuals who had been in contact with wild virus, since it is well known that there is a higher magnitude of serological response to natural infection [Reference Best and Reef1]. Similarly to a Swedish study [Reference Kakoulidou13], our study did not observe an association between time since MMR2 and concentration of rubella antibodies. However, such an association has been observed in other studies [Reference Poethko-Müller and Mankertz14, Reference Davidkin15]. Our finding of lower antibody concentration in males is consistent with another study [Reference LeBaron9] but we are not aware of a plausible biological or epidemiological explanation. Moreover, this resulted in no significant differences in the proportions seronegative.
Table 3. Comparison of selected outcome variables between studies investigating the antibody (IgG) response to vaccination against mumps and rubella
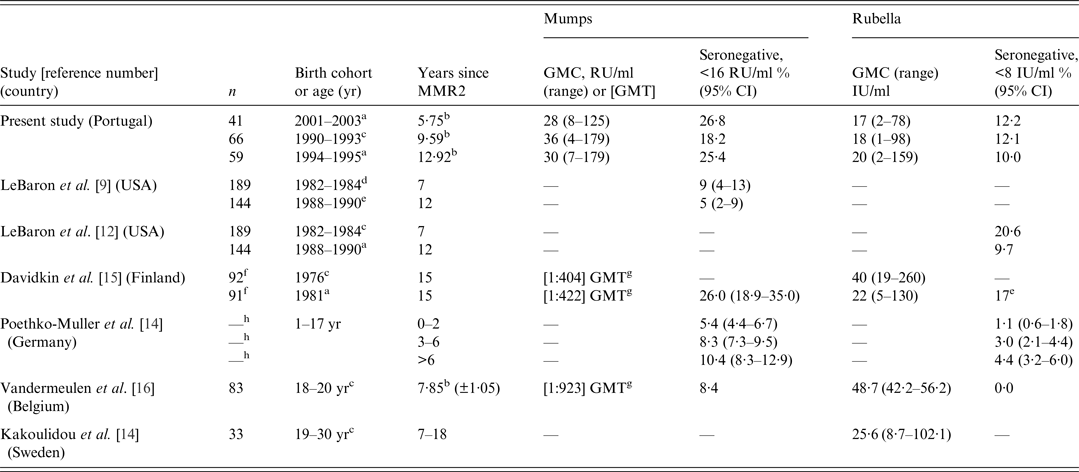
CI, Confidence interval.
a MMR2 at ~5–6 years.
b Average.
c MMR2 at ~10–13 years.
d MMR2 at ~10 years.
e MMR2 at ~5 years.
f These are the numbers of participants initially recruited; for each specific antibody, different (lower) numbers were studied.
g When plaque-reduction neutralization was used to measure antibody concentrations, geometric mean titres (GMT) were reported instead of geometric mean concentrations (GMC).
h Participants with a vaccination card totalled 12 972, but the precise number in each of these groups was not reported.
Our study found no differences in antibody concentrations between the cohorts vaccinated at different ages with MMR2 but that has not been case in other studies. As mentioned before, one study reported higher concentrations of antibodies in those receiving the second dose at age 5–6 years (kindergarten) [Reference LeBaron12]. A Finnish study [Reference Davidkin15] found that those receiving MMR2 at an older age had higher levels of antibodies. We can speculate on the possible advantage of giving the second dose of rubella vaccine as late as possible, but we cannot definitively answer this question. The aim of vaccination against rubella is to prevent CRS and we would like to protect women of reproductive age. As in many other countries, Portuguese women are having children at increasingly older ages. If immunity against rubella does wane over time, vaccinating at a later age might be more efficient in protecting the fetus. However, this issue has no straightforward answer since the strategy to prevent CRS in developed countries, involves achieving herd immunity to eliminate rubella [Reference Best and Reef1, Reference LeBaron12, Reference Gonçalves24, Reference Gao25] through vaccinating boys and girls (with MMR) at earlier ages and maintaining a high vaccination coverage [Reference Cutts, Lessler and Metcal2].
Anti-mumps IgG
It is difficult to compare antibody concentrations since some other studies used plaque-reduction neutralization (PRN) tests and reported titres instead of RU/ml (Table 3). Some authors also mentioned the limitations of enzyme immunoassays (EIA), similar to the one we used, in assessing seronegative status [Reference LeBaron9].
Regarding the proportions of seronegatives, this study showed similar results to those observed in Finland [Reference Davidkin15] while seronegative proportions were much lower in studies conducted in Germany [Reference Poethko-Müller and Mankertz14] and Belgium [Reference Vandermeunlen16], all of which had used EIA tests. In the USA, a study using PRN also found much lower proportions of seronegatives at similar times from MMR2 as this study [Reference LeBaron9].
Our results did not show waning immunity with time since MMR2, in contrast with several other studies [Reference LeBaron9, Reference Poethko-Müller and Mankertz14, Reference Davidkin15, Reference Abrams, Beutels and Hens26]. We cannot draw any definite conclusions about the lack of a long-term effect of the use of Rubini in the NVP and its consequence in terms of epidemics as the association of antibody concentration with vaccine strains used was not statistically significant. Linked to this, we also did not know which individuals had contact with the wild virus, which might have resulted in a more intense serological response [Reference Mclean, Hickman and Seward4].
Bringing forward the recommended age for MMR2 in Portugal does not appear to have affected the persistence of antibodies, which is consistent with findings from other studies [Reference LeBaron9].
In the United States, concerns have been raised about the potential for insufficient herd immunity [Reference LeBaron9]. With our higher proportions of seronegatives those concerns are even more legitimate in Portugal. Meanwhile, issues of correlation between serological, cellular and clinical immunity are not yet fully understood [Reference LeBaron9] and might challenge the interpretations of serological studies in the future.
Consequences for MMR schedule and coverage
Taking into account previous evidence, the results of this study and the logistics needed to change vaccination schedules, it seems reasonable that sustaining very high coverage with both MMR1 and MMR2 is currently more relevant than changing the schedule, for both mumps and rubella.
ACKNOWLEDGEMENTS
The authors are grateful to the support given by the National School of Public Health, Lisbon. The authors acknowledge Dr Leonie Prasad for reviewing a previous version of the manuscript. We thank Dr Ana Manso and Dr António Serra for authorizing the study in the health centre of Sabugal, Dr Jorge Costa for authorizing access to vaccination records in Leiria, Professor José Carlos Gomes, Director of ESS-IPL for all his support in several activities and for authorizing this study in his school, nurses Susana Frade, Ana Gomes, Fátima Soares, Dina Pascoal and Júlia Oliveira, for their cooperation in collecting blood samples and consulting vaccination files. The participants (all cohorts) and parents (cohorts born 1990–1995) are also gratefully acknowledged.
This study was not supported by any specific grant. ELISA kits were purchased by institutions where two of the authors work: Instituto de Ciências Biomédicas Abel Salazar (ICBAS), Universidade do Porto (G. Gonçalves). Instituto Politécnico de Leiria (ESS-IPL), Leiria (J. Frade).
DECLARATION OF INTEREST
None.






