Introduction
Giardia duodenalis (syn. Giardia lamblia and Giardia intestinalis) is an important intestinal protozoan that infects a wide range of mammalian species, for example, humans, wildlife, livestock and companion animals such as dogs and cats (Xu et al., Reference Xu, Jin, Wu, Li, Wang, Li, Feng and Xiao2016; Li et al., Reference Li, Deng, Wu, Huang, Song, Su, Hu, Fu, Zhong and Peng2017b). Generally, infection with Giardia results in self-limited illness with weight loss and malabsorption, and asymptomatic giardiasis is common in developing countries (Hellard et al., Reference Hellard, Sinclair, Hogg and Fairley2000; Thompson, Reference Thompson2000). In 2004, giardiasis was classified as a neglected tropical disease by WHO because of its adverse effects on the growth and cognition development of children (Savioli et al., Reference Savioli, Smith and Thompson2006). Giardiasis has a significant public health impact and affects veterinary health. The genus Giardia is divided into eight assemblages/genotypes (A to H) on the basis of genetic analysis. The zoonotic assemblages A and B can infect humans and many mammalian species, such as wild animals, nonhuman primates, domestic animals and companion animals. Other assemblages are more host-specific. Assemblages C and D infect domestic and wild canines, assemblage E infects domestic ruminants and pigs and assemblage F infects cats. Assemblage G is mostly found in rodents, and assemblage H, in seals (Cacciò et al., Reference Cacciò, Lalle and Svard2018). However, some of these assemblages have also been identified in humans, such as assemblage F in children living under poor environmental conditions in Slovakia, assemblage E in people living in Australia and assemblage C in diarrhoea patients in Shanghai, China (Liu et al., Reference Liu, Shen, Yin, Yuan, Jiang, Xu, Pan, Hu and Cao2014b; Zahedi et al., Reference Zahedi, Field and Ryan2017; Pipiková et al., Reference Pipiková, Papajová, Majláthová, Šoltys, Bystrianska, Schusterová and Vargová2018). In fact, transmission of Giardia from humans to animals or vice versa has been detected in areas where humans have close contact with animals such as lambs (Traub et al., Reference Traub, Monis, Robertson, Irwin, Mencke and Thompson2004; Lebbad et al., Reference Lebbad, Mattsson, Christensson, Ljungström, Backhans, Andersson and Svärd2010). A previous study has also shown the possibility of sexual transmission of Giardia in endemic areas (Escobedo et al., Reference Escobedo, Acosta-Ballester, Almirall, Rodriguez-Morales, Ortíz, Laffita and Chirino2018).
In humans, the number of giardiasis cases has been estimated to be about 28.5 million, with an average infection rate of 2.52%. The annual incidence of Giardia infection accounts for more than 10% of the total number of cases worldwide (Li et al., Reference Li, Deng, Wu, Huang, Song, Su, Hu, Fu, Zhong and Peng2017b). In China, large-scale investigations of G. duodenalis in humans showed infection rates of 6.04% (81/1332) in Huainan, Anhui Province, and 9.46% in Shanghai (Fu et al., Reference Fu, Sun and Su2004; Wang et al., Reference Wang, Xiao, Duan, Ye, Guo, Guo, Liu and Feng2013). The infection rate of Giardia is higher in HIV/AIDS patients, with the highest rate of up to 16.2% in Guangzhou, China (Pand et al., Reference Pand, Chen, Gao, Mai, Han, Xu and Yang2015). In China, assemblages A and B are the main genotypes of Giardia in humans (Li et al., Reference Li, Wang, Wang and Zhang2017a). Recently, assemblage C was identified as the predominant species in diarrhoea patients in Shanghai, China (Liu et al., Reference Liu, Shen, Yin, Yuan, Jiang, Xu, Pan, Hu and Cao2014b), and assemblage E was identified to have a high infection rate (6.8%, 6/88) in humans in Queensland, Australia (Zahedi et al., Reference Zahedi, Field and Ryan2017).
In animals, the prevalence of giardiasis varies greatly in different countries because of the animal species, sample methods, environment and development status. The infection rate has been reported to be as high as 52% in cattle in the United States and 66.4% in pigs in Canada (Hoar et al., Reference Hoar, Paul, Siembieda, Pereira and Atwill2009; Farzan et al., Reference Farzan, Parrington, Coklin, Cook, Pintar, Pollari, Friendship, Farber and Dixon2011). Recently, studies on Giardia in animals have been performed in at least 27 provinces and autonomous regions in China, with a prevalence rate of 0.51–50% in non-human primates, 1.04–22.6% in cattle, 0–27.78% in sheep and goats, 3.71–31.51% in dogs and cats, 1.7–11.1% in wild boar and domestic pigs, 1.9–8.38% in rabbits and 6.03–37.50% in rodents (Fan et al., Reference Fan, Wang, Koehler, Hu and Gasser2017; Li et al., Reference Li, Wang, Wang and Zhang2017a, Reference Li, Deng, Wu, Huang, Song, Su, Hu, Fu, Zhong and Peng2017b; Wang et al., Reference Wang, Zhang, Wu, Li, Qi, Li, Wang, Wang, Zhang, Jian, Ning and Zhang2018; Zhang et al., Reference Zhang, Qi, Jing, Yu, Wu, Chang, Zhao, Wei, Dong and Zhang2018). Molecular methods have been used in several studies, and assemblages A and B have been isolated from animals. Assemblages A and E were found in a dog and cattle, respectively; assemblage B, in rabbits; assemblages C, D and F, in companion dogs and assemblage G, in racehorses (Li et al., Reference Li, Wang, Wang and Zhang2017a, Reference Li, Deng, Wu, Huang, Song, Su, Hu, Fu, Zhong and Peng2017b). The molecular studies of Giardia in animals in China mainly concentrated on cattle, sheep, cats and dogs. There is limited information on the prevalence and genetic characterization of G. duodenalis in pigs. Pigs are one of the main sources of meat products in China. Swine manure may cause environmental contamination through the water or other ways (Thurston-Enriquez et al., Reference Thurston-Enriquez, Gilley and Eghball2005), and a large number of Giardia spores in animal slurry can also enter streams and rivers from pasture run-off. In 2011, researchers investigated pollution by Cryptosporidium and Giardia in the water source of the Huangpu River and showed through genotyping that pigs are one of the sources of pollution (Feng et al., Reference Feng, Zhao, Chen, Jin, Zhou, Li, Wang and Xiao2011). Therefore, it is essential to study Giardia in pigs in the area around Huangpu River.
Although several genes, such as small-subunit (SSU) rRNA, glutamate dehydrogenase (gdh), triose phosphate isomerase (tpi) and beta-giardin (bg), are widely used to identify Giardia with PCR, a single gene may not accurately identify Giardia or fully describe its genetic characterization. Multilocus genotyping (MLG) based on more than three genes is being used to provide more genetic information and contribute to understanding possible zoonotic transmission linkages (Cacciò et al., Reference Cacciò, Beck, Lalle, Marinculic and Pozio2008; Scorza et al., Reference Scorza, Ballweber, Tangtrongsup, Panuska and Lappin2012). The aim of the present study was to assess the prevalence of G. duodenalis in pigs from two farms in Shanghai, which is the largest economic centre of the country, by amplification of gdh, bg and tpi and sequencing and investigate the possible zoonotic potential of G. duodenalis at a genetic level.
Materials and methods
Sample collection
In the two farms, permission to conduct the study was obtained from the managers. In 2014, a total of 93 faecal samples were collected from the farms. The samples were collected from freshly dropped faeces by using a sterile disposable latex glove, placed in clean plastic bags, transported on ice to the laboratory and stored at 4 °C until DNA extraction.
DNA extraction
The faecal samples were washed three times using sterile water, and genomic DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Valencia, USA), according to the manufacturer's protocol. The DNA was eluted in 200 µL of AE elution buffer and stored at−30 °C until use.
Molecular methods
All the samples were analysed for the three loci. A 530 bp fragment of gdh, 530 bp fragment of tpi and 380 bp fragment of bg were amplified using nested PCR (Cacciò et al., Reference Cacciò, De Giacomo and Pozio2002; Sulaiman et al., Reference Sulaiman, Fayer, Bern, Gilman, Trout, Schantz, Das, Lal and Xiao2003; Scorza et al., Reference Scorza, Ballweber, Tangtrongsup, Panuska and Lappin2012). For all three genes, primary PCR was performed with 12.5 µL of 2 × PCR master mix (Promega, Italy), 1 µL of each primer (10 µ m), and 1 µL of DNA in a total reaction volume of 25 µL. For the nested PCR, 1 µL of the first PCR product was used as the template. The PCR cycling conditions were the same for gdh and tpi: initial hot start at 95 °C for 5 min, followed by 35 cycles (94 °C for 50 s, 57 °C for 45 s and 72 °C for 1 min) and a final extension step at 72 °C for 10 min. The secondary PCR cycling conditions were identical to the primary PCR cycling conditions. For bg, the cycling conditions were the same, except the annealing temperature was 60 °C. A Giardia-positive DNA specimen and distilled water were used as the positive and negative controls, and the PCR products were analysed using 2% agarose gel electrophoresis and ethidium bromide staining.
DNA sequencing and data analysis
For accurate analysis, all the genes were amplified at least three times and all PCR-positive products were sequenced in both directions using an ABI 3730 DNA Analyzer (Applied Biosystems, Foster City, USA), secondary primers and a Big Dye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems). ContigExpress was used to evaluate the wave peak and assemble the sequences. The nucleotide sequences were aligned and edited using BLAST, BioEdit (version 7.0.9), GenBank and ClustalX 1.83 (ftp://ftp-igbmc.u-strasbg.fr/pub/ClustalX/).
Results
Prevalence and PCR amplification of G. duodenalis
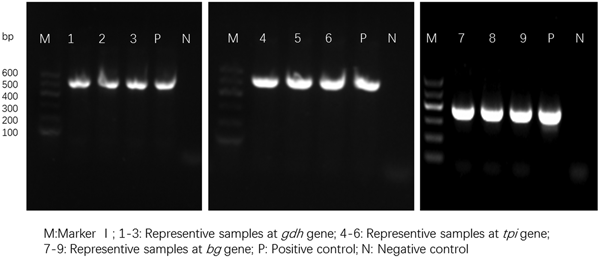
For the 93 samples, nested PCR amplification of gdh, tpi and bg was performed. On the basis of at least one gene, the average prevalence of G. duodenalis infection was 26.88% (25/93) in the pigs (Table 1). In farm 1, gdh, bg and tpi were detected in 11 (17.19%), 11 (17.19%) and 5 (7.81%) samples, respectively. Among the samples, all three loci were successfully amplified in two isolates, whereas gdh and bg were amplified in five isolates. On the basis of one locus, gdh, bg and tpi were successfully amplified in four, four and three isolates. In farm 2, gdh, bg and tpi were detected in five (17.24%), three (10.34%) and two (6.90%) samples. Among the samples, the three loci were successfully amplified in only one isolate, whereas gdh and bg were amplified in one isolate. In total, the prevalence of G. duodenalis was 28.13% (18/64) in farm 1 vs 24.14% (7/29) in farm 2, and gdh showed a higher amplification rate (17.20%, 16/93) than bg (15.05%, 14/93) and tpi (7.53%, 7/93). PCR results of representative samples for the gdh, tpi and bg genes are shown in Fig. 1.
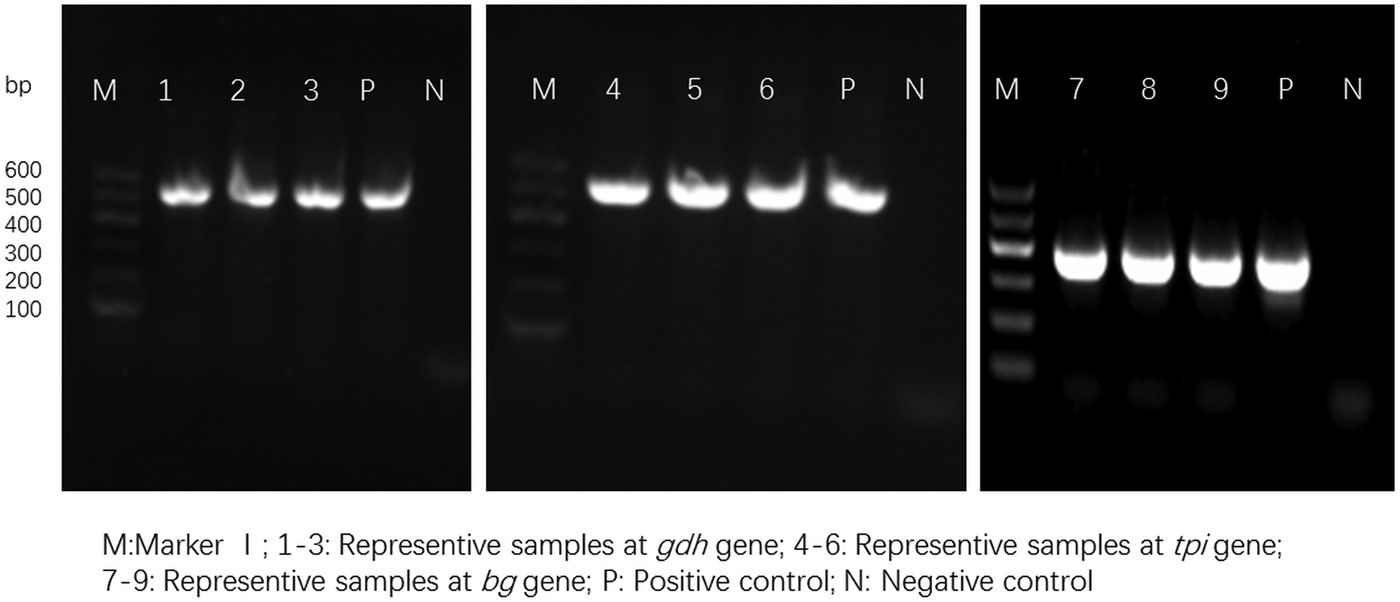
Fig. 1. PCR results of representative samples for the gdh, tpi and bg genes.
Table 1. Infection rate and assemblage distribution of G. duodenalis in pigs
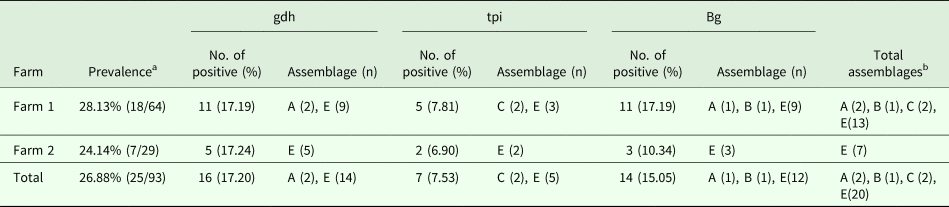
a Prevalence based on one locus.
b Total assemblages indicating that if one isolate had the same assemblage at different loci, it was considered as one assemblage.
Percentage and distribution of G. duodenalis assemblages
In this study, assemblages A, B, C and E were found in the pigs. Using the gdh locus, 16 specimens were identified to be assemblage A and 14, assemblage E, whereas, using the tpi locus, two specimens were identified to be assemblage C and five, assemblage E. The expected fragment of bg was successfully amplified in 14 specimens, and assemblage A (1), assemblage B (1) and assemblage E (12) were identified. In general, assemblage E was predominant in the pigs in the investigated areas. This result is consistent with those obtained in China and other countries.
In addition, the different farms yielded different genotypes. In farm 1, the genotypes were diverse, and assemblages A, B, C and E were identified. However, in farm 2, only assemblage E was detected.
Homology analyses of G. duodenalis assemblages
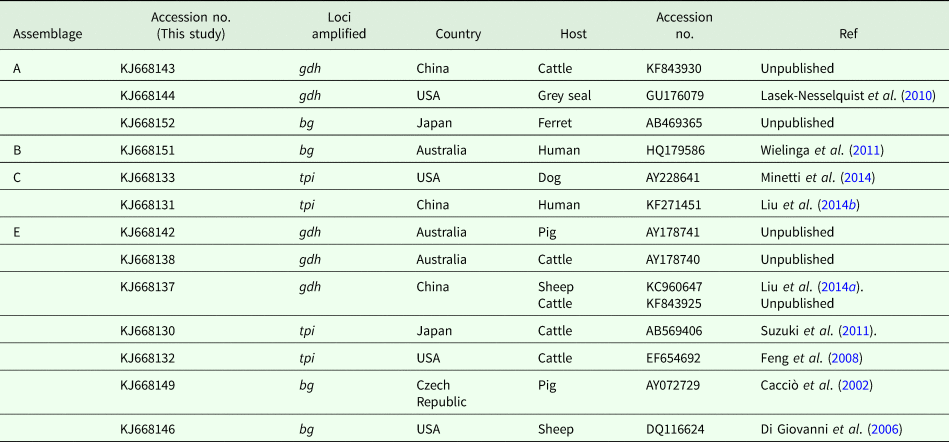
Sequence comparison with G. duodenalis sequences available in the GenBank database revealed that the isolates listed in Table 2 showed 100% homology with the sequences reported previously. One isolate was confirmed to be assemblage A by amplification of gdh (KJ668144) and bg (KJ668152), which have been reported in the grey seal (GU176079) from the United States and ferret (AB469365) from Japan, respectively. The other assemblage A isolate was identical to a cattle-derived isolate (KF843930) from China. The assemblage B isolate (KJ668151) showed 100% homology with human-derived isolates from Australia (HQ179586). Two assemblage C isolates were identical to a dog-derived isolate from the United States (AY228641) and a human-derived isolate from China (KF271451). Six (KJ668142) and four (KJ668138) assemblage E isolates have been found in pig- (AY178741) and cattle-derived (AY178740) isolates from Australia. On the basis of the tpi locus, two isolates were identical to cattle-derived isolates from Japan and the United States. On the basis of the bg loci, eight isolates have been described in pigs from the Czech Republic (AY072729) and one in sheep from the United States (DQ116624).
Table 2. Homology analyses of pig-derived isolates of G. duodenalis assemblages
Genetic diversity of assemblage E
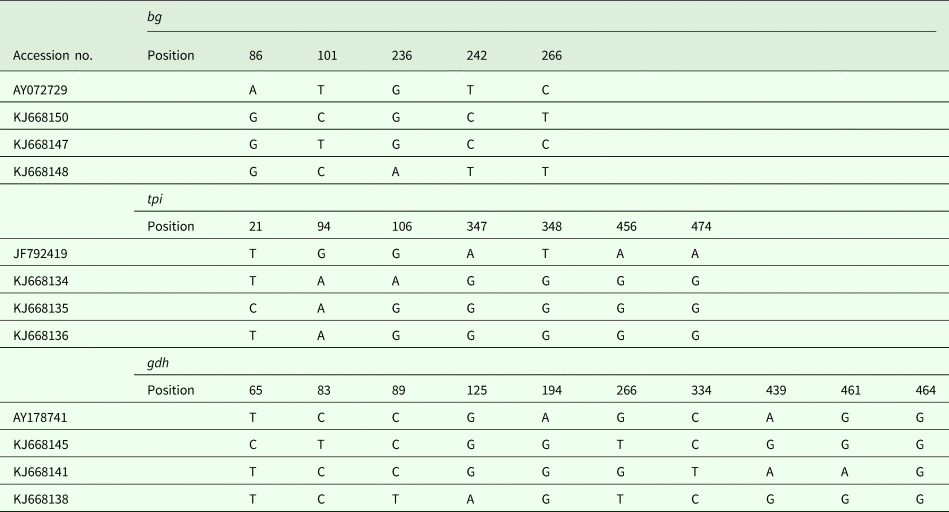
In addition to the above-mentioned sequences, other assemblage E isolates were analysed, and a multiple alignment was performed (Table 3). In this study, intra-genotypic diversity of G. duodenalis assemblage E was observed. On the basis of the bg loci, three subtypes were noted using AY072729 as the reference sequence (Table 3). On the basis of the tpi loci, the obtained isolates could also be divided into three subtypes, with five- to six-base variations at seven nucleotide sites. The subtyping analysis revealed that three isolates have not been described on the basis of the gdh loci, and a detailed description of the single nucleotide polymorphisms is provided in Table 3.
Table 3. G. duodenalis assemblage E subtypes on the basis of the gdh, bg and tpi loci
Discussion
Pigs are complicated hosts of many diseases caused by bacteria, viruses and parasites. Globally, Giardia assemblages A, B, C, D, E and F have been identified in pigs, with assemblage E being the predominant species (Wang et al., Reference Wang, Zhang, Wu, Li, Qi, Li, Wang, Wang, Zhang, Jian, Ning and Zhang2018). In the United Kingdom and Australia, pigs have been implicated as sources of G. duodenalis, and zoonotic genotypes occur frequently. However, only a few studies have investigated the infection and molecular epidemiology of Giardia in pigs in China (Wang et al., Reference Wang, Yuan, Yin, Hu, Song and Zhao2017, Reference Wang, Zhang, Wu, Li, Qi, Li, Wang, Wang, Zhang, Jian, Ning and Zhang2018; Shi et al., Reference Shi, Feng, Wu, Yan, Yang, Zhou and Zhao2018), which is a major pig-raising country. Recently, African swine fever was reported in pigs in different cities in China, causing wide public concern (Ge et al., Reference Ge, Li, Fan, Liu, Li, Wang, Ren, Bao, Liu, Wang, Liu, Zhang, Xu, Wu and Wang2018). Therefore, considering that pigs are the main economic animals in China and the importance of zoonotic Giardia, we performed multilocus genotyping of G. duodenalis in pigs. To the best of our knowledge, this is the first report of the occurrence and genetic characterization of giardiasis in pigs in Shanghai, China. Giardia spp. were identified in 26.88% (25/93) of the pigs by using nested PCR, with 28.13% (18/64) in farm 1 vs 24.14% (7/29) in farm 2. In this study, the infection rate was higher than that detected in pigs in Henan (8%), Sichuan (3.1%), Shanxi (1.7%) and Yunnan (1.55%) and lower than that reported in Canada (50.8%), Western Australia (31.1%) and the United Kingdom (57.1%) (Armson et al., Reference Armson, Yang, Thompson, Johnson, Reid and Ryan2009; Farzan et al., Reference Farzan, Parrington, Coklin, Cook, Pintar, Pollari, Friendship, Farber and Dixon2011; Minetti et al., Reference Minetti, Taweenan, Hogg, Featherstone, Randle, Latham and Wastling2014; Wang et al., Reference Wang, Yuan, Yin, Hu, Song and Zhao2017, Reference Wang, Zhang, Wu, Li, Qi, Li, Wang, Wang, Zhang, Jian, Ning and Zhang2018; Shi et al., Reference Shi, Feng, Wu, Yan, Yang, Zhou and Zhao2018). The results showed that the amplification rate of gdh was higher (16, 17.20%) than that of bg (14, 15.05%) and tpi (6, 6.45%). In fact, infection rates are complicated and related to many factors, such as the selected locus, detection methods, different seasons and farms and the structure of the specimens (Geurden et al., Reference Geurden, Thomas, Casaert, Vercruysse and Claerebout2008). In addition, a different management system, involving differences in animal stocking density, water supply or hygiene regimes, could increase the potential risk of infection by intestinal parasites like Giardia.
Globally, few studies on the genotyping of Giardia have been conducted, with the infection rate being 0–66.4%. The genotypes are mainly assemblages A, B, C, E and F with assemblage E being predominant, except for a study in Canada, in which assemblage B was the main genotype (Farzan et al., Reference Farzan, Parrington, Coklin, Cook, Pintar, Pollari, Friendship, Farber and Dixon2011; Wang et al., Reference Wang, Yuan, Yin, Hu, Song and Zhao2017). In this study, the obtained sequences were all aligned with reference sequences; the specimens were determined to be assemblages A, B, C or E, with assemblage E being more prevalent. This is consistent with the results of previous studies conducted in other countries (Langkjaer et al., Reference Langkjaer, Vigre, Enemark and Maddox-Hyttel2007; Armson et al., Reference Armson, Yang, Thompson, Johnson, Reid and Ryan2009), but different from those of a study performed in Ontario, Canada (Farzan et al., Reference Farzan, Parrington, Coklin, Cook, Pintar, Pollari, Friendship, Farber and Dixon2011). No assemblage swapping was found in the specimens (different assemblages at different loci in the same isolate). Interestingly, different assemblages were found at the two farms. Assemblages A, B, E and canine-specific assemblage C were identified at farm 1, and only assemblage E was found at farm 2. This may be because the two farms are in different parts of Shanghai, with farm 1 in the middle of the city and farm 2 in southwestern Shanghai. In addition, the difference in the number of samples from the farms could have influenced the results, as only 29 specimens were collected from farm 2.
All the assemblage A isolates have been described previously in different hosts. The sequence analysis showed that two isolates typed as assemblages B and C in the study were identical to the human-derived isolates on the basis of bg and tpi, respectively (Wielinga et al., Reference Wielinga, Ryan, Andrew Thompson and Monis2011; Liu et al., Reference Liu, Shen, Yin, Yuan, Jiang, Xu, Pan, Hu and Cao2014b). Similar to our study, assemblage C was found in a pig from the United Kingdom (Minetti et al., Reference Minetti, Taweenan, Hogg, Featherstone, Randle, Latham and Wastling2014). Unexpectedly, the assemblage C isolate (KJ668131) has been reported in diarrhoea patients in the investigated area (Liu et al., Reference Liu, Shen, Yin, Yuan, Jiang, Xu, Pan, Hu and Cao2014b). Thus, the occurrence of assemblages A, B and C isolates is a potential zoonotic risk for humans. In our study, the molecular epidemiological data showed that assemblage E was the most common in the pigs in the investigated areas, and similar results have been reported in many countries (Maddox-Hyttel et al., Reference Maddox-Hyttel, Langkjaer, Enemark and Vigre2006; Langkjaer et al., Reference Langkjaer, Vigre, Enemark and Maddox-Hyttel2007; Armson et al., Reference Armson, Yang, Thompson, Johnson, Reid and Ryan2009; Sprong et al., Reference Sprong, Cacciò and van der Giessen2009).
Sequence analysis of the bg locus of G. duodenalis revealed three subtypes in 12 assemblage E isolates, with two to four nucleotide variations; AY072729 was used as the reference sequence. Intra-genotype variations were also found on the basis of the tpi locus, and three novel isolates had only one or two nucleotide variations within seven sites. However, using JF792419 as the reference sequence, the single nucleotide polymorphisms increased to five or six sites, suggesting that the novel subtypes may represent endemic genetic characterizations in the investigated areas. On the basis of the gdh locus, 11 of 14 isolates have been reported in different animals, and three novel subtypes have been reported for the first time in pigs in Shanghai.
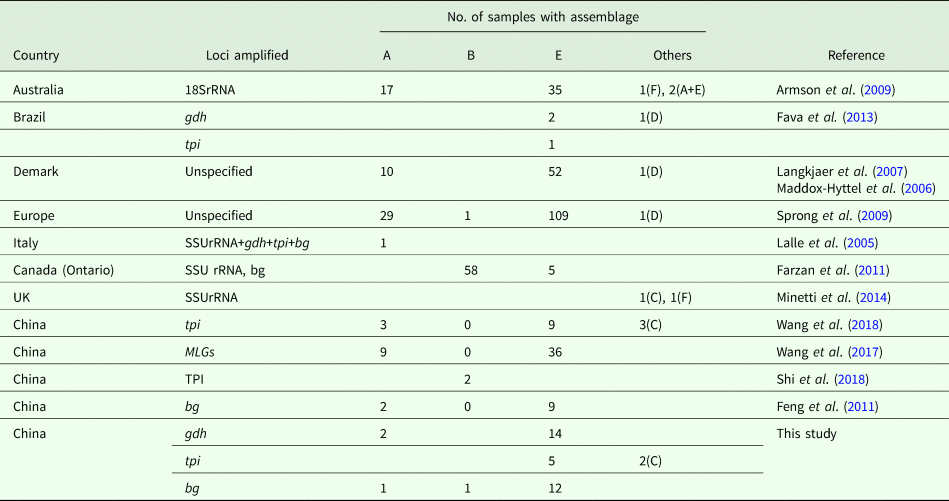
Currently, molecular analysis is being widely used to identify G. duodenalis in pigs (Table 4). In Australia, although assemblage E was the most common Giardia genotype, zoonotic assemblage A and feline-specific assemblage F were identified in pigs, with two mixed infections (A + E) (Armson et al., Reference Armson, Yang, Thompson, Johnson, Reid and Ryan2009). Similarly, assemblage F and a canine-specific assemblage C isolate were found in the United Kingdom (Minetti et al., Reference Minetti, Taweenan, Hogg, Featherstone, Randle, Latham and Wastling2014). In Denmark, assemblage E and zoonotic assemblage A have been identified (Langkjaer et al., Reference Langkjaer, Vigre, Enemark and Maddox-Hyttel2007; Petersen et al., Reference Petersen, Jianmin, Katakam, Mejer, Thamsborg, Dalsgaard, Olsen and Enemark2015). In Canada and Poland, assemblage E and zoonotic assemblage B have been identified (Farzan et al., Reference Farzan, Parrington, Coklin, Cook, Pintar, Pollari, Friendship, Farber and Dixon2011; Stojecki et al., Reference Stojecki, Sroka, Cencek and Dutkiewicz2015). In the study, two pig-derived isolates were also typed as assemblage C on the basis of tpi. The occurrence revealed that the host-adopted assemblages were no longer confined to specific hosts. Likewise, the canine-specific assemblage D was also found in pigs from Demark and Europe, whereas assemblage E was more prevalent. In China, Giardia assemblages A, B, C, E and F have been reported in Shanxi, Yunnan, Henan and Sichuan Provinces (Li et al., Reference Li, Wang, Wang and Zhang2017a, Reference Li, Deng, Wu, Huang, Song, Su, Hu, Fu, Zhong and Peng2017b; Wang et al., Reference Wang, Yuan, Yin, Hu, Song and Zhao2017, Reference Wang, Zhang, Wu, Li, Qi, Li, Wang, Wang, Zhang, Jian, Ning and Zhang2018; Shi et al., Reference Shi, Feng, Wu, Yan, Yang, Zhou and Zhao2018). In fact, contaminated water, food and fomites are considered to be the sources of infection for G. duodenalis (Feng and Xiao L, Reference Feng and Xiao2011). To our knowledge, the pig farms involved in our study were not industrialized pig farms and faeces may pollute the environment through water or other routes during the treatment process; similar findings have been reported by Hutchison et al. (Reference Hutchison, Walters, Avery, Synge and Moore2004). In addition, children or adults in close contact with farm animals are at increased risk of Giardia infection (Hoque et al., Reference Hoque, Hope, Kjellström, Scragg and Lay-Yee2002, Reference Hoque, Hope, Scragg and Kjellström2003).
Table 4. Assemblage distribution of G. duodenalis isolates from pigs worldwide
In 2005, three Egyptians were identified to be infected with G. duodenalis assemblage E, which was the first report in humans (Foronda et al., Reference Foronda, Bargues, Abreu-Acosta, Periago, Valero, Valladares and Mas-Coma2008). In view of the aforementioned infection factors, the pigs infected with G. duodenalis in the two farms may also be considered as a potential source of infectious cysts that affect humans. Because our study was a cross-sectional survey, and the farms were selected on the basis of the willingness of the owners, our data may not reflect the true population prevalence of G. duodenalis in farm animals in Shanghai. However, MLG based on three loci was used to detect the specimens, so our results demonstrate that G. duodenalis is a common intestinal parasite of pigs in the investigated areas. Previous studies have illustrated the difficulties of confirming the assemblage of an isolate by using MLG with different loci; however, the detection method provides clues for understanding assemblage exchange and potential zoonotic transmission (Lebbad et al., Reference Lebbad, Mattsson, Christensson, Ljungström, Backhans, Andersson and Svärd2010; Beck et al., Reference Beck, Sprong, Pozio and Cacciò2012; Scorza et al., Reference Scorza, Ballweber, Tangtrongsup, Panuska and Lappin2012). Recently, Cacciò et al. (Reference Cacciò, Beck, Lalle, Marinculic and Pozio2008) proposed an MLG model for easily defining G. duodenalis assemblage A isolates, and it can be used for appropriate nomenclature of sub-assemblages (subtypes) based on gdh, tpi and bg. Moreover, MLG has been used to identify G. duodenalis assemblages and sub-assemblages in humans, proving it can provide more information on the genetic diversity and transmission dynamics of G. duodenalis (Alyousefi et al., Reference Alyousefi, Mahdy, Xiao, Mahmud and Lim2013; Huey et al., Reference Huey, Mahdy, Al-Mekhlafi, Nasr, Lim, Mahmud and Surin2013).
In conclusion, to the best of our knowledge, this is the first report on pig giardiasis in Shanghai, China, and Giardia assemblage E was prevalent in the pigs in the investigated area. The occurrence of zoonotic assemblages A and B was also detected. In addition, the canine-specific assemblage C was found in two pigs. The finding that pig-derived assemblages B and C have 100% homology with human-derived G. duodenalis isolates at the bg and tpi loci implies the possibility of zoonotic transmission in the investigated areas. A better understanding of the distribution of Giardia in animals will help to establish more targeted measures for its prevention and control. Further studies with a larger number of samples and farms, and evaluation of the farmers in contact with pigs, are needed to investigate G. duodenalis infection and transmission dynamics and assess the zoonotic risk for humans.
Author ORCIDs
Jianping Cao, 0000-0002-1974-0047
Acknowledgements
We thank Mr Yuxin Xu for assistance in collecting the stool samples.
Financial support
This study was supported by the National Key R&D Program of China (Nos. 2016YFC1201900 to JC, 2017YFD0500400 to HL), the Chinese Special Program for Scientific Research of Public Health (No.201502021 to JC), the National Science and Technology Major Program of China (No. 2018ZX10102001-002-004) and Shanghai Municipal Commission of Health and Family Planning (No. 20164Y0225 to HL).
Conflict of interest
None.
Ethical standards
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention. The protocol was approved by the Laboratory Animal Welfare & Ethics Committee (LAWEC), National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (Permit Number: IPD 2012-6). No animals were harmed during the study.