Streptococcus agalactiae or group B streptococcus (GBS) is a leading cause of neonatal sepsis and meningitis. This is often a consequence of exposure to GBS colonizing the maternal vagina and or rectum during the prenatal period. GBS is also recognized as an important cause of bacteraemia in immunocompromised patients and in pregnant and non-pregnant women with underlying medical conditions [Reference Phares1] .
GBS strains are classified serologically by their capsular polysaccharide (CPS) antigens, which have long been recognized as major virulence factors. Ten distinct CPS serotypes (Ia, Ib, II–IX) have been described and their geographical distribution varies worldwide [Reference Ippolito2]. Serotypes Ia, II, III and V predominate in USA and Europe [Reference Phares1, Reference Ippolito2], while VI and VIII appear to be frequent in pregnant women from Japan [Reference Lachenauer3] and serotype IV in the United Arab Emirates [Reference Amin, Abdulrazzaq and Uduman4]. The most recently described serotype, designated serotype IX, was identified in Denmark [Reference Slotved5].
The major surface-localized protein antigens of GBS belong to a family of surface proteins. They are named the alpha-C protein, Rib, Alp2, Alp3, Alp4, and Epsilon and encoded by bca, rib, alp2, alp3, alp4 and epsilon genes, respectively. The genes are characterized by large internal tandem repeat sequences and the proteins are potential virulence factors [Reference Creti6, Reference Kong7]. The protein gene profile increases the potential of GBS subtyping [Reference Creti6].
No epidemiological data has been published on the distribution of serotypes or surface proteins of GBS isolates from pregnant and non-pregnant women in Egypt and this was the objective of the current study.
A cross-sectional study was conducted for a period of 3 months (March–May 2010). During this period, vaginal swab samples were collected from 364 consecutive women attending the Gynecological Clinic at Ismailia General Hospital (400 beds) and the University Hospital of Suez Canal University (415 beds). This included 264 pregnant women and 100 non-pregnant women. One vaginal swab was collected from each woman after obtaining informed consent. The swab was cultured and GBS isolates were identified as described previously [8]. GBS isolates were serotyped (types Ia, Ib, II–VIII) with the Essum GBS serotyping kit (Essum AB, Sweden) according to the manufacturer's instructions and a multiplex PCR [Reference Imperi9] with serotype-specific primers [Reference Kong10] was used to confirm serotyping results. The surface protein genes bca, rib, epsilon, alp 2/3 and alp4 [Reference Creti6] were also detected with a multiplex PCR method and the primers described by Kong et al. [Reference Kong7] were used to discriminate between the alp2 and alp3 genes. Categorical data were compared by χ 2 and Fisher's exact two-tailed tests using PASW Statistic 18 (SPSS Inc., USA). P values ⩽ 0·05 were considered statistically significant.
One-hundred (27·4%) of the 364 women screened yielded GBS. Serotype V (33%) was the most predominant followed by serotypes II (17%), III (15%), Ia (14%), VI (12%) and Ib (8%). One strain of serotype IV (1%) was identified but serotypes VII or VIII were not found.
There was no statistically significant difference in serotype distribution between pregnant and non-pregnant women but for the former group the serotypes in order of frequency were V (40%), III (19%) II (13%), Ib (10%), VI (10%), Ia (8%) and IV (2%). For non-pregnant women, the order of frequency was Ia (24%), II (24%), V (22%), VI (16%), III (8%) and Ib (5%).
Genes encoding Alp3 and Epsilon proteins were the most common with frequencies of 26% and 27%, respectively. Other protein genes identified were alpha-C (18%), rib (16%), and alp2 (10%). One strain carried both alpha-C and epsilon and two strains lacked identifiable genes; none possessed alp4. No statistical difference in complement of surface protein genes was evident between the two groups of women. The order of frequency for strains from pregnant women was alp3 (29%), epsilon (24%), alpha-C (21%), rib (13%), alp2 (11%), while for non-pregnant women the order of frequency was epsilon (32%), rib (22%), alp3 (22%), alpha-C (13%), and alp2 (8%).
The presence of a particular surface protein gene in relation to the serotype was also noted (Table 1). Of the 26 alp3-positive strains, 23 (88%) strains were of serotype V (P < 0·0001) while 11/27 (41%) epsilon-positive strains were of serotype VI (P < 0·0001). Similarly 80% of alp2 strains were of serotype III (P < 0·0001) and rib gene-positive strains were of serotypes II (P = 0·0055) and III (P = 0·0139). Conversely, half of serotype Ia strains carried epsilon (P = 0·0515) and all Ib strains carried the alpha-C gene (P < 0·0001). Other high associations were serotype II with either alpha-C (P = 0·0125) or rib (P = 0·0055), serotype III with alp2 (P < 0·0001) or rib (P = 0·0154) and serotype V with alp3 (P < 0·0001). All but two serotype VI strains harboured the epsilon gene (P < 0·0001).
Table 1. Distribution of surface protein genes in 100 GBS strains of observed serotypes
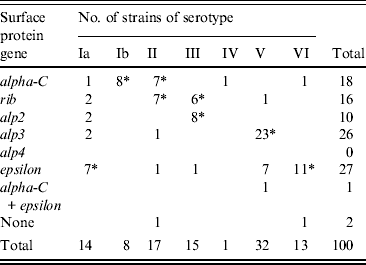
* P values ⩽ 0·05.
The finding of a high frequency of serotype V in this study is consistent with other reports from the USA, Canada, Zimbabwe, The Gambia, Mynamar, and Australia. [Reference Phares1, Reference Ippolito2]. However, this differs from studies in neighbouring Arab states, notably the United Arab Emirates [Reference Amin, Abdulrazzaq and Uduman4] and Saudi Arabia [Reference Al-Huseini11] where serotype V was the least prevalent serotype among colonizing GBS strains in females. Further, discrepant with the United Arab Emirates [Reference Amin, Abdulrazzaq and Uduman4] but in agreement with recent studies from Kuwait [Reference Al-Sweih12] and Saudi Arabia [Reference Al-Huseini11], serotype IV in the current study was one of the least common serotypes in both pregnant (3%) and non-pregnant (8%) women.
Evidently, GBS serotype prevalence varies with geographical location [Reference Ippolito2]. Although serotype distribution in our population was rather similar to that reported elsewhere, surprisingly serotype VI had a relatively high prevalence (12%) and was marginally less frequent than Ia, II and III which are usually reported as the most prevalent serotypes [Reference Ippolito2]. Serotype VI is generally considered to be uncommon except in Japan where serotypes VI and VIII predominate in GBS colonizing strains [Reference Lachenauer3]. Moreover, serotypes VI and VII are rarely or not reported in neighbouring Arabian or African countries or elsewhere [Reference Ippolito2, Reference Amin, Abdulrazzaq and Uduman4, Reference Al-Huseini11, Reference Al-Sweih12]; however, a recent study from Malaysia [Reference Dhanoa, Karunakaran and Puthucheary13] found serotype VI to be the dominant colonizing GBS strain (17%) in pregnant women. It may be that we are witnessing an increasing emergence of this serotype as documented previously with serotype V and more recently for serotype IV [Reference Ippolito2, Reference Hickman14]. This underlines the premise that GBS seroprevalence is not only dependant on geographical location but also on changes over time as several serotype prevalence shifts have been documented in the past decades [Reference Hickman14].
The surface proteins of GBS are likely to play an important role in the pathogenesis of GBS infection [Reference Creti6, Reference Kong7]. Creti et al. [Reference Creti6] noted a relationship between serotypes and surface protein genes and found an association of serotypes Ia, Ib and II with the alpha-C protein, of serotype III with Rib, and serotypes V and VIII with Alp3, but it was not absolute. In the present study, the alp3 gene predominated in serotype V strains (70%) while almost all serotype VI strains carried the epsilon gene. Other notable associations were alpha-C and serotype Ib and alp3 with serotype III. However, none of the associations was exclusive as different serotypes often carried the same protein gene and the same gene occurred in different serotypes. This feature may enhance the further differentiation of strains for epidemiological studies, but studies validating their stability and potential as strain markers are needed before the combination of markers can be applied to subdivide strain populations.
This is the first study to report the prevalence of GBS serotypes and surface proteins in Egypt; however, a notable limitation is the relatively small initial sample size and hence the numbers of strains in combinations of serotypes and surface protein profiles. Nevertheless, we have shown similar type distributions in our GBS strains as reports from other parts of the world with the notable exception of the relatively high frequency of serotype VI in Egyptian strains. Moreover, there appears to be potential for surface protein gene profiling for the further characterization of GBS strains of some serotypes to aid epidemiological studies and possibly inform the development of GBS vaccines suitable for use in a range of geographical areas.
DECLARATION OF INTEREST
None.


