The incidence of allergic disease has increased rapidly around the world in the past decades(Reference Eder, Ege and von Mutius1,Reference Ronmark, Bjerg and Perzanowski2) . This phenomenon might be attributed to the environmental factors elevating the incidence of infection during childhood and minimising the contact with microbes, which might affect immune system function and is correlated with the incidence of allergic disease(Reference Chang and Pan3,Reference Harris, Mills and White4) . Probiotics play a vital role in modulating systemic immune responses and contain crucial micronutrients in pregnant and lactating women, neonate and young children(Reference Cross5). A previous observational study illustrated that probiotic milk products were associated with a low risk of atopic eczema and rhinoconjunctivitis, whereas the effect was marginal and yet controversial(Reference Bertelsen, Brantsaeter and Magnus6).
Probiotic supplementation in pregnant women modulates the microbial milk composition, breast milk immunity and immunity-modulating molecules and might transfer into the neonate(Reference Rautava, Luoto and Salminen7). Besides, the biological effects of probiotics might be affected by strain type(Reference Viljanen, Savilahti and Haahtela8), and the commonly used probiotic strains include Lactobacillus, Bifidobacterium and Saccharomyces (Reference Ouwehand, Salminen and Isolauri9). A previous meta-analysis by Dugoua et al. indicated that Lactobacillus and Bifidobacterium were not associated with the risk of Caesarean section, birth weight and gestational age, while none of the randomised controlled trials (RCT) investigated the effect of Saccharomyces (Reference Dugoua, Machado and Zhu10). Doege et al. also concluded that lactobacilli supplementation significantly reduced the risk of atopic eczema, while a mixture of various bacterial strains did not yield a benefit on atopic eczema in children aged 2–7 years(Reference Doege, Grajecki and Zyriax11). However, several other indexes were not calculated. Subsequently, the present meta-analysis was conducted for large-scale analysis of available RCT to determine the efficiency of probiotic supplementation in pregnant women on immune-related outcomes and adverse events occurred in pregnancy and neonatal. Nevertheless, additional stratified analyses based on strains’ types were also conducted.
Experimental methods
Data sources, search strategy and selection criteria
This systematic review adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement. The ethics approval was not necessary for the present study(Reference Moher, Liberati and Tetzlaff12). The electronic databases including PubMed, Embase and the Cochrane Library were searched to select the RCT published from database inception to August 2018 that investigated the probiotics v. placebo in pregnant women, and the MeSH (Medical Subject Headings) terms included ‘probiotics’ OR ‘lactobacillus’ OR ‘bifidobacterium’ OR ‘bifidobacteria’ OR ‘saccharomyces’ AND ‘pregnancy’ AND ‘randomized controlled trials’. The details of search strategy in PubMed and Embase are summarised in online Supplementary material S1. The included studies were restricted to human cohorts and the English language. Furthermore, the reference lists from the retrieved RCT were searched manually for any new potential eligible studies.
Two authors independently performed a literature search and study selection processes according to PICOS (participants, intervention, control, outcomes and study design) criteria, and any disagreement was resolved by an additional author. The study was included if they met the following inclusion criteria: (1) Participants: pregnant women; (2) Intervention: probiotics including Lactobacillus, Bifidobacterium or Saccharomyces; (3) Control: placebo; (4) Outcomes: the study should report at least one of the following outcomes: atopic eczema, eczema, allergic disease, IgE-associated allergic disease, asthma, sensitisation, Caesarean section, gestational age, birth weight, death, necrotising enterocolitis (NEC), gastrointestinal symptoms, pre-eclampsia and sepsis; (5) Study design: the study should be designed as RCT.
Primary and secondary outcomes
The primary outcomes of the present study were immune-related outcomes, including atopic eczema, eczema, allergic disease, IgE-associated allergic disease, asthma and sensitisation, while the secondary outcomes were pregnancy and neonatal outcomes, including Caesarean section, gestational age, birth weight, death, NEC, gastrointestinal symptoms, preeclampsia and sepsis. The definitions of primary and secondary outcomes depend on the individual trial.
Data collection
Two authors independently collected the information from the retrieved studies according to a standardised form to ensure the homogeneity of the extracted results. The following data items were collected: the first author’s name, publication year, country, sample size, intervention, control and reported outcomes. Any disagreement in the assimilated data was resolved by an additional author until a consensus was reached.
Quality assessment
The authors independently evaluated the quality of eligible RCT using Jadad scale and Cochrane risk of bias tool(Reference Jadad, Moore and Carroll13,Reference Higgins and Green14) . The Jadad scale was based on random sequence generation, allocation concealment, blinding, completeness of follow-up and the use of intention-to-treat analysis, and the scale system ranged from 0 to 5(Reference Jadad, Moore and Carroll13). The Cochrane risk of bias tool was conducted based on random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias(Reference Higgins and Green14). The conflicts in the quality assessment were resolved by group discussion in reference to the original study.
Statistical analysis
Relative risks (RR) and weighted mean differences (WMD) with corresponding 95 % CI were employed to calculate the dichotomous and continuous data, respectively. All pooled results were evaluated using a random-effects model(Reference Ades, Lu and Higgins15,Reference DerSimonian and Laird16) . The heterogeneity among the included studies for the summary effect estimates was computed using I 2 and Q statistics, and P-value <0·10 indicated significant heterogeneity(Reference Deeks, Higgins and Altman17,Reference Higgins, Thompson and Deeks18) . The robustness of the pooled results and the impact of a single study from overall analysis were assessed using sensitivity analysis(Reference Tobias19). Subgroup analyses were conducted for outcomes reported from more than five studies depending on the strain type. Publication bias was calculated using Egger(Reference Egger, Davey Smith and Schneider20) and Begg(Reference Begg and Mazumdar21) tests. The P-values for pooled results were two-tailed, and P < 0·05 was regarded as statistically significant. All statistical analyses were performed using STATA software (version 10.0; Stata Corporation).
Quality of evidence
The quality of evidence for primary outcome was assessed using GRADE recommendations, which is based on the methodological quality and the reliability of results. Moreover, each outcome assessed by GRADE recommendations was divided into high, moderate, low and very low quality(Reference Guyatt, Oxman and Vist22). Each outcome available from the included studies should be considered based on the four criteria: (1) risk bias; (2) comparability; (3) heterogeneity and (4) statistical power.
Results
Literature search
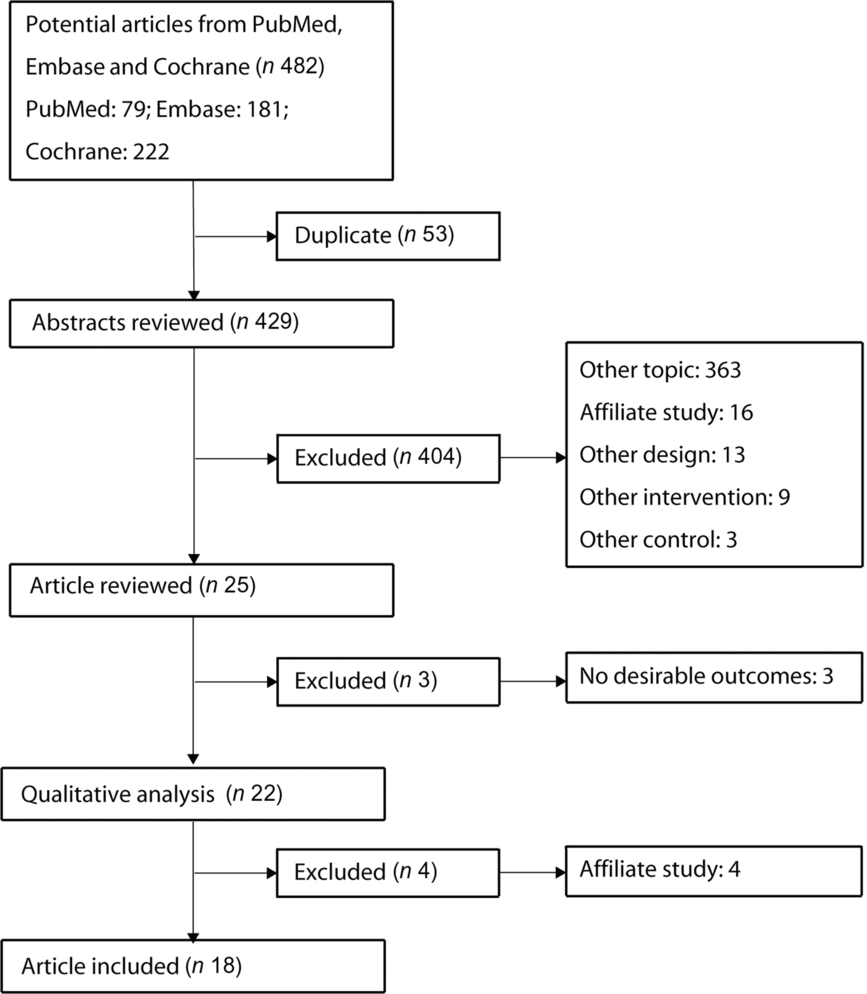
Fig. 1 shows the flow chart of the literature search and study selection processes. The electronic searches retrieved 482 papers based on search terms used in the present study, and fifty-three duplicate studies were excluded. The remaining 429 studies were subjected to abstract review, following which twenty-five studies fulfilled the inclusion criteria. Subsequently, four studies were excluded due to the same population, while another three studies did not report any interesting outcomes. Manual searches of the reference lists from eligible RCT did not yield any new eligible study. Finally, eighteen RCT with 4356 pregnant women were included in this quantitative analysis(Reference Kalliomäki, Salminen and Arvilommi23–Reference Wickens, Barthow and Murphy40).

Fig. 1. Flow chart of study selection process.
Study characteristics
A total of eighteen RCT, published between 2001 and 2017, were included in this meta-analysis, and the sample size of individual trial ranged from fifty-eight to 925 pregnant women. Five trials were conducted in Finland, one in Sweden, one in Germany, one in the Netherlands, one in the UK, one in Norway, two in New Zealand, one in Ireland, one in Italy, one in Israel, one in China, one in Korea and one in India. Seven of the included studies focused on pregnant women receiving Lactobacillus, while the remaining eleven trials administered a mixture of various bacterial strains in pregnant women. Nine RCT had a Jadad score of 4, eight RCT had a score of 3 and the remaining one RCT had a score of 2 (Table 1). The details regarding the Cochrane risk of bias are presented in online Supplementary material S2.
Table 1. Baseline characteristics of studies included in the systematic review and meta-analysis

CFU, colony-forming units; ATCC, American Type Culture Collection.
Atopic eczema and eczema
The risk of atopic eczema in pregnant women receiving probiotics was described in seven trials, and the evidence was downgraded to moderate quality owing to the potential information bias (online Supplementary material S3). The summary RR indicated that probiotics significantly reduced the risk of atopic eczema as compared with placebo (RR 0·68; 95 % CI 0·58, 0·81; P < 0·001; Fig. 2), and no evidence of heterogeneity was observed (I 2 = 0·0 %; P = 0·739). The pooled result was robust and not altered by sequential exclusion of the individual trial (online Supplementary material S4). Subgroup analyses suggested that these significant differences persisted whether Lactobacillus or a mixture of various bacterial strains was administered to pregnant women (Table 2).

Fig. 2. Effect of probiotics on the risk of atopic eczema and eczema.
Table 2. Subgroup analyses for investigated outcomes
(Relative risks (RR) or weighted mean differences (WMD) and 95 % confidence intervals)

LGG, Lactobacillus rhamnosus GG.
The risk of eczema in pregnant women receiving probiotics was observed in seven trials. Quality of evidence was downgraded to moderate quality for the balance of baseline characteristics between groups (online Supplementary material S3). Notably, probiotics were associated with a reduced risk of eczema as compared with placebo (RR 0·79; 95 % CI 0·68, 0·91; P = 0·002; Fig. 2), and insignificant heterogeneity was detected (I 2 = 27·6 %; P = 0·218). Sensitivity analysis indicated that the conclusion was not affected by sequential exclusion of individual trials (online Supplementary material S4). Subgroup analysis found that the significant difference primarily occurred in pregnant women receiving a mixture of various bacterial strains (RR 0·76; 95 % CI 0·66, 0·87; P < 0·001), while this significant effect was not observed in women receiving Lactobacillus.
Allergic disease and IgE-associated allergic disease
A total of five and three trials were available for allergic disease and IgE-associated allergic disease, respectively. Moreover, no significant differences were detected between probiotics and placebo with respect to the risk of allergic disease (RR 0·92; 95 % CI 0·79, 1·08; P = 0·303; Fig. 3) and IgE-associated allergic disease (RR 0·98; 95 % CI 0·55, 1·74; P = 0·946; Fig. 3). No evidence of heterogeneity was found for allergic disease (I 2 = 0·0 %; P = 0·972), and moderate heterogeneity was observed for the IgE-associated allergic disease (I 2 = 49·4 %; P = 0·138). Quality of evidence was downgraded twice to low quality for allergic disease owing to the balance of baseline characteristics between groups, and reported results are different from evidence regarding the outcome. Similarly, evidence was downgraded twice to low quality for IgE-associated allergic disease due to the balance of baseline characteristics between groups and moderate heterogeneity (online Supplementary material S3). Sensitivity analysis indicated that the conclusions were not altered after sequential exclusion of individual trials (online Supplementary material S4). Subgroup analysis for the allergic disease was conducted, and the results were consistent with the overall analysis whether the pregnant women received Lactobacillus or a mixture of various bacterial strains (Table 2).

Fig. 3. Effect of probiotics on the risk of allergic disease and IgE-associated allergic disease.
Asthma and sensitisation
The number of trials available for asthma and sensitisation was 3 and 7, respectively. Notably, pregnant women who received probiotics v. placebo did not yield any benefits on the risk of asthma (RR 0·87; 95 % CI 0·57, 1·32; P = 0·501; Fig. 4) and sensitisation (RR 0·88; 95 % CI 0·76, 1·02; P = 0·082; Fig. 4). No evidence of heterogeneity was detected for asthma (I 2 = 0·0 %; P = 0·500) and sensitisation (I 2 = 0·0 %; P = 0·660). The quality of evidence was downgraded to very low for asthma because of the balance of baseline characteristics between groups, reported results are different from evidence regarding the outcome, and studies included relatively few patients. Then evidence for sensitisation was downgraded to moderate quality owing to the balance of baseline characteristics between groups (online Supplementary material S3). Sensitivity analysis indicated that the risk of asthma was stable, while the risk of sensitisation was significantly reduced after excluding the trial conducted by Dotterud et al. (Reference Dotterud, Storro and Johnsen32) (online Supplementary material S4). Subgroup analysis indicated that Lactobacillus administration was associated with a low risk of sensitisation (RR 0·77; 95 % CI 0·60, 0·98; P = 0·032; Table 2).

Fig. 4. Effect of probiotics on the risk of asthma and sensitisation.
Caesarean section
The risk of Caesarean section in pregnant women receiving probiotics was available from fourteen trials, and the evidence was downgraded to moderate quality owing to potential information bias (online Supplementary material S3). The summary RR did not indicate any significant difference for the incidence of Caesarean section between probiotics and placebo (RR 0·90; 95 % CI 0·80, 1·02; P = 0·091; Fig. 5); also, no evidence of heterogeneity was observed among the included studies (I 2 = 0·0 %; P = 0·906). Sensitivity analysis indicated that probiotics significantly reduced the incidence of Caesarean section after excluding the trial conducted by Wickens et al. (Reference Wickens, Barthow and Murphy40) (online Supplementary material S4). Subgroup analysis indicated that the supplementation of a mixture of various bacterial strains was associated with a low incidence of Caesarean section (RR 0·85; 95 % CI 0·73, 0·98; P = 0·031), while Lactobacillus had no significant effect on Caesarean section (Table 2).

Fig. 5. Effect of probiotics on the incidence of Caesarean section.
Gestational age and birth weight
The gestational age of pregnant women receiving probiotics was available from nine trials, and the quality of evidence was downgraded to moderate quality owing to potential information bias (online Supplementary material S3). Notably, the gestational age was significantly longer in pregnant women receiving probiotics with insignificant heterogeneity (I 2 = 11·5 %; P = 0·339) among included studies (WMD 0·09; 95 % CI 0·04, 0·15; P = 0·001; Fig. 6). Sensitivity analysis indicated that the pooled result was robust and not altered by excluding any specific trial (online Supplementary material S4). A significant difference was observed in the gestational age mainly in women receiving Lactobacillus (Table 2).

Fig. 6. Effect of probiotics on gestational age.
The birth weight of pregnant women receiving probiotics was available from thirteen trials. However, no significant difference was observed between probiotics and placebo with respect to birth weight (WMD 0·01; 95 % CI −0·05 to 0·07; P = 0·851; Fig. 7), and significant heterogeneity (I 2 = 94·0 %; P < 0·001) was detected among included trials. The quality of evidence was downgraded to low owing to potential information bias and high heterogeneity (online Supplementary material S3). The conclusion was not affected by the exclusion of any specific trial (online Supplementary material S4). The results of the subgroup analysis were consistent with the overall analysis (Table 2).

Fig. 7. Effect of probiotics on birth weight.
Severe adverse events
The summary results for death, NEC, gastrointestinal symptoms, pre-eclampsia and sepsis are shown in Fig. 8. Notably, the pregnant women receiving probiotics showed a significantly reduced risk of death (RR 0·34; 95 % CI 0·13, 0·91; P = 0·031) and NEC (RR 0·38; 95 % CI 0·18, 0·81; P = 0·012). However, no significant differences were observed for the risk of gastrointestinal symptoms (RR 0·71; 95 % CI 0·35, 1·46; P = 0·350), pre-eclampsia (RR 1·49; 95 % CI 0·85, 2·63; P = 0·165) and sepsis (RR 0·73; 95 % CI 0·28, 1·93; P = 0·532). Moreover, no evidence of heterogeneity for death (I 2 = 0·0 %; P = 0·546), NEC (I 2 = 0·0 %; P = 0·765), gastrointestinal symptoms (I 2 = 0·0 %; P = 0·794) and pre-eclampsia (I 2 = 0·0 %; P = 0·830) was detected, while significant heterogeneity was noted for sepsis (I 2 = 64·8 %; P = 0·092).

Fig. 8. Effect of probiotics on severe adverse events. NEC, necrotising enterocolitis.
Publication bias
Publication bias for investigated outcomes was assessed by Egger and Begg tests and is presented in Table 3. No significant publication bias was observed for atopic eczema (P-value for Egger 0·546; P-value for Begg 1·000), eczema (P-value for Egger 0·777; P-value for Begg 1·000), allergic disease (P-value for Egger 0·139; P-value for Begg 0·462), sensitisation (P-value for Egger 0·698; P-value for Begg 0·548), Caesarean section (P-value for Egger 0·327; P-value for Begg 0·443), gestational age (P-value for Egger 0·658; P-value for Begg 0·466) and birth weight (P-value for Egger 0·120; P-value for Begg 0·855).
Table 3. Summary results for publication biases

Discussion
This comprehensive meta-analysis included 4356 pregnant women from eighteen RCT worldwide and found that probiotics yielded significant benefits for atopic eczema, eczema, gestational age, death and NEC. The results of sensitivity analyses indicated that probiotics might affect the incidence of sensitisation, Caesarean section and gestational age. The risk of eczema, sensitisation, Caesarean section and gestational age differ according to the type of strains.
The summary results for atopic eczema and eczema were significantly reduced in pregnant women. The included trials pointed out that probiotics administered to the pregnant women elevated the IgE concentration in the cord blood and increased the level of TGF-β2 in breast milk. These factors indicated that the early improved immunological effects play a role in the progression of atopy and atopic disease. The risk of atopy was affected by immunoprotective factors that interact with genetic predisposition and early sensitisation. Abrahamsson et al. (Reference Abrahamsson, Jakobsson and Bottcher25) demonstrated that eczema in women administered Lactobacillus did not benefit due to the similar prevalence of eczema between probiotics and placebo, thereby indicating that the effect of probiotics was pronounced in pregnant women with allergic disease(Reference Liu, Wang and Chuang41). Strikingly, the risk of allergic disease, IgE-associated allergic disease and asthma between probiotics and placebo was not statistically significant, which might be due to small number of trials included in this meta-analysis, and the summary results were determined by a single trial. Finally, the probiotic supplements in pregnant women might play a major role in the incidence of sensitisation and the improved immunological function.
Probiotics supplementation might play a vital role in the incidence of Caesarean section, and the reduced incidence of Caesarean section was primarily observed in women, who received a mixture of various bacterial strains. Gestational age in women received probiotics was significantly longer than placebo. No significant difference was detected between probiotics and placebo regarding birth weight. These results were correlated with the immunological function and environmental factors.
The summary results indicated that the risk of death and NEC was significantly reduced in pregnant women, who received probiotics, whereas the risk of gastrointestinal symptoms, pre-eclampsia and sepsis was not statistically significant. These results were obtained from two trials that specifically addressed low birth weight newborns. Samanta et al. concluded that a mixture of various bacterial strains reduces the incidence and death due to NEC and improves feed tolerance(Reference Samanta, Sarkar and Ghosh28). Benor et al. suggested that the probiotic supplementation in postpartum might decrease the risk of NEC, whereas the risk of sepsis and mortality rates are not statistically significant(Reference Benor, Marom and Ben Tov37). This phenomenon might be attributed to the direct transfer of probiotics from the maternal gut to the infantile gut(Reference Schanler, Fraley and Lau42), and probiotic supplementation could improve the immunological properties of breast milk(Reference Newburg and Walker43).
Nevertheless, the present study had several limitations. (1) The quality of various studies focused on allocation concealment, blinding and the use of intention-to-treat analysis could affect the reliability of pooled results. (2) The present study based on published studies and grey literature was not searched, which might overestimate the effect size; (3) Numerous factors could affect the pregnancy outcomes, whereas the characteristics of individuals were not available in most of the included studies; (4) The present analysis based on pooled data and individual data was not available.
In conclusion, probiotics for pregnant women provides additional benefits on atopic eczema, eczema, gestational age, death and NEC. However, the outcomes of allergic disease, IgE-associated allergic disease, asthma, sensitisation, Caesarean section, birth weight, gastrointestinal symptoms, pre-eclampsia and sepsis between probiotics and placebo were not statistically significant. The summary results of sensitisation, Caesarean section and gestational age were not stable and need further large-scale RCT to substantiate the findings. Furthermore, whether the treatment effects of probiotics in pregnant women differ according to the characteristics of the women should be explored in subsequent RCT.
Acknowledgements
This research is funded by Study on Drug resistance and drug resistance mechanism of Group B streptococcus colonized in the birth canal of pregnant women (no. 2019YJ0083).
K. L. carried out the studies, participated in collecting data and drafted the manuscript. J. Y. performed the statistical analysis and participated in its design. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114519003374