Age-related macular degeneration (AMD), the leading cause of legal blindness in people aged over 65 years in industrialised countries, is a progressive disorder primarily affecting the macula, the central region of the retina involved with central vision(Reference Jager, Mieler and Miller1). Early AMD is characterised clinically by yellowish deposits known as soft drusen accumulations and pigmentary abnormalities in the retinal pigment epithelium (RPE) and Bruch's membrane, whereas late-stage manifestations encompass atrophy of photoreceptors and the RPE underlying it, choroidal neovascularisation, subretinal haemorrhage, detachment of RPE and retinal scarring(Reference Muni, Altaweel and Tennant2). Currently, it has been reported that more than ten million people in the USA and approximately fifty million worldwide suffer from AMD(Reference Klein, Peto and Bird3). In the UK, almost 200 000 people aged 75 years or older were visually impaired due to AMD(Reference Evans, Fletcher and Wormald4). Owing to the sharp rise in the elderly population, the disease has brought a huge burden for the health care system and had a profound impact on the quality of life and independence of older individuals. It is estimated that by the year 2020 the number of patients with late AMD will be increased by more than 50 % to almost three million in the USA alone(Reference Friedman, O'Colmain and Muñoz5).
To date, the exact pathogenic mechanism of AMD has not been completely identified, and AMD is regarded as a complicated disease caused by the actions and interactions of multiple environmental risk factors and genetic factors(Reference Khan, Shahid and Thurlby6–Reference Schmidt, Haines and Postel8). Although new therapies with anti-vascular endothelial growth factor agents have been shown to slow progressive visual loss effectively in certain types of neovascular AMD, these agents are costly and there are no treatments for geographic atrophy at present(Reference Muni, Altaweel and Tennant2, Reference Subramanian, Ness and Abedi9). Thus, identifying modifiable risk factors that could prevent or delay the onset of early AMD is considered to be far more preferable and of critical importance. Apart from being related to ageing, AMD has been associated with several modifiable risk factors, including smoking, sunlight exposure and diet(Reference Thornton, Edwards and Mitchell10–Reference Cai, Nelson and Wu14).
Dietary antioxidants, particularly lutein and zeaxanthin, are hypothesised to have the capacity to modulate defence and repair systems that operate in response to oxidative damage and inflammation on the basis of the notion that the retina is highly susceptible to oxidative stress because of its high concentration of oxygen and PUFA, in combination with an intense exposure to light, which have been implicated in the development of AMD(Reference Cai, Nelson and Wu14–Reference Margrain, Boulton and Marshall16). As the major components of macular pigment, lutein and its structural isomer, zeaxanthin, are thought to have the beneficial effects on preventing the onset and progression of AMD(Reference Krishnadev, Meleth and Chew17). Laboratory data suggested an important role for these two carotenoids in protecting the neural retina from photo-oxidative damage and the development of common visually disabling disorders by absorbing blue light and by quenching reactive oxygen species through powerful antioxidant activity(Reference Haegele, Gillette and O'Neill18–Reference Junghans, Sies and Stahl20). Currently, a number of epidemiological studies have evaluated the relationship between dietary intake of these xanthophyll carotenoids and the risk of AMD(Reference Flood, Smith and Wang21–Reference Tan, Wang and Flood24). Although some studies have suggested a possible protective effect with the consumption of lutein and zeaxanthin in diet, others failed to show this benefit.
Given the public health importance of clarifying the potential role of lutein and zeaxanthin in the prevention of AMD, we conducted a systematic review of the evidence to evaluate the relationship between dietary intake of lutein and zeaxanthin and the risk of AMD. We also investigated whether this association was differed by subtype of AMD.
Methods
Selection of studies
The electronic databases MEDLINE, EMBASE, ISI Web of Science, CINAHL and the Cochrane Central Register of Controlled Trials in the Cochrane Library were searched for publications through April 2010 using the keywords lutein, zeaxanthin, xanthophyll or carotenoid in conjunction with each of the following words: AMD, age-related maculopathy, neovascular AMD, exudative AMD, choroidal neovascularisation and geographic atrophy. Language restrictions were not imposed. We also performed a manual search of references cited by the published original studies and relevant review articles on the topic for additional studies, and contacted authors and experts in the field to identify any ongoing studies.
All retrieved articles were examined by performing an initial screen of identified abstracts and titles. Any studies that did not address the association between lutein and zeaxanthin intake and AMD were excluded, and the full texts of the remaining articles were further checked for their suitability for the present meta-analysis. The full-text articles of all references selected after application of these criteria were reviewed by using the same criteria. The present systematic review and meta-analysis was limited to cohort studies. Randomised clinical trials would have been considered, but none were found. For inclusion, a study had to meet the following criteria: (1) the primary outcome was clearly defined as AMD; (2) dietary intake of lutein and zeaxanthin was quantified; (3) relative risks (RR) or relative odds and their CI (or sufficient data to calculate these) were reported; (4) potential confounders were controlled for by matching or multivariable analysis in the studies. If a series of articles was published from the same study, the report with the most updated data was selected for analysis, although previous articles could be reviewed to supplement missing data where applicable; in the case of duplicate publication, only one publication was included. All literature search and article review were conducted independently in a standardised manner by two investigators (L. M. and Y.-Q. W.). Wherever discrepancies between investigators occurred for inclusion or exclusion, a third investigator (X.-M. L.) was involved to adjudicate disagreements or uncertainties.
Data abstraction and study quality assessment
All data were independently abstracted in duplicate by two investigators (L. M. and Y.-Q. W.) using a standardised protocol. All disagreements between investigators were resolved by referencing the original publication and by discussion with a third investigator (X.-M. L.). We also contacted the primary authors to request additional information. Study characteristics recorded were as follows: first author's name, year of publication, country of origin, study design, characteristics of the study population (sample size, sampling methods, source of population and distribution of age, sex, ethnicity and BMI), diagnosis method of AMD, classification and grading systems for AMD, dietary assessment tool, follow-up duration, the RR or relative odds overall and in each subgroup and the corresponding CI or standard errors, and the confounding factors matched or adjusted in the studies. If a study provided several risk estimates, the most completely adjusted estimate was extracted.
The outcomes of interest were early AMD and late AMD. The early AMD was defined by the presence of drusen, pigmentary abnormalities in RPE or both, whereas the late AMD, including neovascular AMD and geographic atrophy, was defined by the presence of choroidal neovascularisation, detachment of RPE or geographic atrophy. The AMD cases were ascertained in the studies retrieved using fundus photograph or medical record review of visual acuity, on the basis of the criteria from the International Classification and Grading System (ICGS), the Wisconsin Age-related Maculopathy Grading System (WARMGS) or the modified Age-Related Eye Disease Study (AREDS).
Information on key indicators of study quality was also extracted, and assessment of methodological quality of each study was carried out by two of the investigators (L. M. and Y.-Q. W.) independently, according to the Newcastle–Ottawa Scale(Reference Wells, Shea and O'Connell25). The Newcastle–Ottawa Scale judges the quality of studies based on three aspects of the study: selection of study groups (four criteria), comparability of study groups (one criterion) and assessment of the outcome (three criteria). Studies that fulfilled five or more of the Newcastle–Ottawa Scale criteria were considered to be categorised as good quality. Studies that met four or fewer of this criteria were considered fair quality or poor quality. Discrepancies regarding quality parameters were also decided by discussion and consensus.
Statistical analyses
For all studies, RR or relative odds and their 95 % CI were extracted or derived from data reported in articles. Because the absolute risk of AMD was low, relative odds were considered an approximation of RR. Pooled RR estimates were calculated using both fixed effects and DerSimonian and Laird random effects models, weighting individual study results by the inverse of their variances. Forest plots were used to visually assess the RR estimates and corresponding 95 % CI across studies. Heterogeneity across studies was assessed by conducting Q tests (significance level of P < 0·10) and quantifying the degree of heterogeneity by estimating the I 2 statistic(Reference Higgins, Thompson and Deeks26). I 2 values of less than or equal to 25, 50 and ≥ 75 % represent low, moderate and high heterogeneity, respectively. In case of significant heterogeneity, the sources of heterogeneity were explored and sensitivity analyses were performed. Variables included in the subgroup analyses were population source (population based v. volunteer based), country of origin (USA v. not USA), mean age of participants ( ≤ 65 v. >65 years), method of diagnosis (fundus photography v. visual acuity criteria) and classification criteria of AMD (ICGS v. WARMGS v. AREDS modified). Sensitivity analyses were conducted to examine the contribution of each individual study by iteratively eliminating each study from the meta-analysis and comparing the point estimates including and excluding the study. To assess the potential of publication bias, we performed both the Egger test and Begg test and examined relative symmetry of individual study estimates around the overall estimate using a funnel plot in which log RR were plotted against their corresponding standard errors(Reference Egger, Davey Smith and Schneider27, Reference Sterne and Egger28). Additionally, we initially intended to conduct the dose–response meta-analysis to evaluate the relationship between dietary intake of lutein and zeaxanthin and AMD risk. Nevertheless, most studies had to be excluded for no CI, no distribution of cases and control subjects by exposure level or insufficient dose data on each category of lutein and zeaxanthin intake. Thus, we were unable to evaluate their associations in the dose–response meta-analysis. All statistical analyses were conducted by using RevMan version 5.0 (Cochrane Collaboration; Oxford, UK) and Stata version 8.2 (Stata Corporation, College Station, TX, USA). All P values were two-sided, with statistical significance set at a level of 0·05.
Results
Literature search
The search strategy yielded a total of 3465 citations. After excluding duplicates and screening the titles or abstracts, full-text versions of the remaining seventy-three articles were then retrieved for detailed evaluation. Of these seventy-three articles, five articles (six studies) were included in the systematic review and meta-analysis(Reference van Leeuwen, Boekhoorn and Vingerling23, Reference Tan, Wang and Flood24, Reference Van den Langenberg, Mares and Klein29–Reference Cho, Hankinson and Rosner31) (Fig. 1).

Fig. 1 Flow diagram of study selection process. AMD, age-related macular degeneration.
Study characteristics
The characteristics of these studies are presented in Table 1. Of the six longitudinal cohort studies, four were conducted in the USA(Reference Van den Langenberg, Mares and Klein29–Reference Cho, Hankinson and Rosner31), one in Australia(Reference Tan, Wang and Flood24) and one in the Netherlands(Reference van Leeuwen, Boekhoorn and Vingerling23). The number of subjects ranged from 1709 to 71 494, comprising a total of 2477 incident AMD cases and 0·12 million participants. The study population in three studies included both men and women(Reference van Leeuwen, Boekhoorn and Vingerling23, Reference Tan, Wang and Flood24, Reference Van den Langenberg, Mares and Klein29), two consisted entirely of women(Reference Moeller, Parekh and Tinker30, Reference Cho, Hankinson and Rosner31) and one consisted of only men(Reference Cho, Hankinson and Rosner31). The duration of follow-up ranged from 5 to 18 years. Three were population-based studies(Reference van Leeuwen, Boekhoorn and Vingerling23, Reference Tan, Wang and Flood24, Reference Van den Langenberg, Mares and Klein29), whereas three studies consisted of volunteers(Reference Moeller, Parekh and Tinker30, Reference Cho, Hankinson and Rosner31). Most of the studies had follow-up rates of 76·6 % or more, with the exception of the studies by Cho et al. (Reference Cho, Hankinson and Rosner31), which did not report them. The association between dietary intake of lutein and zeaxanthin and the risk of early AMD was the primary outcome of interest for all studies, whereas four studies also reported late AMD(Reference Tan, Wang and Flood24, Reference Moeller, Parekh and Tinker30, Reference Cho, Hankinson and Rosner31). Diagnosis of AMD was based on fundus photography in four of the studies(Reference van Leeuwen, Boekhoorn and Vingerling23, Reference Tan, Wang and Flood24, Reference Van den Langenberg, Mares and Klein29, Reference Moeller, Parekh and Tinker30) and on review of medical records in two(Reference Cho, Hankinson and Rosner31). Three studies used the ICGS criteria to establish AMD(Reference van Leeuwen, Boekhoorn and Vingerling23, Reference Cho, Hankinson and Rosner31), whereas the WARMGS criteria and the AREDS modified criteria were applied in two studies(Reference Tan, Wang and Flood24, Reference Van den Langenberg, Mares and Klein29) and one study(Reference Moeller, Parekh and Tinker30), respectively. Dietary intake information was collected using a FFQ in all the studies. All of the studies adjusted for age and smoking, fewer adjusted for alcohol intake (four studies)(Reference van Leeuwen, Boekhoorn and Vingerling23, Reference Van den Langenberg, Mares and Klein29, Reference Cho, Hankinson and Rosner31), sex (three studies)(Reference van Leeuwen, Boekhoorn and Vingerling23, Reference Tan, Wang and Flood24, Reference Van den Langenberg, Mares and Klein29), energy intake (three studies)(Reference Van den Langenberg, Mares and Klein29, Reference Cho, Hankinson and Rosner31), BMI (three studies)(Reference van Leeuwen, Boekhoorn and Vingerling23, Reference Cho, Hankinson and Rosner31), history of AMD (two studies)(Reference Tan, Wang and Flood24, Reference Moeller, Parekh and Tinker30), CVD (two studies)(Reference van Leeuwen, Boekhoorn and Vingerling23, Reference Moeller, Parekh and Tinker30) or postmenopausal hormone use (two studies)(Reference Moeller, Parekh and Tinker30, Reference Cho, Hankinson and Rosner31).
Table 1 Characteristics of the cohort studies of dietary intake of lutein and zeaxanthin and risk of age-related macular degeneration (AMD)

WARMGS, Wisconsin Age-related Maculopathy Grading System; NR, not reported; ICGS, International Classification and Grading System; SBP, systolic blood pressure; AREDS, Age-Related Eye Disease Study; BCVA, best corrected visual acuity.
* Study quality was judged based on Newcastle–Ottawa Scale(Reference Wells, Shea and O'Connell25).
† The Health Professionals' Follow-Up Study conducted by Cho et al. (Reference Cho, Hankinson and Rosner31).
‡ The Nurses' Health Study conducted by Cho et al. (Reference Cho, Hankinson and Rosner31).
Lutein and zeaxanthin intake and early age-related macular degeneration
All studies(Reference van Leeuwen, Boekhoorn and Vingerling23, Reference Tan, Wang and Flood24, Reference Van den Langenberg, Mares and Klein29–Reference Cho, Hankinson and Rosner31) reporting on the relationship between dietary lutein and zeaxanthin intake and early AMD risk were considered for inclusion in meta-analysis. Among the selected studies, all but one(Reference Cho, Hankinson and Rosner31) found an association between intake of lutein and zeaxanthin and a reduced risk of early AMD, and only one study was statistically significant(Reference Tan, Wang and Flood24). There was significant heterogeneity across the studies (I 2 = 52 %; P for heterogeneity 0·06), and the random effects pooled RR for early AMD comparing the highest with the lowest category of lutein and zeaxanthin intake was 0·96 (95 % CI 0·78, 1·17; Fig. 2).

Fig. 2 Forest plot of relative risk (RR) and 95 % CI for highest v. lowest category of dietary lutein and zeaxanthin intake and early age-related macular degeneration risk. * The Health Professionals' Follow-Up Study conducted by Cho et al. (Reference Cho, Hankinson and Rosner31). † The Nurses' Health Study conducted by Cho et al. (Reference Cho, Hankinson and Rosner31).
To explore the study heterogeneity, we performed stratified analyses across a number of participant characteristics (Table 2). These factors did not significantly alter the shape of association between lutein and zeaxanthin and early AMD risk, although population source and definition of AMD seemed to be slightly related with the results. Population-based studies tended to report a slightly stronger association of lutein and zeaxanthin intake with early AMD incidence, whereas the pooled estimate of volunteer-based studies was larger in magnitude (pooled RR 1·07; 95 % CI 0·80, 1·45). Likewise, inconsistencies in diagnosis method and separate criteria of AMD might somewhat affect the findings for lutein and zeaxanthin intake and early AMD risk. In sensitivity analyses, exclusion of any single study from the analyses did not markedly influence the overall results.
Table 2 Stratified analysis of the association between dietary intake of lutein and zeaxanthin and age-related macular degeneration (AMD)
(Number of studies, pooled relative risk (RR) and 95 % confidence intervals)
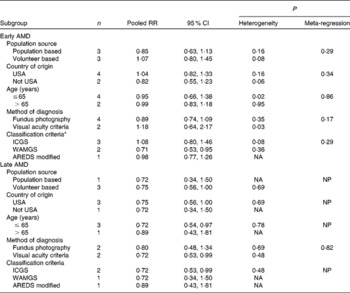
ICGS, International Classification and Grading System; WARMGS, Wisconsin Age-related Maculopathy Grading System; AREDS, Age-Related Eye Disease Study; NA, not applicable because only one study; NP, meta-regression was not possible.
* Meta-regression was performed for the first two categories.
Visual inspection of the funnel plot for the studies evaluating lutein and zeaxanthin intake and its association with early AMD revealed symmetry (Fig. 3(a)). The Egger test (P = 0·76) and Begg test (P = 0·85) suggested no significant asymmetry of the funnel plot, indicating the absence of substantial publication bias.

Fig. 3 Funnel plots with 95 % CI for (a) early age-related macular degeneration (AMD) risk and (b) late AMD risk. RR, relative risk; se, standard error.
Lutein and zeaxanthin intake and late age-related macular degeneration
Four studies(Reference Tan, Wang and Flood24, Reference Moeller, Parekh and Tinker30, Reference Cho, Hankinson and Rosner31) were included in the analysis of the relationship between dietary lutein and zeaxanthin intake and late AMD risk. The point estimates of the RR were consistently less than 1 in all studies, and none of studies reported statistically significant associations. A forest plot showing results of the comparison between the highest and lowest categories of lutein and zeaxanthin intake is shown in Fig. 4. There was little evidence of heterogeneity among studies, with an I 2 of 0 % (P value for heterogeneity = 0·86). Overall, compared with individuals in the lowest intake of lutein and zeaxanthin, those in the highest intake had a significantly reduced risk of developing late AMD, with a fixed effects pooled RR of 0·74 (95 % CI 0·57, 0·97). In a subgroup analysis of the three studies(Reference Tan, Wang and Flood24, Reference Cho, Hankinson and Rosner31) that also reported on the RR of neovascular AMD, we estimated a reduction of 32 % in neovascular AMD risk associated with high lutein and zeaxanthin intake (pooled RR 0·68; 95 % CI 0·51, 0·92), and there was no statistically significant heterogeneity among these studies (I 2 = 0 %; P for heterogeneity = 0·38). In addition, stratified analyses showed consistency in the direction of effect when studies were grouped by the characteristics of participants. The sensitivity analyses showed minimal influence on the overall pooled estimate and heterogeneity for any single study.

Fig. 4 Forest plot of relative risk (RR) and 95 % CI for highest v. lowest category of dietary lutein and zeaxanthin intake and late age-related macular degeneration risk. * The Health Professionals' Follow-Up Study conducted by Cho et al. (Reference Cho, Hankinson and Rosner31). † The Nurses' Health Study conducted by Cho et al. (Reference Cho, Hankinson and Rosner31).
Fig. 3(b) displays a funnel plot for the visual assessment of publication bias. The funnel plot was symmetrical, and neither the Egger test (P = 0·97) nor the Begg test (P = 1·00) suggested publication bias.
Discussion
The present meta-analysis involved data on evaluating the effects of lutein and zeaxanthin on AMD prevention published in six cohort studies. Results from the present study suggested that high intake of lutein and zeaxanthin was significantly associated with a reduction in risk of late AMD. No significant relationship was found for dietary intake of these carotenoids and early AMD. We conducted some stratified analyses across participant characteristics, with essentially no change in the findings of the present study.
Several biological mechanisms have been proposed for the potential protective effect of lutein and zeaxanthin on preventing the onset of AMD. As the major components of macular pigment, lutein and zeaxanthin are uniquely concentrated at the macula, indicating that these carotenoids may exert their effects on protecting the macula from age-related loss of visual function and macular disease(Reference Khachik, Bernstein and Garland32). Both lutein and zeaxanthin, possessing a series of unconjugated double bonds, are believed to be very effective antioxidants(Reference Ma and Lin33). They have been shown to help quench singlet oxygen, scavenge reactive free radicals and inhibit lipid peroxidation of membrane phospholipids, and thus may prevent or delay the development of AMD(Reference Rapp, Maple and Choi34, Reference Stahl and Sies35). In addition, the spectrum of lutein and zeaxanthin includes a broad absorption band, with a peak at approximately 450 nm; therefore, these carotenoids have an important role in absorbing and attenuating the damaging blue light before it reaches the photoreceptors(Reference Krinsky, Landrum and Bone36). Results from animal studies also showed that macular xanthophylls could prevent or retard some of the destructive processes that ultimately led to AMD(Reference Thomson, Toyoda and Langner19, Reference Thomson, Toyoda and Delori37). Furthermore, vascular defect and inflammation may be partly responsible for the pathogenesis of AMD, particularly of neovascular AMD(Reference Donoso, Kim and Frost38, Reference Seddon, Gensler and Milton39). There was evidence that lutein and zeaxanthin had the capacity to reduce thickening of the arteries and maintain the normal vascular function of retina and choroid(Reference Izumi-Nagai, Nagai and Ohgami15, Reference Kowluru, Menon and Gierhart40).
As there were no randomised clinical trials regarding the effect of dietary lutein and zeaxanthin on AMD prevention at present, the greatest interpretative weight associated with results could be obtained from the cohort design. Several other types of observational studies had previously examined the association between the intake of these carotenoids and the risk of AMD(Reference Seddon, Anani and Sperduto41–Reference Bone, Landrum and Dixon45). In contrast with the results from the cohort studies, almost all case–control and cross-sectional studies reported statistically significant associations between lutein and zeaxanthin intake and AMD risk, indicating that the combination of different study designs in the present meta-analysis would bias the present results towards the positive outcomes. Furthermore, case–control and cross-sectional studies might be inherently biased by various factors. It is generally considered that cohort studies provide stronger evidence for evaluating a relationship than other observational studies, because the cohort studies could largely reduce the likelihood of selection bias and reverse causation. Therefore, only cohort studies were included in the present systematic review and meta-analysis.
Results from the present analysis showed that lutein and zeaxanthin intake was not significantly associated with a decrease in the risk of developing early AMD. Among the six available studies, only one(Reference Tan, Wang and Flood24) found a significant association between dietary lutein and zeaxanthin and the incidence of early AMD, whereas the others found no associations that were consistent with the present finding. Given the evidence that the older participants, who had a poorer survival than the younger participants did, were more likely to be AMD cases, the potential for survival bias should be considered. Such bias would increase with the average age of the sample and tend to under-report the strength of the association(Reference Zhang, Shu and Chow46). However, findings from previous studies were not consistent. Moeller et al. (Reference Moeller, Parekh and Tinker30) found that associations of dietary lutein with early AMD were strengthened in younger, compared with older subjects. The present results showed no significant differences in RR stratified by age of participants, suggesting that the age of samples was not likely to be a strong contributor to heterogeneity. It is worth noting that meta-analyses of published data do not permit an adequate evaluation of age interactions. Further evaluations using person-specific data pooling are needed to better evaluate this possibility. We also repeated some analyses stratified by other participant characteristics, with essentially no change in these findings. Moreover, we conducted a sensitivity analysis and also found that the shape of association remained unchanged. The consistencies between these findings indicated that the associations were robust and combining studies with different participant characteristics did not bias the present results. In contrast with the findings for early AMD, we found a statistically significant relationship between lutein and zeaxanthin intake and the risk of late AMD. These inconsistent relationships from different stages of AMD might be partly explained by differences in the degree of macular pigment damage and ascertainment of AMD. Previous studies had shown that no significant differences in macular pigment optical density were found between eyes with and without early AMD or between the various stages of early AMD(Reference Berendschot, Willemse-Assink and Bastiaanse47, Reference Jahn, Wustemeyer and Brinkmann48). Results from studies that compared the macular pigment optical density of eyes with and without late AMD were not consistent; however, most indicated declines in the optical density of macular pigment among subjects with late AMD(Reference Beatty, Murray and Henson49, Reference Wüstemeyer, Jahn and Nestler50). Similar results had also been found in the peripheral retina of autopsy specimens from donor eyes with AMD(Reference Bone, Landrum and Mayne51), indicating that the loss of macular pigment might reflect the accumulation of damage accrued over an entire lifespan. As an indicator of the macular pigment status of the retina, dietary intake of lutein and zeaxanthin is more likely to be related with late AMD, rather than early AMD(Reference Bone, Landrum and Dixon45). On the other hand, an alternative explanation for the discrepancies between the relationships of early AMD and late AMD could be due to the variety of diagnosis methods and classification criteria of AMD in different studies. Most of the studies support the notion that fundus photography is highly more accurate for detection of AMD, whereas the definition of AMD based on visual acuity criteria is subject to inter-individual variability(Reference Cho, Hankinson and Rosner31, Reference Seddon, Anani and Sperduto41). As AMD often progresses without any symptoms, many patients with AMD at early stage are not easy to distinguish only by routine ophthalmic examination. Thus, the inconsistencies of AMD assessment methods in the original studies may cause random misclassifications, which would be more likely to underestimate the true association, in particular, of early AMD. In addition, the present finding was in agreement with the subgroup analyses by subtype of late AMD. Results from the present study showed that dietary intake of lutein and zeaxanthin had a significant inverse association with neovascular AMD risk and this pooled RR was even stronger, suggesting these carotenoids might serve as a function in modulating inflammatory response and promoting flow of blood to and from the macular region(Reference Izumi-Nagai, Nagai and Ohgami15, Reference Choi, Kim and Hong52).
The present meta-analysis has several strengths. The present review included a large number of people from different studies. Most of the studies had a large sample size and a long duration of follow-up. Almost all the studies had adjusted for age and smoking in the analyses. These relatively high-quality studies combined should give more reliable assessment of the relation between lutein and zeaxanthin and risk of AMD. Additionally, the association was essentially consistent among subgroups stratified by characteristics of participants, indicating that the conclusions of the present study were not dependent on arbitrary decisions in the present meta-analysis. Finally, the present results were unlikely to result from publication bias, as indicated by the funnel plots and other analyses.
The present study also has several limitations that merit consideration. First, the present study was based on observational studies and might have the problems of potential bias and confounding effects associated with such studies. Second, inconsistencies in methods of diagnosing AMD might have contributed to the inconsistent results among studies. Although case diagnosis of AMD in all the studies had been validated on the basis of the generally accepted standard methods, the accuracy of visual acuity criteria for detection of AMD was limited compared with fundus photography, because early signs of AMD were asymptomatic in most people(Reference Freeman, El-Bradey and Plummer53). In the present analysis, the RR of the both forms of AMD associated with lutein and zeaxanthin did not materially change after exclusion of studies that did not use fundus photography for diagnosis. However, such bias could not be ruled out completely, which might have led to underestimation or overestimation of the association. Furthermore, the validated classification and grading systems for AMD were inconsistently applied between studies because of the different countries and periods in which the studies were performed. The present results were also likely to be affected by different separate criteria of AMD. Third, another limitation of the present study concerned the assessment methods of dietary intakes of lutein and zeaxanthin. Although all studies included in the present meta-analysis stated that the FFQ used to assess dietary intake had been validated, dietary intake was measured only at baseline and might not reflect changes in intake of these carotenoids during follow-up. Differences in nutrient databases used across the studies could also constitute potential sources of variability in the values of lutein and zeaxanthin(Reference Van den Langenberg, Brady and Nebeling54). Fourth, even though several confounding factors had been adjusted for in all the studies incorporated, the possibility of other uncontrolled or potential residual confounding could not be fully excluded in the present meta-analysis. Moreover, lutein and zeaxanthin intake tended to be associated with some healthy lifestyles or dietary patterns that might be protective against AMD(Reference Seddon, George and Rosner55). Consequently, it was difficult to separate beneficial effects of dietary lutein and zeaxanthin intake from effects due to healthy lifestyle or dietary habits. This might be a possible explanation for the inconsistent findings from different population source. Fifth, it is possible that populations with specific genetic backgrounds or nutritional status may affect the analysis of the relationship(Reference Seddon, Cote and Page56). The present results were mainly based on studies carried out in Western populations, which limited the generalisation of findings. More research is necessary to be conducted in different populations to examine variations between populations. Finally, as with any meta-analysis, the potential for publication bias is a concern. Despite no publication bias examined in the present study, it was still difficult to fully rule out such bias because there was not a sufficient number of studies to detect it adequately.
In conclusion, the present systematic review and meta-analysis demonstrates that, on the basis of evidence available to date, dietary intake of lutein and zeaxanthin is not significantly associated with a decrease in the risk of developing early AMD, whereas an increase in the intake of xanthophylls may have beneficial effects for late AMD. It should also be noted that there are only a few studies that have examined this association, which limits the power of meta-analysis. Therefore, further well-designed large studies with prospective cohort design are required before definitive conclusions can be drawn regarding the potential effect of dietary lutein and zeaxanthin on AMD prevention.
Acknowledgements
The present study was supported by a grant from the National Natural Science Foundation of China (NSFC-30872113) and Academic Award for Excellent Doctorial Candidates of the Ministry of Education of China. X.-M. L., H.-L. D. and L. M. proposed the study concept and design; X.-M. L. and H.-L. D. supervised the study; L. M. and Y.-Q. W. conducted the study; L. M. and Y.-Q. W. collected the data; L. M., Y.-Q. W. and X.-R. X. carried out analysis and interpretation of the data; L. M., Y.-Q. W. and Y.-B. H. carried out statistical analysis; Y.-Q. W., H.-L. D., Y.-B. H., Y.-M. H., X.-R. X, Z.-Y. Z. and X.-M. L. provided technical or material support; L. M. and Y.-B. H. drafted the manuscript; and Y.-Q. W., H.-L. D., Y.-B. H. and X.-M. L. aided in critical revision of the manuscript. The authors declare no conflict of interest.








