Introduction
Herpes simplex virus type 1 (HSV-1) infection is a lifelong infection that is acquired primarily orally and during childhood [Reference Fatahzadeh and Schwartz1]. Although the infection is nearly always mild or asymptomatic, it can cause serious disease, including severe neurological, corneal and mucocutaneous complications [Reference Fatahzadeh and Schwartz1, Reference Brady and Bernstein2]. The historical pattern of HSV-1 epidemiology appears to be changing in Western countries, whereby HSV-1 oral acquisition is decreasing among children, but genital acquisition is increasing among youth, mainly due to oral sex [Reference Ayoub, Chemaitelly and Abu-Raddad3–Reference Roberts, Pfister and Spear8]. To address HSV-1 disease burden and its evolving epidemiology, the World Health Organization (WHO) and other global partners are leading initiatives to better understand HSV-1 epidemiology and to develop a vaccine that prevents acquisition of infection [Reference James7, Reference Broutet9, Reference Gottlieb10].
HSV-1 epidemiology is well characterised in many Western countries [Reference Ayoub, Chemaitelly and Abu-Raddad3, Reference Chemaitelly5, Reference Xu11, Reference Yousuf12], but remains insufficiently understood in Australia, New Zealand and Pacific Island nations. Accordingly, we carried out a study to delineate trends and patterns to inform policy, programming and resource allocation, for the purpose of addressing the disease burden of this infection. The study implemented an established analytical approach that has been developed, tested and refined over years of investigation and applications for a range of infections [Reference AlMukdad13–Reference Smolak18].
We characterised HSV-1 epidemiology in this region by systematically reviewing available measures for HSV-1 antibody prevalence (seroprevalence), HSV-1 seroincidence, proportion of HSV-1 detection in clinically diagnosed genital ulcer disease (GUD), and proportion of HSV-1 detection in laboratory-confirmed genital herpes. We further estimated pooled means for HSV-1 seroprevalence and for proportions of HSV-1 detection in GUD and in genital herpes, and investigated associations and temporal trends for HSV-1 seroprevalence and for proportion of HSV-1 detection in genital herpes.
Methods
The methodology used in this study was based on methodology we developed previously in a series of systematic reviews assessing HSV-1 epidemiology in other regions and countries [Reference Yousuf12, Reference Khadr15, Reference Chaabane19–Reference Harfouche, Chemaitelly and Abu-Raddad21]. A description of this methodology is shown in Box 1 and is outlined below.
Box 1. Description of study methodology.

Abbreviations: ELISA, enzyme-linked immunosorbent type-specific assay; GUD, genital ulcer disease; HSV-1, herpes simplex virus type 1; HSV-2, herpes simplex virus type 2.
Data sources, search strategy, study selection and eligibility criteria
This systematic review was informed by the Cochrane Collaboration Handbook [Reference Higgins and Green22], and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Table S1) [Reference Moher23, Reference Page24]. The included Pacific Island nations were selected as informed by the United Nations regional geoscheme and the WHO definition of the Pacific region (Box 1) [25, 26]. Search strategies are shown in Table S2. Screening processes and inclusion and exclusion criteria are explained in Box 1.
Four outcomes were assessed: (1) HSV-1 seroincidence, whether defined as the occurrence of infection per person-time or as a cumulative risk over a specific duration, (2) HSV-1 seroprevalence defined as the proportion of individuals tested that were seropositive, (3) proportion of GUD cases in which HSV-1 was isolated as the cause of GUD and (4) proportion of genital herpes cases in which HSV-1 was isolated as the cause of the genital herpes.
Data extraction, synthesis and quality assessment
Data extraction and double extraction were performed independently by four authors (SM, MH, UF and LA), using a predefined list of variables (Box 1). The validity of each HSV-1 diagnostic assay in each potentially relevant study was investigated in consideration of known limitations of HSV serology and potential cross reactivity between HSV-1 and HSV-2 antibodies [Reference Ashley27, Reference Ashley-Morrow28]. This was done with support of Professor Rhoda Ashley-Morrow of the University of Washington, an expert in HSV-1 diagnostic methods. Only studies that used valid and reliable assays were included. Each included study was subsequently appraised for precision and risk of bias (ROB), as informed by the Cochrane approach [Reference Higgins and Green22] and described in Box 1.
Both overall measures and stratified measures were extracted from relevant studies (Box 1). Since our aim was to characterise the natural heterogeneity that exists in HSV-1 epidemiology, measures were extracted and stratified by key epidemiological factors known to affect the natural epidemiology of this infection [Reference Yousuf12, Reference Khadr15, Reference Chaabane19–Reference Harfouche, Chemaitelly and Abu-Raddad21]. Meta-regression analyses were further conducted on these stratified measures to estimate effects of these epidemiological factors on both HSV-1 seroprevalence and proportion of HSV-1 detection in genital herpes.
Meta-analyses
DerSimonian-Laird random-effects models [Reference Borenstein29] with the Freeman-Tukey double arcsine transformation [Reference Freeman and Tukey30] were used to conduct meta-analyses, after examining the transformation's applicability to the dataset [Reference Schwarzer31]. Pooled mean estimates were calculated for HSV-1 seroprevalence and for proportions of HSV-1 detection in GUD and genital herpes. The meta package [Reference Schwarzer32] was used to perform these analyses in R, version 4.0.4 (Box 1) [33].
Since our study approach implicitly treats each outcome for a specific stratum or subgroup as an independent measure, studies reporting outcomes for more strata or subgroups had larger weights in the pooled estimate than studies reporting outcomes for less strata or subgroups, even when the study sample size was comparable. In a sensitivity analysis, pooled estimates were calculated by first generating a single estimate for each study by pooling estimates of all strata and subgroups in that study, and then pooling the single estimates across all studies. Accordingly in this analysis studies had weights that are independent of the number of reported stratum or subgroup outcomes.
Meta-regressions
Univariable and multivariable random-effects meta-regression analyses were conducted to investigate associations with HSV-1 seroprevalence and with proportion of HSV-1 detection in genital herpes, as well as to explain the between-study heterogeneity (Box 1). Random-effects meta-regression allows for residual heterogeneity (between-study heterogeneity not explained by the covariates) by assuming that the true effects follow a normal distribution around the linear predictor [Reference Harbord and Higgins34]. The metareg package [Reference Harbord and Higgins34, Reference Higgins and Thompson35] was used to calculate log-transformed seroprevalence and log-transformed proportions and to investigate associations in Stata/SE version 16.1 (Box 1) [36].
Results
Search results and scope of evidence
Screening and study selection processes are described in Figure 1. The search retrieved 1347 records: 546 in PubMed and 801 in Embase, of which 19 were deemed relevant. Bibliographic screening identified two additional relevant studies [Reference Birch37, Reference Smith38]. Altogether, 21 publications met the inclusion criteria from which data were extracted. Only one study was identified among the 21 Pacific Island nations, conducted in Vanuatu [Reference Haddow39].

Fig. 1. Flow chart of article selection for the systematic review of HSV-1 infection in Australia, New Zealand and Pacific Islands, per PRISMA guidelines [Reference Moher23]. Abbreviation: HSV-1, herpes simplex virus type 1.
Extracted HSV-1 measures included one cumulative seroconversion rate and one seroincidence rate in Australia, 13 overall seroprevalence measures (27 stratified) in Australia, one overall seroprevalence measure in Vanuatu (one stratified), four overall proportions of HSV-1 detection in clinically diagnosed GUD (four stratified) in Australia, and ten overall proportions of HSV-1 detection in laboratory-confirmed genital herpes (26 stratified) in Australia and New Zealand. There were no seroprevalence studies in New Zealand and no GUD or genital herpes studies addressed any of the 21 Pacific Island nations.
Cumulative seroconversion and seroincidence overview
One cohort study was identified that reported both cumulative seroconversion rate and seroincidence rate for HSV-1 infection among men who have sex with men (MSM) in Australia [Reference Jin40]. Cumulative seroconversion rate was estimated at 9.7% over a median follow-up duration of 2 years [Reference Jin40]. The seroincidence rate was estimated at 5.6 per 100 person-years [Reference Jin40].
Seroprevalence overview
Overall HSV-1 seroprevalence measures are listed in Table S3. All extracted measures were from studies conducted in Australia (n = 13), apart from the one study conducted in Vanuatu [Reference Haddow39]. Most studies were published prior to 2005 (number of measures (n) = 8; 61.5%) and used convenience sampling (n = 12; 92.3%).
Stratified HSV-1 seroprevalence measures in Australia for the different populations are summarised in Table 1. Seroprevalence across all populations (n = 27) ranged between 9.0–100.0% with a median of 75.5%. Seroprevalence among healthy general-population adults (n = 14) ranged between 32.5–100.0% with a median of 80.9%. Seroprevalence among clinical-population adults (n = 8) ranged between 9.0–73.5% with a median of 69.0%. Seroprevalence among MSM (n = 5) ranged between 54.2–87.7% with a median of 77.5%. No HSV-1 seroprevalence measures among children were identified in any of the countries.
Table 1. Pooled mean estimates for HSV-1 seroprevalence in Australia and Pacific Islands

Abbreviations: CI, confidence interval; HSV-1, herpes simplex virus type 1.
a Q: Cochran's Q statistic is a measure assessing heterogeneity in pooled outcome measures, here HSV-1 seroprevalence.
b I 2: A measure assessing the magnitude of between-study variation due to true differences in HSV-1 seroprevalence across studies rather than to sampling variation.
c Prediction interval: A measure quantifying the 95% interval of the distribution of true HSV-1 seroprevalence around the estimated pooled mean.
d No meta-analysis was done due to the small number of studies (n < 3).
Pooled mean estimates for HSV-1 seroprevalence
Pooled mean seroprevalence across all populations in Australia was 76.4% (95% confidence interval (CI) 67.5–84.3%; Table 1). Pooled means for seroprevalence among healthy adults and clinical adults were 84.8% (95% CI 74.3–93.1%) and 59.1% (95% CI 41.2–75.9%), respectively. Pooled mean seroprevalence for MSM was 75.7% (95% CI 64.8–85.1%). Pooled mean seroprevalence was 70.2% (95% CI 47.4–88.7%) among individuals <35 years of age and 86.9% (95% CI 79.3–93.0%) among individuals ≥35 years.
In the sensitivity analysis pooling estimates by first generating a single estimate for each study and then pooling the estimates across all studies, pooled mean seroprevalence across all populations in Australia was 66.3% (95% CI 48.5–81.9%). Pooled mean seroprevalence was 77.1% (95% CI 51.9–94.8%) among healthy adults, 52.2% (95% CI 26.3–77.5%) among clinical adults, and 75.7% (95% CI 73.4–78.0%) among MSM.
The majority of meta-analyses reflected heterogeneity (P value < 0.001) with wide prediction intervals (Table 1). The variation in seroprevalence resulted from true differences in seroprevalence as opposed to sampling variation (I 2 > 50%). Forest plots of meta-analyses by population type are shown in Figure S1.
Sources of between-study heterogeneity and predictors of HSV-1 seroprevalence
Univariable and multivariable meta-regression analyses for HSV-1 seroprevalence are shown in Table 2 and Table S4. Due to collinearity between year of data collection and year of publication, four multivariable models were conducted, two using a categorical variable for time and two using time as a continuous linear variable.
Table 2. Univariable and multivariable meta-regression analyses for HSV-1 seroprevalence in Australiaa
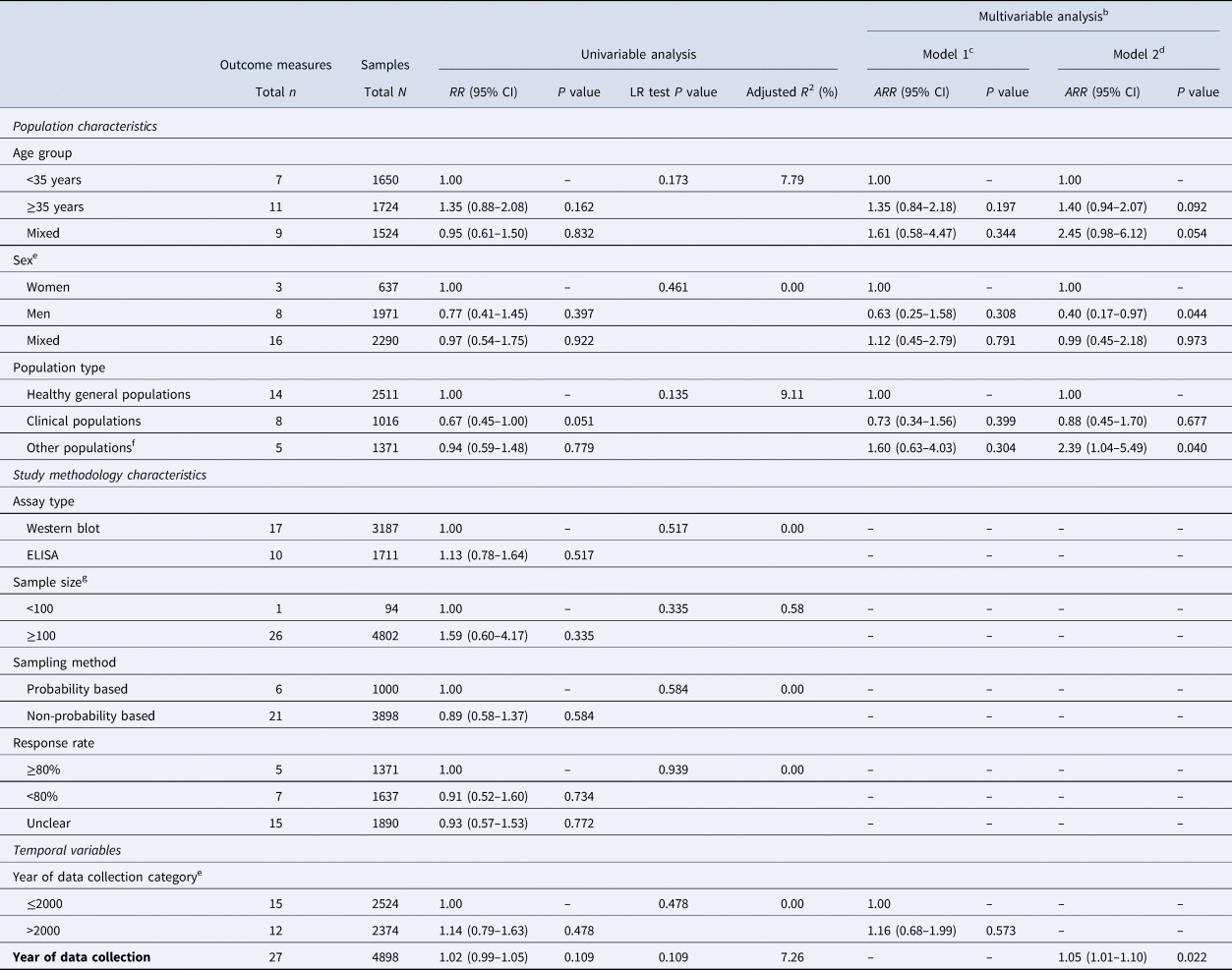
Abbreviations: ARR, adjusted risk ratio; CI, confidence interval; ELISA, enzyme-linked immunosorbent type-specific assay; HSV-1, herpes simplex virus type 1; RR, risk ratio.
a The only available study from the Pacific Islands nations (Vanuatu) was excluded from this analysis.
b Two multivariable models were conducted, one for year of data collection as a categorical variable and one for year of data collection as a linear term.
c Variance explained by the final multivariable model 1 (adjusted R2) = 4.96%.
d Variance explained by the final multivariable model 2 (adjusted R2) = 29.72%.
e Although sex and year of data collection category variables did not have a statistically significant association with the outcome in the univariable analysis (P value > 0.2), they were included in the multivariable analysis because of epidemiological relevance.
f Other populations included only men who have sex with men.
g Sample size denotes the sample size of each study population found in the original publication.
The model that included age group, sex, population type, and year of data collection as a continuous linear variable, explained 30% of seroprevalence variation (Table 2). Men had 0.40-fold (95% CI 0.17–0.97) lower seroprevalence than women. Seroprevalence increased by 1.05-fold (95% CI 1.01–1.10) per year. There was no evidence for differences in seroprevalence between healthy general populations and clinical populations, but there was some evidence of higher seroprevalence among MSM.
The other three multivariable models showed similar results, but evidence for the associations did not reach statistical significance (Table 2 and Table S4).
HSV-1 detection in clinically diagnosed GUD and in laboratory-confirmed genital herpes
Overall proportions of HSV-1 detection in clinically diagnosed GUD and in laboratory-confirmed genital herpes are listed in Table S5 and their stratified measures are summarised in Table 3. Proportion of HSV-1 detection in GUD (n = 4) ranged from 1.1–30.0% with a median of 7.5% and a pooled mean of 8.2% (95% CI 0.4–22.9%).
Table 3. Pooled mean proportions of HSV-1 detection in clinically diagnosed genital ulcer disease and in laboratory-confirmed genital herpes in Australia and New Zealand

Abbreviations: CI, confidence interval; GUD, genital ulcer disease; HSV-1, herpes simplex virus type 1.
a Q: Cochran's Q statistic is a measure assessing heterogeneity in pooled outcome measures, here proportions of HSV-1 detection.
b I 2: A measure assessing the magnitude of between-study variation due to true differences in proportions of HSV-1 detection across studies rather than to sampling variation.
c Prediction interval: A measure quantifying the 95% interval of the distribution of true proportions of HSV-1 detection around the estimated pooled mean.
Proportion of HSV-1 detection in genital herpes (n = 26) ranged from 5.6–80.0% with a median of 33.2% and a pooled mean of 30.5% (95% CI 23.3–38.3%). In Australia (n = 23), the proportion ranged from 5.6–80.0% with a median of 36.0% and a pooled mean of 32.5% (95% CI 24.8–40.7%). In New Zealand (n = 3), it ranged from 25.6–53.0% with a median of 30.4% and a pooled mean of 17.5% (95% CI 3.3–39.5%). Among women (n = 9), the proportion ranged from 19.0–80.0% with a median of 38.8% and a pooled mean of 40.7% (95% CI 27.5–54.7%). Among men (n = 9), it ranged from 5.6–60.5% with a median of 25.0% and a pooled mean of 24.5% (95% CI 13.9–36.9%).
All meta-analyses showed evidence of heterogeneity (P value < 0.001) and wide prediction intervals (Table 3). Most of the heterogeneity was due to true differences in these proportions rather than sampling variation (I 2 > 50%). Forest plots for meta-analyses of proportion of HSV-1 detection in GUD and proportion of HSV-1 detection in genital herpes are illustrated in Figure S2.
Sources of between-study heterogeneity and predictors of HSV-1 detection in genital herpes
Results of univariable and multivariable meta-regressions for the proportion of HSV-1 detection in laboratory-confirmed genital herpes are shown in Table 4. The model including age group, sex, country and year of data collection as a continuous linear variable explained 42% of the variation in the proportion of HSV-1 detection. Individuals ≥35 years of age had 0.49-fold (95% CI 0.27–0.91) lower proportion of HSV-1 detection compared to individuals <35 years. Proportion of HSV-1 detection increased by 1.04-fold (95% CI 1.00–1.08) per year. The second model, including year of data collection as a categorical variable, showed similar overall results (Table 4).
Table 4. Univariable and multivariable meta-regression analyses for HSV-1 detection in laboratory-confirmed genital herpes in Australia and New Zealand

Abbreviations: ARR, adjusted risk ratio; CI, confidence interval; HSV-1, herpes simplex virus type 1; LR, likelihood ratio; RR, risk ratio.
a Two multivariable models were conducted, one for year of data collection as a categorical variable and one for year of data collection as a linear term.
b Variance explained by the final multivariable model 1 (adjusted R2) = 32.97%.
c Variance explained by the final multivariable model 2 (adjusted R2) = 41.54%.
d Although sex and year of data collection category variables did not have a statistically significant association with the outcome in the univariable analysis (p-value>0.2), they were included in the multivariable analysis because of epidemiological relevance.
Quality assessment
Quality assessment results for the seroprevalence measures are presented in Table S6. Briefly, all studies (100.0%) had high precision. One study (7.1%) had low ROB in the sampling method domain, and one study (7.1%) had low ROB in the response rate domain. In contrast, 13 studies (92.9%) had high ROB in the sampling method domain, and two studies (14.3%) had high ROB in the response rate domain. None of the studies had low ROB in both quality domains, while one study (7.1%) had high ROB in both quality domains. The ROB assessment for the response rate domain was ‘unclear’ for 11 studies (78.6%).
Discussion
About 80% of the Australian population is infected with HSV-1, a higher level than that observed in Western countries (74% in Europe, 58% in the United States of America (USA) and 51% in Canada) [Reference Ayoub, Chemaitelly and Abu-Raddad3, Reference Chemaitelly5, Reference Yousuf12, Reference AlMukdad41], but comparable to levels in Asia (77%) [Reference Khadr15] and Latin America and the Caribbean (LAC) (85%) [Reference Sukik20], and lower than those in Africa (96%) [Reference Harfouche, Chemaitelly and Abu-Raddad21] and the Middle East and North Africa (89%) [Reference Chaabane19]. HSV-1 seroprevalence also appears to be increasing, contrary to other global regions where seroprevalence is decreasing or has remained stable for the last three decades [Reference Ayoub, Chemaitelly and Abu-Raddad3, Reference Chemaitelly5, Reference Yousuf12, Reference Khadr15, Reference Chaabane19–Reference Harfouche, Chemaitelly and Abu-Raddad21, Reference McQuillan42], perhaps because of increasing immigration from regions with higher HSV-1 seroprevalence. The only other country in which evidence suggests increasing HSV-1 seroprevalence is Canada [Reference AlMukdad41], another country of increasing immigration from regions with higher HSV-1 seroprevalence.
Seroprevalence was lower in men than women, an observation not seen elsewhere, except in Europe [Reference Yousuf12] and Canada [Reference AlMukdad41], and contrary to the global pattern of no differences in seroprevalence by sex [Reference Khadr15, Reference Chaabane19–Reference Harfouche, Chemaitelly and Abu-Raddad21]. While none of the included studies were on children <15 years of age, one study was conducted on senior high school students (age range of 15–20 years), and showed a low HSV-1 seroprevalence of 32.5% [Reference Bowden43]. There was no evidence for differences in seroprevalence between healthy general populations and clinical populations, just as observed in other regions [Reference Yousuf12, Reference Khadr15, Reference Chaabane19–Reference Harfouche, Chemaitelly and Abu-Raddad21, Reference AlMukdad41], reflecting that this infection is a general population infection.
The proportion of HSV-1 (versus HSV-2) detection in genital herpes in Australia and New Zealand was relatively high at 31%, comparable to levels observed in other Western countries (34% in Europe, 33% in the USA and 37% in Canada) [Reference Yousuf12, Reference AlMukdad41, Reference Dabestani44], but considerably higher than those in other regions (19% in Asia, 11% in LAC and 1% in Africa) [Reference Khadr15, Reference Sukik20, Reference Harfouche, Chemaitelly and Abu-Raddad21]. The proportion of HSV-1 detection in genital herpes was also increasing year by year, as in other Western countries [Reference Ayoub, Chemaitelly and Abu-Raddad3, Reference Bernstein4, Reference Roberts, Pfister and Spear8, Reference Yousuf12, Reference AlMukdad41, Reference Dabestani44]. These results are consistent with results of a study of HSV-2 infection in Australia and New Zealand that estimated HSV-2's contribution to genital herpes at 72% and decreasing year by year [Reference AlMukdad45].
These findings suggest that HSV-1 epidemiology is transitioning in Australia and New Zealand towards less oral acquisition in childhood, but more genital acquisition among youth, as is occurring in other Western countries [Reference Ayoub, Chemaitelly and Abu-Raddad3–Reference Roberts, Pfister and Spear8, Reference Yousuf12, Reference AlMukdad41, Reference Dabestani44]. This shift in epidemiology is associated with increasing adverse psychosexual effects among youth such as negative consequences for sexual relations and quality of life, depression, anxiety and low self-esteem [Reference Fisman46–Reference Mindel and Marks49]. This conclusion is supported by the higher proportion of HSV-1 detection in genital herpes among younger compared to older adults (Table 4). This conclusion also suggests that the level of HSV-1 seroprevalence among children in Australia and New Zealand is substantially lower than among adults, but no data among children were available to confirm this conjecture.
This study has limitations. There were hardly any data from Pacific Island nations; only one seroprevalence study was available in these countries. No seroprevalence studies were found in New Zealand. While there were multiple studies in Australia, none of them included children. Most seroprevalence studies were two-decades old, with no studies in recent years, where seroprevalence is more likely to be decreasing, especially among youth, as in other Western countries [Reference Ayoub, Chemaitelly and Abu-Raddad3, Reference Chemaitelly5, Reference Yousuf12, Reference McQuillan42]. Overall, the number of studies was not large enough to allow precise quantification of different potential associations through meta-regression analysis. Studies varied in methodological characteristics, including assay type, sample size, sampling method and response rate. Despite these limitations, the meta-regressions explained a substantial fraction of the heterogeneity observed across studies in terms of epidemiological factors such age (Tables 2 and 4 and Table S4).
Conclusions
HSV-1 seroprevalence is high in Australia and higher than that in other Western countries. HSV-1 epidemiology in Australia and New Zealand may be transitioning towards less oral acquisition in childhood, but more genital acquisition among youth. HSV-1 infection is already the cause of nearly one-third of genital herpes cases, particularly among youth. There is an immediate need for expansion of HSV-1 epidemiological research in Pacific Island nations, in addition to Australia and New Zealand. This research should include studies on different populations with a focus on levels of infection in children. Research and surveillance are also needed to monitor the aetiology of GUD and genital herpes in this part of the globe.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268823000183
Author contributions
SM, MH, UF and LA conducted the systematic search, data extraction and data analysis. SM wrote the first draft of the paper. LJA conceived the study and led the data extraction and analyses and interpretation of the results. All authors contributed to drafting and revising the manuscript.
Financial support
This work was supported by the Qatar National Research Fund [NPRP 9-040-3-008] and by pilot funding from the Biomedical Research Program at Weill Cornell Medicine in Qatar. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article. Statements made herein are solely the responsibility of the authors.
Conflict of interest
The authors declare no competing interests.
Data availability statement
All relevant data are presented in the manuscript and its Supplementary Material file.








