Introduction
Pediatric medical device technology lags significantly behind adult technology [1]. The primary reason for this lag is a smaller market with few incentives for the medical device industry or investors to pursue development in pediatrics. Beyond limited market size, there are a number of additional barriers to pediatric medical device development. Designing devices specifically for children is challenging due to size considerations and continuously changing anatomy and physiology. Testing pediatric devices presents yet another challenge. Small numbers of pediatric patients with a given medical condition can make it difficult, costly, and time consuming to run clinical trials. This in turn leads to complicated regulatory clearance or approval mechanisms and reimbursement models [2]. Providers are left with limited options to prevent, treat, or palliate pediatric disease. One common approach is to use adult products off label, which increases risks of complications and does not provide for optimal therapy [Reference Sutherell, Hirsch and Beekman3,Reference Jenkins4].
Nationally, efforts to close the innovation gap have been led by the Food and Drug Administration (FDA) through the Pediatric Device Consortia (PDC) Program [Reference Ulrich5]. The PDC program funds nonprofit consortia that provide innovators of pediatric devices with seed funding and expertise needed to advance through the product development lifecycle [6]. Indeed, this national effort has been successful in narrowing the gap in pediatric medical device development as described in depth by Chowdhury et al. [Reference Chowdhury7].
While the FDA’s PDC program is a national-level program with significant federal resources ($31.4M in project funding awarded since 2009 [Reference Chowdhury7]), there are opportunities for academic institutions to create successful pediatric-focused innovation programs to complement the FDA initiative. Institutional programs are positioned to support local pediatric technologies in alignment with unique missions and approaches, drawing on specific strengths of the institution and regional ecosystem. In our experience, promising health innovations that receive institutional funding and expert guidance are typically more competitive for follow-on funding sources to advance development.
At the University of Minnesota (UMN), we recognized a gap in pediatric medical technology innovation that mirrored the national lag. To augment the FDA’s initiative and address the innovation gap at our own institution, the UMN created the Pediatric Device Innovation Consortium (PDIC), an academic innovation initiative designed to facilitate and accelerate development of pediatric health innovations at an institutional and local level. The PDIC has a distinct mission and approach, including an emphasis on global health technologies for low resource settings and the inclusion of patient and caregiver perspectives to drive innovation. In addition to supporting FDA-regulated medical devices, the PDIC also supports pediatric health innovations that do not meet the FDA definition of a medical device, such as open source methods. Located in a thriving medical device ecosystem, the PDIC has leveraged local expertise in fulfillment of its mission. Here we highlight unique characteristics of the PDIC program, and describe key components of an academically centered pediatric medical device development program that can be replicated at other institutions with an interest in pediatric innovation.
The PDIC Model
Mission
The PDIC was created in 2011 to assist university, community, and industry innovators of pediatric medical technologies. Specifically, the goals of the PDIC are to
1. identify unmet pediatric medical needs and opportunities for innovation;
2. support the development of pioneering medical solutions that improve care for the pediatric population; and
3. form innovative partnerships that advance pediatric device development.
Initially, the primary function of the PDIC was to connect innovators with guidance from industry experts through the formation of a multidisciplinary project advisory team. While guidance was beneficial to innovators, the ability to financially support promising technologies was also needed to meet PDIC goals. To fill this need, in 2014, the PDIC partnered with the Office of Discovery and Translation (ODAT) within the Clinical and Translational Science Institute (CTSI) at UMN to develop a targeted funding program [Reference Wells, Rebuffoni and LeBien8]. ODAT was established in 2011 to create and administer translational funding programs at UMN. Historically, very few applications for pediatric medical technologies were submitted to ODAT funding programs (which were open to all technologies), and none of the projects were selected for funding. Funding applications submitted to ODAT for pediatric technologies were typically less competitive when compared with large-market technologies due to the development challenges described earlier. For example, it is difficult for a translational grant program such as ODAT with limited resources to assess the impact when comparing a project that addresses a relatively rare but devastating childhood disease to a project that addresses a condition such as Alzheimer’s disease that impacts millions of individuals. Additionally, we observed that pediatricians are often the first to recognize innovation gaps, but they may lack the device-development experience, time, and institutional support or resources to advance the development of a new solution, so they are less likely to put forth a funding application for review.
The PDIC/ODAT partnership was created to level the playing field for pediatric health innovations competing for institutional funding through ODAT programs. As part of this partnership, ODAT contributes institutional funding and many other key structural components, such as providing all program and project management support. Drawing on the mission and expertise of the PDIC, ODAT is better able to meet the innovation needs of an underserved population.
Funding Programs
With ODAT support, the PDIC has developed and implemented three different funding programs since 2014 that target specific subpopulations of innovators or innovation partners. Two of the programs focus on faculty/clinicians and industry while the third focuses on patients/caregivers, who see unmet pediatric needs from a unique perspective. Implementation of each program was strategic to fill a gap identified within the UMN innovation ecosystem. See Fig. 1 and below for an overview of the three funding programs.

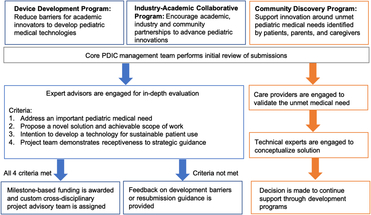
Fig. 1. Pediatric Device Innovation Consortium (PDIC) funding programs with project selection and guidance processes. The PDIC has developed three different funding programs. Each is designed to facilitate pediatric innovation from a unique perspective: faculty/clinicians (Device Development) industry (Industry–Academic Collaborative), and patients/caregivers (Community Discovery). Applications submitted to all three programs are first reviewed by the core PDIC management team. Device Development and Industry–Academic Collaborative Programs support development of a defined technology, and have similar processes represented by blue boxes and arrows. Following the initial review, applications undergo an in-depth review by internal and external experts. Projects that meet criteria receive funding and development support, whereas projects that do not meet criteria receive feedback and guidance. The Community Discovery process, represented by orange boxes and arrows, supports conceptualization of solutions for unmet needs. Following the initial review, submissions of unmet needs are validated by external experts, explored for potential solutions and may receive further technology development support through the Device Development or Industry–Academic Collaborative programs.
The Device Development Program was created by the PDIC in 2014 to provide a specific support mechanism for innovators of pediatric technologies. Aside from funding, this program utilizes a project team approach to fill gaps in expertise, allowing for a broader and more diverse range of applicants with varying levels of technology development experience.
To leverage the strengths of the UMN pediatric research community and local medical device industry, the PDIC expanded its programs in 2016 to encourage collaboration between academic faculty and industry or community partners. The Industry–Academic Collaborative Program promotes partnerships to advance pediatric device innovations. Typically, these projects involve external intellectual property that is advanced through collaboration with academic experts such as pediatricians and engineers. Projects supported by this program include close involvement with the UMN Technology Commercialization office to provide the necessary clarity around technology ownership and contractual rights.
Looking beyond medical providers, academia, and the medical device industry, the PDIC recognized that the voices least represented in our programs were those of the patients, parents, and caregivers. In response to this gap, the PDIC launched the Community Discovery Program in 2016. This program solicits descriptions of unmet medical needs and challenges from the perspective of patients and caregivers that may be improved with new technology developed and supported by the PDIC. Members of the community submit descriptions of needs directly to the PDIC staff or through the PDIC website at http://www.thepdic.org/community.
Process
Applications submitted to any of the programs are first reviewed by the core PDIC management team. The subsequent review processes have been tailored to each funding program. In general, the processes for the Device Development and Industry–Academic Collaborative Programs are similar to one another because innovators are requesting funding to advance development of a defined technology. The process for the Community Discovery Program differs because caregiver or community members have identified an unmet need, but a defined technology has not yet been conceptualized (pre-concept). The PDIC allows for follow-on funding to further advance development of PDIC projects. See Fig. 1 for an overview of the project selection and guidance processes for each funding program.
Device Development and Industry–Academic Collaborative Programs
Proposals are formally reviewed by a project advisory team of academic and industry-based pediatric technology experts using an iterative review process. Typically, projects selected for PDIC funding meet the following criteria: the innovation addresses an important pediatric health need; the project proposes a novel solution and achievable scope of work; the applicants intend to develop an innovation for sustainable patient use; the project team demonstrates receptiveness to strategic guidance; PDIC funding can make a meaningful impact in the advancement of the innovation; the project has the potential to positively benefit UMN.
In addition to the above evaluation criteria, the PDIC evaluation process categorizes projects in accordance with the stages of the product life cycle, in order to determine the type and level of strategic guidance or services needed from the PDIC. These categories include pre-concept (identification of an unmet pediatric medical need), concept for a new device, prototype development, preclinical testing, clinical testing, manufacturing, marketing, and commercial use/patient access.
Once approved for funding, projects are supported by a custom cross-disciplinary project advisory team to guide the investigators in addressing known and unforeseen development barriers. The project advisory team helps to develop a product development roadmap for the product, and assesses achievement of stated project milestones to determine continuation of funding resources. Utilization of a project advisory team positions the project for success and ensures good stewardship of program funding allocated to product development.
Promising projects that are not funded receive guidance to address specific issues that are not fundamental to the technology or development pathway, but may make them more competitive upon reapplication to the PDIC or other funding programs. Guidance may include addressing cost/time limitations to achieve project deliverables, or lack of clarity in the project goals and scope of work.
Projects with more significant issues that are fundamental to the technology and/or development pathway receive specific review feedback to educate innovators on barriers that must be addressed to successfully develop their technology. Examples of these issues include a misunderstanding of the clinical problem to be addressed, product/market fit, or a project focused on scientific research rather than translational product development.
Community Discovery Program
For pre-concept projects, the PDIC management team engages care providers such as patients, parents, therapists, and others to fully understand and validate the unmet medical problem. Medical issues addressed through the Community Discovery Program typically encompass quality of life/quality of care unmet needs that may be low tech but high impact for care providers. For projects that present opportunities for PDIC innovation, individuals with the necessary technical expertise are engaged to conceptualize a possible solution and receive support through PDIC development programs.
Structure and Support
The structure of the PDIC is critical to achieving its mission. A director to create and drive the program mission, dedicated program staff to facilitate and execute program operations, cross-disciplinary project advisory experts, and access to a network of service providers are all essential to success. PDIC staff salaries are supported through the UMN CTSI. PDIC project funding, including consulting funds for experts and advisors, comes from the UMN Medical School.
The PDIC offers a continuum of support, ranging from consultations to strategic guidance and funding. Consultations are informal discussions that do not include a formal request for PDIC support. Certain projects are in need of in-depth strategic guidance before PDIC funding would be beneficial or appropriate. Strategic guidance is driven by the PDIC core management team and may include project advisory team members or ad hoc consultants to fill a key knowledge gap. Strategic guidance always includes direct effort from the PDIC staff and may include funding for consultants or service providers. Project funding is issued in milestones for a limited scope of work. The opportunity for follow-on funding enables a milestone-based approach without limiting advancement of promising innovations. PDIC funding inherently comes with project advisory team guidance. Details of how the PDIC has operationalized structure and support are further defined in the Discussion section. See Fig. 2 for an overview of the operational structure of the PDIC.
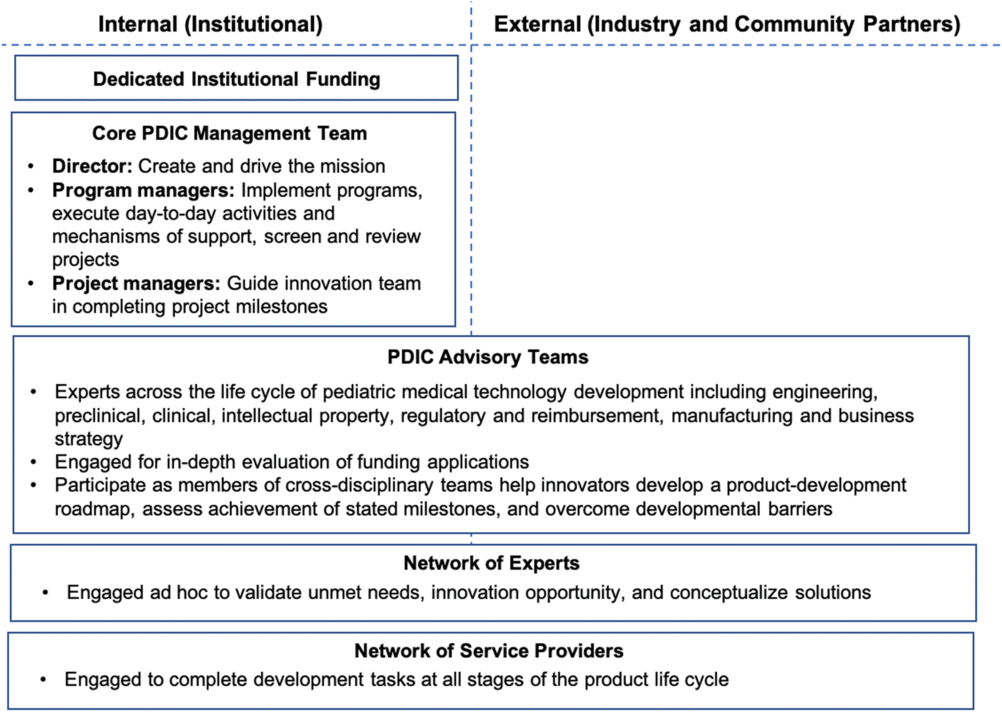
Fig. 2. Pediatric Device Innovation Consortium (PDIC) operational structure and support network. The PDIC leverages both internal (institutional) and external (industry and community) resources to support development of pediatric medical technologies. Internal resources include dedicated institutional funding and a core PDIC management team with a director and dedicated program staff to facilitate and execute program operations. The PDIC draws on both internal and external experts for various operational functions, including project and opportunity evaluation, product development strategies, and completion of milestones.
Results
Case Studies
Example 1: The PDIC supported a proof-of-concept study for a tissue-engineered pediatric right ventricular outflow tract (RVOT) graft. The PDIC-funded scope of work to create the product and test it in an animal model was successful, and the results from this pioneering work were published in Nature Communications [Reference Syedain9]. The project advisory team met with the investigators throughout the course of the project, and the PDIC management team has continued to provide commercialization guidance for this project 3 years after completion. This technology was licensed to a UMN start-up company, Vascudyne, in 2017, has received follow-on funding (including a PDC award), is seeking FDA approval to conduct a clinical trial, and serves as a success story of PDIC funding and expert guidance.
Example 2: The PDIC received a submission via the Community Discovery Program from a speech pathologist in the community describing an unmet need related to the challenges of communicating important information to children with language and literacy impairments. The lack of understanding and awareness in this particular population often heightens the child’s confusion and anxiety and negatively impacts compliance. The PDIC conducted an evaluation of both the unmet need and existing solutions, and determined that there was an opportunity for innovation. The PDIC is currently working with a community-based innovator to co-create an app-based software solution that uses customized images and specialized user interface methods to communicate sequenced events for the target population. This project exemplifies PDIC’s unique ability to support projects originating as unmet needs that do not come into our programs with a defined device concept.
Example 3: The PDIC has supported development of a digital solution for an unmet need to address pediatric dental anxiety. Through the Community Discovery Program, the PDIC first supported the creation of the prototype, which was then licensed to a UMN start-up, Let’s Yonder, in 2017. After start-up formation, the PDIC further supported software development in conjunction with the UMN through the Industry–Academic Collaborative Program. The software is currently being used in dental clinics, and the company made their first sale at the end of 2018. This project shows how submissions of unmet needs that come through the Community Discovery Program can transition into PDIC development programs and ultimately to patient use.
Impact
The ultimate goal of PDIC support is to provide patient benefit through the development of novel, pediatric-specific health innovations. Similar to the FDA’s PDC program, the PDIC measures return-on-investment based on whether funding translates to achievement of product development milestones and patient access. In these traditional outcome measures, 70% of supported projects have advanced to a later stage in development as a result of PDIC support, with several advancing across multiple milestones. Additionally, two technologies have been licensed to start-up companies and two technologies have reached patient access. Figure 3 shows the 22 funded projects and the progress they made during PDIC support on a continuum from pre-concept to patient access.
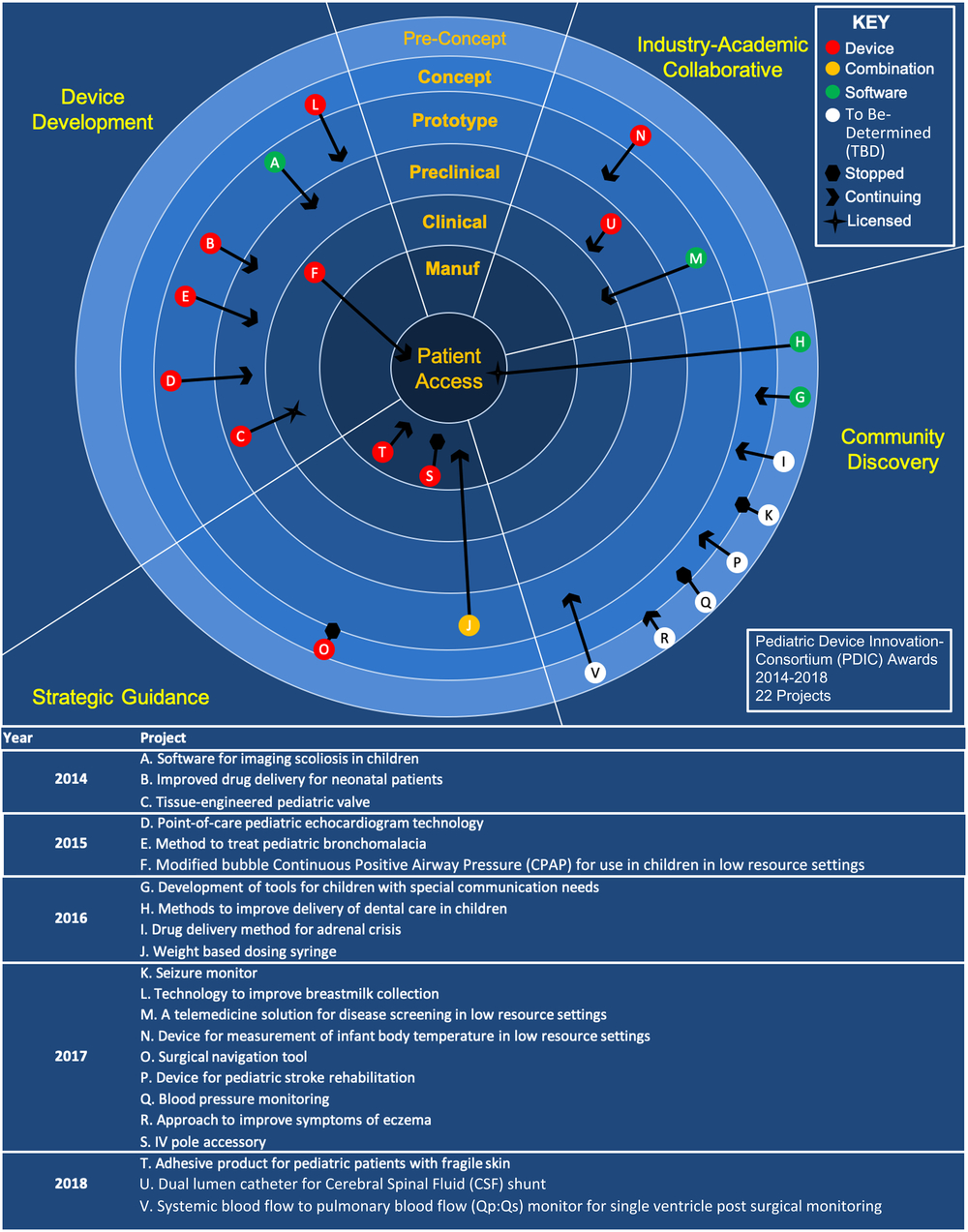
Fig. 3. Pediatric Device Innovation Consortium (PDIC) supported projects and advancement along the product life cycle. PDIC-supported projects from 2014 to 2018 by funding program or support mechanism, product type, and progression through product development stages. A brief description for each project A–V can be found below the diagram. Projects are categorized by the program and year that the submission was initially received. For projects noted as “Stopped,” the PDIC determined that available support was not sufficient to advance product development efforts or the technology presented a barrier that was determined to be unaddressable. The data shown in this figure are specific to PDIC-supported projects and are displayed in a format similar to previously published data that include other non-PDIC translational funding programs [Reference Syedain8].
As an institutional gap funding program, serving as a bridge between academic innovation and industry development, the PDIC also measures programmatic impact based on the ability to positively influence the development of pediatric health innovations at our institution. The following section describes focus areas of program impact with associated indicators. Each of ODAT’s funding programs has unique aims, processes, and amounts of funding support. Therefore, comparison of outcomes across programs is not an appropriate measure of individual program goal achievement. PDIC program impact is measured against historic PDIC metrics beginning at the implementation of PDIC programs.
Positively influence the institutional rate of pediatric technology innovation
Prior to the implementation of pediatric-specific innovation programs, only 2% (3/125) of total applications historically submitted to ODAT addressed a pediatric medical need. The PDIC/ODAT partnership and development of pediatric-specific innovation programs in 2014 increased the number of project applications, which was further increased by the expansion of PDIC programs in 2016 to include the Industry–Academic Collaborative and Community Discovery programs. From 2014 to 2018, the PDIC received a total of 49 project applications, supported 22 projects, and awarded more than $500,000 in project funding. Within a 4-year time period, our metrics reflect an upward trend in applications for pediatric technology as evidenced by a 9% increase since 2014 (Fig. 4). These metrics indicate that creating dedicated programs with sufficient expertise and resources can significantly increase the rate of pediatric technology innovation at the institutional level.
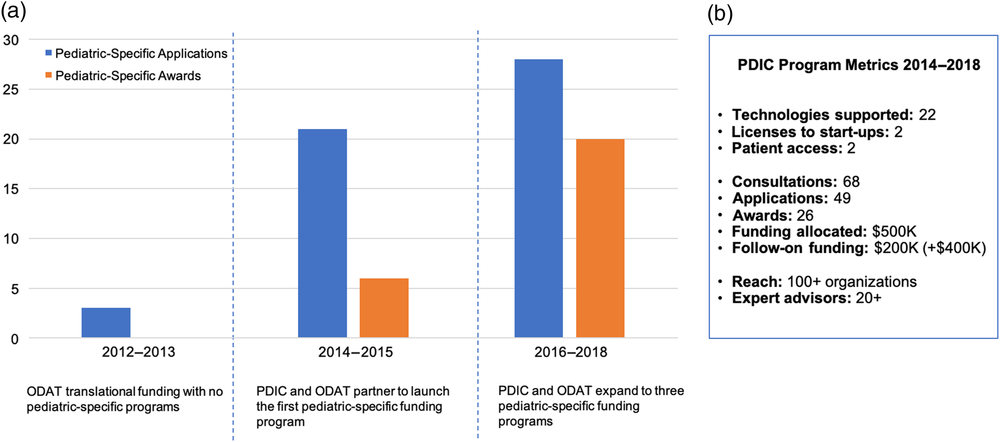
Fig. 4. Institutional pediatric innovation and Pediatric Device Innovation Consortium (PDIC) program metrics. A. The PDIC has positively influenced the institutional pediatric innovation rate. Prior to partnering with the PDIC and developing pediatric-specific funding programs, from 2012 to 2013, Office of Discovery and Translation (ODAT) received very few pediatric-specific translational funding applications and none were competitive for funding. Partnering with the PDIC and developing a pediatric-specific funding program in 2014 increased the number of pediatric-specific translational funding applications and awards from 2014 to 2015. The creation of two additional pediatric-specific funding programs further increased the number of pediatric-specific translational funding applications and awards from 2016 to 2018. B. A summary of key metrics to measure the impact of the PDIC funding programs from 2014 to 2018.
Foster a culture of development-based innovation
The PDIC encourages development-based innovation by raising awareness of PDIC programs and facilitating engagement of stakeholders, partners, and collaborators both within and outside the university. In the past 4 years, the PDIC has provided no-cost consultations to 68 innovators, disseminated information about the PDIC model at local and national conferences, and engaged 100+ government, hospital, academic, industry, and community organizations through annual PDIC-hosted events, including a collaborative event with a PDC program.
Attract expertise and leverage funding opportunities to best support technologies
The PDIC supports development of pediatric technologies by leveraging capital and a network of experts. Since 2014, the network of PDIC advisors has grown to over 20 experts across product development areas. PDIC-supported projects have attracted more than $200,000 in follow-on funding from sources such as philanthropic donors, and the FDA PDC program. Additional funding totaling more than $400,000 is pending. Partnerships and collaborations forged by the PDIC are aimed at augmenting the capital and expertise of the PDIC to best support technologies in the program portfolio.
Strength in these areas demonstrates alignment between our programmatic efforts and the mission of the PDIC. It also highlights our role in bridging a gap between academic innovation and the industry-level resources required to develop technologies for patient use.
Discussion
Lessons Learned
Through experience gained in developing the PDIC and supporting innovators, we have identified common challenges and opportunities that may be useful for other institutions interested in developing programs for pediatric technology development.
Mission:
A mission to develop pediatric medical technologies for patient access must be aligned at the project, program, and institutional levels.
Programs:
Programs should be positioned to support a range of innovators from an academic engineer capable of creating new devices to a pediatrician who recognizes unmet needs.
Process:
The ability to accept new submissions on a continuous basis helps to provide timely support, in-depth feedback and advice for promising technologies.
An iterative review process with internal and external experts, and established review criteria, increases the level of confidence that resources are allocated in alignment with program goals and mission.
Structure and Support:
A core management team with technology development experience is recommended to manage day-to-day activities, screen and review projects, and execute mechanisms of support.
A network of internal and external service providers is essential to complete development tasks at all stages of the product life cycle, such as device design, prototyping, animal studies, and regulatory applications.
Dedicated institutional funding is needed to financially support promising technologies. Partnerships and collaborations with other institutions and organizations offer opportunities to leverage limited institutional funding and further extend the breadth of expertise.
An early understanding of potential development barriers, through support from a multidisciplinary project advisory team, minimizes the risk of innovation “blind spots” that may prevent technologies from achieving patient access.
Future Directions
The PDIC management team is currently evaluating opportunities for growth by assessing the key programmatic and structural components, including methods to effectively promote public–private collaborations in the development of pediatric medical technology, and approaches to allow scalability to support a larger volume of projects.
Conclusions
Approaches to accelerate innovation of pediatric technologies in an academic setting will continue to evolve. PDIC’s efforts to improve available medical technology for pediatric patients aim to increase efficiency in care delivery, reduce healthcare costs, increase patient satisfaction, as well as improve patient outcomes. Medical device development is rapid and iterative, and encompasses a wide range of disciplines. The PDIC model reflects the realities of device innovation at an academic institution for smaller markets, and accommodates clinician innovators who may lack the broad range of experience or resources required to advance medical device innovation alone. The PDIC programs described in this paper demonstrate a model that shows how an academic institution can increase support for pediatric innovation and contribute to the national effort to close the pediatric technology gap.
Acknowledgments
Material reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to acknowledge Karen Kaehler, M.S., M.B.A. from the UMN Technology Commercialization office for providing key data for this paper.
Disclosure
The authors have no conflict of interest to report.