Recommendations on dietary intake have been part of public health initiatives in the Western world for over a century( Reference Schneeman 1 ). During the past two decades, the WHO has issued dietary recommendations for prevention of chronic diseases( 2 ). The Nordic countries (Denmark, Finland, Iceland, Norway and Sweden) have provided joint recommendations on nutrient intakes since 1980: the Nordic Nutrition Recommendations (NNR)( 3 ). Based on scientific evidence and revised every eight years, the last time in 2004, the NNR mirror the nutritional needs of the Nordic population. They form the basis for national dietary guidelines and aim to promote overall good health and prevent chronic diet-associated diseases. In a recent study, adherence to the NNR was found to be unrelated to upper respiratory tract infections( Reference Fondell, Christensen and Balter 4 ). However, no study to date has examined the NNR in relation to cancer risk.
Among men in the Nordic countries, prostate cancer (PC) is by far the cancer with the highest incidence( Reference Engholm, Ferlay and Christensen 5 ). The aetiology of PC is still poorly understood, although both environmental and genetic factors are thought to be involved( Reference Lichtenstein, Holm and Verkasalo 6 , Reference Mucci, Signorello and Adami 7 ). Because incidence changes following migration from a low- to a high-risk country( Reference Mucci, Signorello and Adami 7 – Reference Shimizu, Ross and Bernstein 10 ), lifestyle factors such as diet are likely to play an important role. Several individual foods or nutrients have previously been associated with risk of PC( Reference Mucci, Signorello and Adami 7 , Reference Hori, Butler and McLoughlin 11 , 12 ). Among nutrients included in the NNR, Ca has been shown to increase the risk( 12 , Reference Skinner and Schwartz 13 ), whereas conflicting results suggest a possible protective effect of vitamin E and Se, especially among men with low serum levels of these nutrients( Reference Hori, Butler and McLoughlin 11 , 12 , Reference Ma and Chapman 14 ). Dietary fat may also be associated with PC risk( Reference Ma and Chapman 14 – Reference Leitzmann, Stampfer and Michaud 16 ). However, considering the interplay between different components of the diet, it may be more informative to study the effect of overall diet. There is also growing evidence for potential gene–diet interactions in the development of PC( Reference Hedelin, Balter and Chang 17 – Reference Steinbrecher, Rohrmann and Timofeeva 21 ).
In a large population-based case–control study in Sweden, we investigated if adherence to the NNR is associated with PC. We also explored potential effect measure modification by genetic and lifestyle factors. We created a dietary score to assess NNR adherence and a genetic risk score to study gene–diet interactions. To the best of our knowledge, no study on dietary recommendations and PC has been performed previously.
Materials and methods
Study population
The Cancer of the Prostate in Sweden (CAPS) is a population-based case–control study including incident, histologically confirmed PC cases identified from four of the six regional cancer registries in Sweden( Reference Chang, Hedelin and Adami 22 – Reference Lindmark, Zheng and Wiklund 24 ). Cases resided in the central and northern parts of Sweden and were 35–79 years old at enrolment (January 2001–September 2002). Clinical data were obtained for 95 % of all cases via linkage to the National Prostate Cancer Registry. Advanced PC cases were defined as those meeting at least one of the following criteria: TNM (tumour-node-metastasis) stage T3/T4, N1 or M1; Gleason score 8–10; or serum prostate-specific antigen (PSA) level at diagnosis ≥100 ng/ml. Localized cases did not meet any of the above criteria. Population controls were randomly selected from the Swedish Population Registry and frequency-matched to cases by 5-year age categories and region of residence.
In total 1895 eligible cases were invited to participate, of whom 1499 (79 %) completed a baseline questionnaire and 1400 (74 %) donated a blood sample. Of 1684 eligible controls, 1130 (67 %) completed the questionnaire and 879 (52 %) donated blood. In total, 1352 cases (71 %) and 858 controls (51 %) both completed the questionnaire and donated blood. An average time of 5 months elapsed between diagnosis and date for sending out the questionnaire. The study was performed according to the guidelines laid down in the Declaration of Helsinki and was approved by the ethics committees at Karolinska Institutet and Umeå University in Sweden. All participants gave their written informed consent at enrolment.
Exposure assessment
A semi-quantitative FFQ assessed the usual intake during the previous year of 106 items including foods, beverages and alcohol, with additionally three questions on dietary fat and ten questions on dietary supplements( Reference Chang, Hedelin and Adami 22 , Reference Chang, Smedby and Zhang 25 , Reference Westerlund, Steineck and Balter 26 ). Macro- and micronutrient intakes assessed by the FFQ have been validated against repeated 24 h recall interviews in 248 Swedish men, with Spearman correlation coefficients between 0·3 and 0·8 (e.g. r = 0·3 for Fe, r = 0·8 for alcohol; P < 0·05)( Reference Messerer, Johansson and Wolk 27 ). The intake of food items has been validated against weighed food records, with Pearson correlation coefficients ranging from 0·2 to 0·6 (e.g. r = 0·2 for broccoli, r = 0·5–0·6 for dairy products; P < 0·05)( Reference Chang, Smedby and Zhang 25 ). To calculate nutrient and energy intakes, the FFQ dietary information was linked to the Swedish National Food Administration database( Reference Bergström, Kylberg and Hagman 28 ), containing about 1500 food products. For these calculations we created aggregated codes corresponding to 253 food and beverage items. Dietary supplement data were not included in nutrient calculations.
Physical activity was assessed in the questionnaire as levels of occupational, household and recreational activity at different ages (15, 30, 50 and 65 years, and age at study entrance). Questionnaire validity has been tested against 7 d activity diaries (Spearman correlation r = 0·56)( Reference Norman, Bellocco and Bergstrom 29 ). Questionnaire data were converted into minutes of daily moderate and/or vigorous physical activity using the most recent age category preceding age at study entrance. Moderate and/or vigorous activity at work was defined as: ‘Mostly sitting’/‘Sitting half of the time’/‘Mostly standing’ = 0 min/d; ‘Mostly walking, lifting, carrying a little’ = 1 h/d for 5 d/week; ‘Mostly walking, lifting, carrying a lot’ = 3 h/d for 5 d/week; ‘Heavy physical labour’ = 5 h/d for 5 d/week. Walking/bicycling and physical exercise were reported in minutes per day. To obtain total minutes of daily physical activity, we calculated the sum of occupational activity, walking/bicycling and physical exercise.
Adherence to the Nordic Nutrition Recommendations
The NNR include reference values for energy intake, recommendations for macronutrient (fat, carbohydrates and protein) composition expressed as a percentage of total energy intake (excluding energy from alcohol), and recommendations for intakes of alcohol, fibre, salt, individual vitamins and minerals, and for levels of physical activity. NNR adherence was expressed as a score based on nine main variables: fat, carbohydrates, protein, fibre, vitamins, minerals, salt, alcohol and physical activity. Micronutrient intakes were energy-adjusted by the residual method, adding to the residual the predicted nutrient intake at an energy intake of 10 600 kJ, an adequate reference level for men aged 61–74 years according to the NNR. Recommendation levels and groupings of variables are listed in Table 1.
Table 1 Grouping of individual recommendations of the Nordic Nutrition Recommendations (NNR) score; recommendation levels and cut-off points for adherence; mean intakes and percentage of adherence in the study population: the Cancer of the Prostate in Sweden (CAPS) study (n 2326)
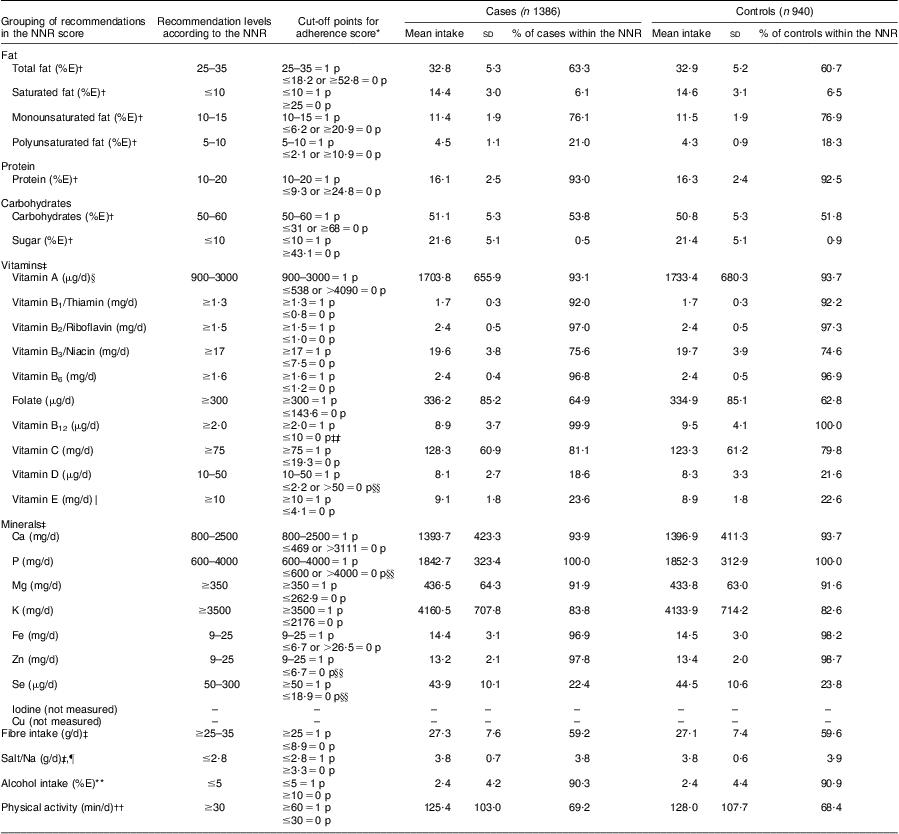
%E, percentage of total energy intake.
*1 point (1 p) was accredited for intakes within the recommendations (perfect adherence) and 0 points (0 p) for intakes outside the median of the ten highest or ten lowest intakes in the study population (non-adherence). A proportional score between 0 and 1 was estimated for intakes between the recommendations and the median cut-off points (intermediate adherence) as described in Fig. 1.
†Alcohol was not included in total energy intake when calculating percentage of energy.
‡Vitamins, minerals, fibre and Na are energy-adjusted by the residual method, adding to the residual a constant equivalent to the predicted nutrient intake at a reference energy intake of 10 600 kJ.
§Vitamin A: measured as the sum of retinol, β-carotene and other carotenoids. Upper intake level is for retinol only.
∥Vitamin E: measured as α-tocopherol, which is the most active form of tocopherols.
¶A cut-off value of 3·3 g/d was chosen instead of the median among the ten highest intakes due to the generally high Na intakes in the Swedish population.
**A cut-off value of 10 %E was chosen instead of the median among the ten highest intakes (41 %E), due to the large variability in alcohol consumption in the population and the detrimental effects of higher alcohol intakes.
††The NNR recommends physical activity of at least moderate intensity for a minimum of 30 min/d and preferably more than 60 min/d. Adherence cut-off points were set higher than the minimum recommendation level so as to obtain a sufficient number of men in the low adherence group.
‡‡An arbitrary level of 1·0 μg vitamin B12/d was chosen as cut-off value for 0 points, since only three men had intakes below the NNR.
§§The median of the ten highest intakes was below the recommended upper intake level, or no men had intakes above the upper intake level.
Adherence to each dietary recommendation was graded on a continuous scale from 0 to 1 (Fig. 1). One point was given for intakes within the NNR (perfect adherence). Zero points (non-adherence) were given for intakes below and above the extreme cut-off points, defined as the median of the ten lowest or ten highest intakes in the study population (Table 1). For intermediate degrees of adherence, a proportional score between 0 and 1 was calculated according to equations (1) and (2) in Fig. 1; accordingly, the score approaches 1 when intake is close to the NNR and 0 when intake is far from the NNR. Due to high activity levels in the study population, adherence cut-off points for physical activity were set at ≥60 min/d for perfect adherence and ≤30 min/d for non-adherence, and a proportional score was calculated for intermediate adherence according to equation (1) in Fig. 1.
Within variables that included several individual recommendations (fat, carbohydrates, vitamins and minerals), the individual scores were summed and divided by the number of recommendations included; thus, each of the nine main variables was given equal weight in the final score. The scores of the main variables were summed into a total adherence score ranging from 0 to 9 points. The score was categorized using the following cut-off points (chosen in order to attain sufficient numbers of participants in each group while avoiding too narrow intervals): ≤6·7 points for low adherence; 6·7–7·6 points for medium adherence; and >7·6 points for high adherence.
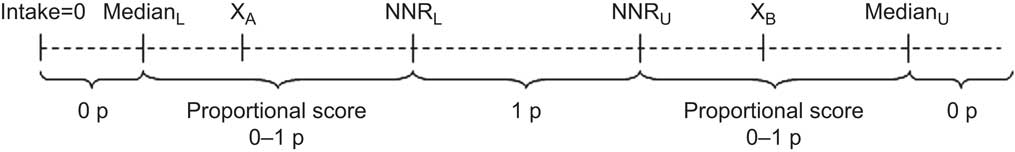
Fig. 1 Schematic illustration of adherence score calculation for each dietary component of the Nordic Nutrition Recommendations (NNR). The dashed line represents the intake range. NNRL and NNRU are respectively the lower and upper recommendation cut-off points defined in the NNR; for intakes within these levels, 1 point (1 p) was accredited (perfect adherence). MedianL and MedianU are respectively the lower and upper extreme cut-off points, defined as the median among the ten lowest and ten highest intakes in the study population; for intakes outside the median cut-off points, 0 points (0 p) were accredited (non-adherence). A proportional score between 0 and 1 (0–1 p) was calculated for intakes between the NNR and the median cut-off points (intermediate adherence) according to equations (1) and (2) below. XA and XB represent actual intake levels within the proportional score range. For lower limits, the score varies from 0 to 1:
![\[--><$$> {\rm{\tf="Helv_R" Proportional}}\,{\rm{\tf="Helv_R" score}} \,{\tf="Helv_R" =}\, {\rm{\tf="Helv_R"(}}{{{\rm{\tf="Helv_R"X}}}_{\rm{\tf="Helv_R"A}}}{\rm{ \tf="Helv_R"- Media}}{{{\rm{\tf="Helv_R"n}}}_{\rm{\tf="Helv_R"L}}}{\rm{\tf="Helv_R"){\tf="Helv_R"/}(\tf="Helv_R"NN}}{{{\rm{\tf="Helv_R"R}}}_{\rm{\tf="Helv_R"L}}}{\rm{ \tf="Helv_R"- Media}}{{{\rm{\tf="Helv_R"n}}}_{\rm{\tf="Helv_R"L}}}{\rm{)}} \eqno\rm<$$><!--\]](https://static.cambridge.org/binary/version/id/?pub-status=live)
For upper limits, the score varies from 1 to 0:
![\[--><$$> {\rm{\tf="Helv_R"Proportional}}\,{\rm{\tf="Helv_R"score}} \,{\tf="Helv_R"=}\, {\tf="Helv_R"1}{\rm{ \tf="Helv_R"- [\tf="Helv_R"(}}{{{\rm{\tf="Helv_R"X}}}_{\rm{\tf="Helv_R"B}}}{\rm{ \tf="Helv_R"- NN}}{{{\rm{\tf="Helv_R"R}}}_{\rm{\tf="Helv_R"U}}}{\rm{\tf="Helv_R"){\tf="Helv_R"/}(Media}}{{{\rm{\tf="Helv_R"n}}}_{\rm{\tf="Helv_R"U}}}{\rm{ \tf="Helv_R"- NN}}{{{\rm{\tf="Helv_R"R}}}_{\rm{\tf="Helv_R"U}}}{\rm{\tf="Helv_R")]}} \eqno\rm<$$><!--\]](https://static.cambridge.org/binary/version/id/?pub-status=live)
Assessment of genetic risk score
The analysis of genetic data in CAPS has been described elsewhere( Reference Lindmark, Zheng and Wiklund 24 ). Altogether thirty-six SNP have previously been associated with PC risk( Reference Gronberg, Aly and Wiklund 30 ). Based on thirty-four of these SNP, we created a genetic risk score by taking the ratio of the number of risk alleles for each individual to the total number of alleles successfully analysed in that individual. The score ranged between 0 and 1 and was categorized as low, medium and high genetic risk using approximate tertiles among the controls. Regarding the included SNP we refer to Table 2 in Grönberg et al. ( Reference Gronberg, Aly and Wiklund 30 ), excluding rs12543663, rs1016343 and rs13252298 in the chromosomal region 8q24, and adding rs4054823 in the 17p12 region.
Table 2 Characteristics of participants by disease status in the Cancer of the Prostate in Sweden (CAPS) study (n 2326)
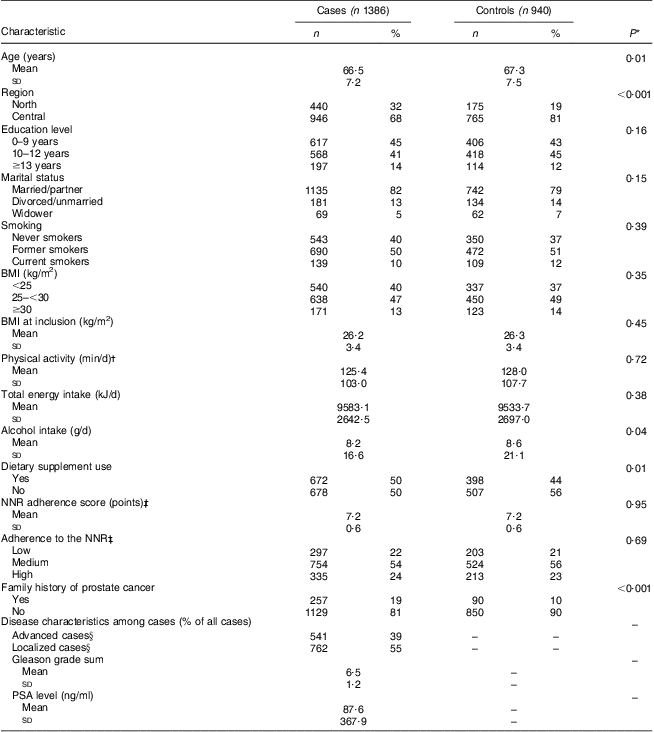
NNR, Nordic Nutrition Recommendations; PSA, prostate-specific antigen.
*P values were obtained by the χ 2 test for categorical variables and the Wilcoxon–Mann–Whitney test for continuous variables. P < 0·05 was considered statistically significant.
†Activity of at least moderate intensity.
‡Adherence to the NNR score, range 0–9 points: low, ≤6·7 points; medium, 6·7–7·6 points; high, >7·6 points.
§Advanced cases: tumour stage T3/T4 or N1 or M1; or Gleason sum ≥8; or PSA ≥100 ng/ml. Localized cases: not meeting any of the aforementioned criteria. Percentage values do not add up to 100 % due to missing disease characteristics data on eighty-three cases.
Statistical methods
Participants with incomplete dietary data (n 67) or unreasonable energy intakes (<3300 kJ/d or >21 000 kJ/d; n 27) were excluded from analyses. Participants with partly missing data for physical activity, except those reaching at least 60 min of daily moderate and/or vigorous activity in spite of their missing data, were also excluded (n 264). The final analyses included 2326 participants (1386 cases and 940 controls). For gene–diet interaction analyses, due to missing genetic information the total data set was limited to 1950 participants (1220 cases and 730 controls). Differences between baseline characteristics of cases and controls were compared using the Wilcoxon–Mann–Whitney test for continuous variables and the χ 2 test for categorical variables. To investigate potential selection bias, comparison of baseline characteristics was performed both for those included in and excluded from the analyses. Moreover, to evaluate the quality of the NNR score we estimated Pearson correlation coefficients between the total score, its individual components and energy intake.
The association between PC and NNR adherence was estimated using unconditional logistic regression, generating odds ratios and 95 % confidence intervals with indicator variables for low, medium and high adherence. The total score was also modelled continuously to assess the effect of a 1-point increment. To evaluate the individual effects of the NNR score components, we modelled each individual recommendation using absolute intake cut-off points for categorization into low, medium and high intake. Ca is likely to be associated with PC and was included in the analysis. However, no other vitamins or minerals were analysed separately as the large number of single nutrients would have increased the risk of chance findings. We also modelled each of the nine main variables categorized into two or three adherence levels, depending on how evenly the score was distributed. To illustrate the shape of the exposure–disease relationship by a smoothed function of adherence, we fitted restricted cubic splines for the continuous NNR score and for each score component. Analyses were performed for total PC and for advanced and localized tumours separately.
All models were adjusted for the matching factors age and region. Multivariate models also included education, smoking, BMI, total energy intake and family history of PC. Selection of potential confounders to include was based on subject matter knowledge and on the change in β coefficients (≥10 %). The following covariates were considered as potential confounders but not included in the final model: employment status, marital status, coffee intake, fatty fish intake, phyto-oestrogen intake, snuff use and dietary supplement use. Potential effect measure modification by a priori selected covariates (genetic risk score, family history of PC, BMI, smoking and dietary supplement use) was assessed by including multiplicative interaction terms in logistic regression models as well as by stratification using interaction indicator variables.
Wald tests and likelihood ratio tests were used to assess the statistical significance of observed effects (P < 0·05, two-sided tests). The Hosmer–Lemeshow and the Pearson χ 2 goodness-of-fit tests were performed to assess model fit. Analyses were performed using the statistical software packages SAS version 9·2 (SAS Institute Inc., Cary, NC, USA) and STATA version 12 (StataCorp LP, College Station, TX, USA).
Results
Table 1 presents the mean absolute intakes and percentage of adherence to each individual recommendation of the NNR in cases and controls. The mean intakes did not reach the recommended levels for SFA, PUFA, sugar, vitamins D and E, Se and Na, resulting in low adherence to these recommendations. As an example, <7 % of the participants followed the recommendations for SFA, sugar and Na. In contrast, almost all participants reached the recommended levels for vitamin B12 and P, and >90 % were within recommended intakes of protein, vitamins A, B1, B2 and B6, Ca, Mg, Fe, Zn and alcohol. Pearson correlation coefficients between the total NNR score and its components ranged from r = 0·01 (Na) to r = 0·64 (e.g. physical activity r = 0·64, fibre r = 0·49, fat r = 0·39; data not shown). Correlations between each individual component ranged from r = 0·46 (fibre v. vitamins) to r = 0·50 (fat v. carbohydrates), and correlations with energy intake were in the range r = 0·02–0·17 (data not shown).
Compared with controls, cases were younger, were more likely to reside in the north of Sweden and to take dietary supplements, had a lower alcohol intake and more frequently had a family history of PC (Table 2). There were no major differences with regard to BMI, education level, marital status, smoking status, total energy intake or physical activity. Participants excluded from the analyses were more likely to be controls (56 %) than the included participants (40 %; data not shown). Among the excluded participants, cases were somewhat older, more likely to reside in the central parts of Sweden and to take dietary supplements, more frequently had a family history of PC, were to a higher extent living alone/widowers and current/former smokers, and had higher energy and alcohol intakes (data not shown). The mean NNR score and the distribution between the adherence groups were similar in cases and controls. The total mean score was 7·2 (sd 0·6, range 4·1–8·6); 22 % of the participants had low NNR adherence, 55 % had medium adherence and 24 % had high adherence.
As illustrated in Fig. 2, we found no statistically significant association between the NNR and PC, but a suggestive increased relative risk for localized disease. There was no evidence of association between the continuous NNR score and PC, nor in restricted cubic spline models (data not shown).
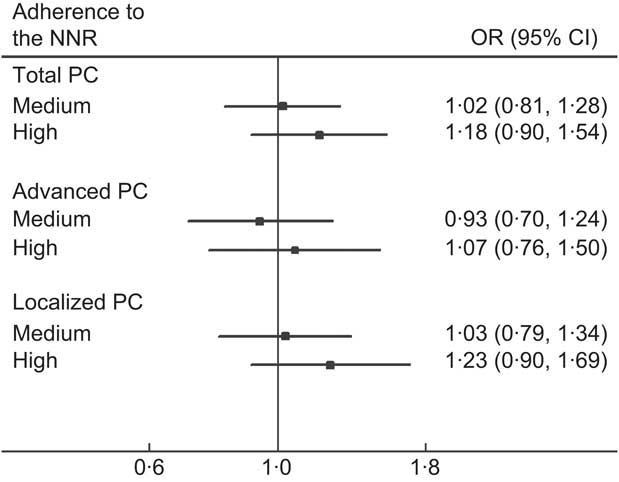
Fig. 2 Adherence to the Nordic Nutrition Recommendations (NNR) and relative risk of prostate cancer (PC) in the Cancer of the Prostate in Sweden (CAPS) study (n 2233), by disease subtype. Adherence to the NNR, range 0–9 points: low adherence (reference group), ≤6·7 points; medium adherence, 6·7–7·6 points; high adherence, >7·6 points. Advanced PC: tumour stage T3/T4 or N1 or M1; or Gleason sum ≥8; or prostate-specific antigen ≥100 ng/ml. Localized PC: tumours meeting none of the aforementioned criteria. Multivariate OR and 95 % CI (shown by horizontal bars) derived from unconditional logistic regression adjusted for age (in 5-year intervals), region (north; central), education (0–9 years; 10–12 years; ≥13 years), smoking status (never; former; current), BMI (quartile distribution of controls), energy intake (quartile distribution of controls) and family history of PC (yes; no). P for trend was not significant for any subgroup (P > 0·05). No major differences between simple and multivariate models were observed
Among individual NNR score components, we observed a 65 % increased relative risk of localized disease for high v. low intake of PUFA (OR = 1·65, 95 % CI 1·01, 2·69; Table 3). There was no statistically significant association with total, advanced or localized PC for any of the other components. Restricted cubic splines on the components did not show any associations, nor did analyses on the individual effects of adherence to each of the nine main variables of the score (data not shown).
Table 3 Individual components of the Nordic Nutrition Recommendations (NNR) score and relative risk of prostate cancer (PC), by disease subtype, in the Cancer of the Prostate in Sweden (CAPS) study
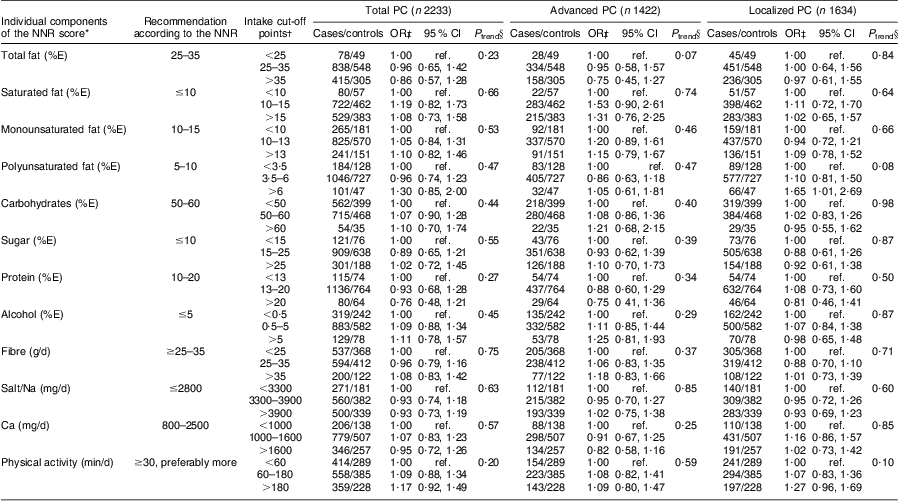
%E, percentage of total energy intake; ref. referent category.
*Vitamins and minerals were not included in the analyses due to the large number of individual nutrients that could increase the risk of chance findings.
†Cut-off points were chosen to mirror the NNR levels as close as possible while retaining enough participants in each group. Higher cut-off points were chosen for physical activity due to a right-skewed distribution in the study population. Low intake was chosen as reference for all components, since few participants were within recommended intakes for several components.
‡Multivariate-adjusted OR and 95 % CI derived from unconditional logistic regression. Adjusted for age (in 5-year intervals), region (north/central), education (0–9 years; 10–12 years; ≥13 years), smoking status (never; former; current), BMI (quartile distribution of controls), energy intake (quartile distribution of controls) and family history of PC (yes; no). No major differences between simple and multivariate models were observed.
§P for trend per 1-step increment across intake or activity categories.
Formal interaction tests showed no statistically significant gene–diet interaction (Table 4). However, stratification by indicator variables showed an increased relative risk of PC for medium (OR = 1·39, 95 % CI 0·92, 2·08) and high (OR = 1·91, 95 % CI 1·15, 3·19) compared with low NNR adherence among participants with a high genetic risk score. No association was seen in those with a medium or low genetic risk score. We observed no statistically significant interaction for family history of PC, BMI, smoking or dietary supplement use. However, stratification by indicator variables showed non-significant positive associations between NNR adherence and malignancy among men with a family history of PC, obese men and former smokers, but not among men with no family history, BMI < 30 kg/m2 or never/current smokers. When separating advanced and localized disease, the results were similar to those for total PC but overall stronger for localized PC (data not shown).
Table 4 Adherence to the Nordic Nutrition Recommendations (NNR) and prostate cancer (PC) risk, stratified by genetic and lifestyle factors, in the Cancer of the Prostate in Sweden (CAPS) study

ref., referent category.
*Adherence to the NNR score, range 0–9 points: low, ≤6·7 points; medium, 6·7–7·6 points; high, >7·6 points.
†Multivariate-adjusted OR and 95 % CI for total PC derived from logistic regression models using interaction indicator variables. Adjusted for age (in 5-year intervals), region (north; central), education (0–9 years; 10–12 years; ≥13 years), energy intake (quartile distribution of controls), BMI (quartile distribution of controls), smoking (never; former; current) and family history of PC (yes; no). No major differences between simple and multivariate models were observed. Results for advanced and localized PC were similar as for total PC.
‡Genetic risk score (range 0–1) was categorized based on approximate tertiles among the controls: low risk, ≤0·40; medium risk, 0·40–0·46; high risk, >0·46. The score was based on thirty-four SNP previously associated with PC risk and was calculated as the ratio of the number of risk alleles for each individual to the total number of alleles successfully analysed in that individual.
Discussion
We found no overall association between the NNR and PC risk in this Swedish study of men with generally good adherence to the NNR. Among individual components of the NNR score, a high intake of PUFA was associated with an increased relative risk of localized PC. A non-significant interaction with the genetic risk score was suggested.
Our findings are in line with several previous studies showing no association between overall diet and PC risk( Reference Jackson, Walker and Simpson 31 – Reference Wu, Hu and Willett 33 ). However, two case–control studies have reported a positive association for empirically derived dietary patterns composed of red and processed meat, refined grains, and processed food and beverages( Reference Ambrosini, Fritschi and de Klerk 34 , Reference Walker, Aronson and King 35 ). No previous study has investigated the relationship between dietary recommendations and PC, but among studies looking at overall cancer, some have found an inverse association( Reference Dixon, Subar and Peters 36 – Reference Reedy, Mitrou and Krebs-Smith 40 ) whereas others did not see an association( Reference Estaquio, Castetbon and Kesse-Guyot 41 – Reference von Ruesten, Illner and Buijsse 45 ).
A plausible explanation for the observed lack of an association between the NNR and PC is the overall good dietary habits of the study population. The exposure distribution is narrow and skewed towards high adherence scores; hence the small differences between adherence groups may have blunted any potential association with PC. To detect a weak association between overall diet and cancer, a large proportion of participants with an informative distribution of the dietary score is needed, which may be difficult to achieve in relatively well-nourished populations such as the Swedish. Also, the main goal of the NNR is to set adequate levels of nutrient intake sufficient for normal growth and function, and these levels may be too low to convey a cancer protective effect. Moreover, the suggestive increased relative risk of localized PC may be explained by the fact that health-conscious individuals are both more likely to eat healthily and to undergo an early diagnosis of PC compared with less health-conscious individuals, which may lead to an overestimation of the risk of early-diagnosed localized disease.
The results for individual score components suggest that a high intake of PUFA, although largely within recommended levels, may increase the risk of localized PC. High intakes of total fat, SFA and the polyunsaturated α-linolenic acid have previously been shown to increase PC risk( Reference Kolonel 15 , Reference Leitzmann, Stampfer and Michaud 16 ). However, our results may be biased by high diagnostic intensity among health-conscious localized cases, as described above. Low statistical power also increases the likelihood of a false positive finding. Regarding Ca, it has been proposed as a probable risk factor for PC, displaying a dose–response relationship with effects seen at doses of 1·5 g/d( 12 , Reference Giovannucci, Liu and Stampfer 46 ). However, comparing the highest (>1·6 g/d) with the lowest (<1·0 g/d) group of Ca intake in the CAPS study, we observed no association with PC.
No overall gene–diet interaction in relation to PC was found. However, stratified analysis suggested a positive association between the NNR and PC only among participants with a high genetic risk score. Previous evidence implies that gene–diet interactions may be involved in PC development. For instance, the relationships between PC risk and dietary intake of fatty fish( Reference Hedelin, Chang and Wiklund 18 , Reference Reese, Fradet and Witte 20 ), phyto-oestrogens( Reference Hedelin, Balter and Chang 17 ) and antioxidants( Reference Li, Kantoff and Giovannucci 19 , Reference Steinbrecher, Rohrmann and Timofeeva 21 ) seem to be modified by genetic polymorphisms. Nevertheless, our analyses are explorative and the results should be interpreted with caution since residual confounding may exist within strata and the results are based on few participants due to narrow distributions of both the NNR score and the genetic risk score.
An interaction between diet and BMI is biologically plausible as changes in body composition lead to metabolic changes potentially affecting the bodily response to dietary factors. Overweight and obesity affect e.g. levels of insulin, insulin-like growth factor 1 and sex-hormone binding globulin, hormones possibly associated with PC risk( Reference Freedland and Platz 47 – Reference Roddam, Allen and Appleby 49 ). In the current study, obese participants had a suggestive but non-significant increase in PC risk with higher compared v. lower NNR adherence. We need to consider that under-reporting of energy intake is generally more common among obese than non-obese individuals( Reference Nielsen, Nielsen and Toubro 50 , Reference Willett 51 ), which is indicated in our study by a lower reported energy intake among obese compared with non-obese men. However, the reported energy intake was similar between cases and controls in all BMI categories, so any potential exposure misclassification is likely to be non-differential, causing a dilution of effect. As regards smoking, we found no formal interaction, although a positive association between the NNR and PC was suggested among former smokers. A previous study found that a high dairy intake reduced the risk of PC, especially aggressive disease, among current but not former smokers( Reference Neuhouser, Barnett and Kristal 52 ). The results on interaction with BMI, smoking and family history of PC should be cautiously interpreted due to lack of statistical significance. Regarding dietary supplement use, a higher proportion of supplement users was recently reported among PC cases compared with population controls, particularly among men with a healthy dietary pattern( Reference Westerlund, Steineck and Balter 26 ). However, no interaction with use of dietary supplements was shown in our data.
Strengths of our study include the population-based design, large sample size, complete and rapid case ascertainment, and information on PC subtypes. The CAPS study includes mainly non-PSA detected cases due to the low level of PSA testing at the time of enrolment( Reference Hedelin, Klint and Chang 23 ), resulting in cases with clinically relevant disease. The study population is ethnically homogeneous, which reduces the risk of confounding bias by population stratification. In addition, an extensive questionnaire provided detailed exposure information and the possibility to adjust for numerous demographic, anthropometric and lifestyle factors. No major differences were observed between simple and multivariate analyses.
The current findings should also be interpreted in light of potential limitations. Case–control studies are limited by possible selection bias due to cases being more prone to participate than eligible controls. The study has a high response rate; however, a number of participants were excluded from the analyses due to missing data. Since the included and excluded participants differed in some baseline characteristics and the proportion of controls was higher among the latter, our results may potentially be biased by selection of health-conscious controls. Furthermore, although the CAPS questionnaire has been validated, we nevertheless cannot rule out potential measurement error as FFQ commonly underestimate the intake of energy and nutrients( Reference Willett 51 ). The reported energy intake differs little between cases and controls, so any potential bias is likely to have diluted our results. Besides, the use of energy-adjusted nutrient intakes minimizes the influence of such measurement error. Recall bias may occur, although a previous study comparing original and repeated dietary recall interviews found no overall difference in recall between PC cases and non-cases( Reference Wilkens, Hankin and Yoshizawa 53 ). The likelihood that reverse causation may have affected our results is small since studies show that men, particularly those ≥60 years, tend not to change their dietary habits, not even after a cancer diagnosis( Reference Mroz, Chapman and Oliffe 54 , Reference Patterson, Neuhouser and Hedderson 55 ).
Another potential drawback is misclassification of exposure due to the scoring method used, especially since categorization was made in two steps. To minimize such a potential effect we tested different scoring models, all yielding similar results. Initially we used a categorical 1–3 scoring system, accrediting 3 points for intakes within the NNR, 2 points for intakes <20 % from the NNR and 1 point for intakes >20 % from the NNR. We chose the continuous 0–1 scoring system as it was considered less subject to misclassification due to arbitrary categorizations than the categorical system. Reducing or increasing the number of adherence categories did not change the results substantially. Since the NNR score is based on externally defined cut-off values, the intake level in the study population may for certain score components be below or above the predefined cut-off point, resulting in reduced discriminating power for those components. In the CAPS population, adherence to the SFA, sugar and salt recommendations was extremely low, whereas almost all participants were within recommended intakes of protein, alcohol and several micronutrients. Moreover, strong inter-correlations between components of a dietary score may also influence the discriminative power of each component and their relative contribution to the total score. The correlations with the NNR score and the inter-correlations between components were low to moderate, so that no individual component was driving the score. We also tested different ways of grouping the macro- and micronutrients within the total score, and seeing no major differences in the results, we decided to hold fat, protein and carbohydrates separate, as well as vitamins and minerals, each giving equal weight to the total score. Furthermore, different NNR scoring models have previously been tested by Fondell et al. ( Reference Fondell, Christensen and Balter 4 ), showing no substantial differences. In summary, we believe that any exposure misclassification due to the scoring method may have influenced our results only marginally.
We recognize the potential problem of over-controlling for factors that may themselves contribute to the dietary score, such as total energy intake. However, the NNR score and its components did not correlate with energy intake, and when modelling the main effect with and without this variable, no substantial change in the estimates occurred.
Conclusions
We found no support for an association between NNR adherence and PC in the Swedish CAPS study. The hypothesis of a potential gene–diet interaction in the aetiology of PC is unclear and deserves further attention in other study populations. We also encourage continued evaluations of dietary recommendations, such as the NNR, in relation to health status.
Acknowledgements
The current study was funded by the Swedish Cancer Society (Grant #08 0470). The authors declare no conflicts of interest. E.M. planned the study, analysed the data and wrote the manuscript. C.G., R.B., T.M.-L.A. and L.A.M. participated in planning of the study and the data analysis. J.A., H.G., H.-O.A. and K.B. were involved in the design and conducting of the CAPS study. K.B. obtained the funding, planned and supervised the study. All authors participated in critical revision of the manuscript and approved the final version. The authors thank Maria Hedelin for preparation of nutrient analyses; Elinor Fondell for collaboration on the NNR score; Fredrik Wiklund for creating the genetic risk score; and the CAPS study participants, the Regional Cancer Registries, and all urologists and other persons involved in the planning, coordination and data collection of the CAPS study. This paper was presented in part at the 11th European Nutrition Conference, Madrid, Spain, 26–29 October 2011, and published (in part) in: 11th European Nutrition Conference (FENS). Ann Nutr Metab (2011) 58, Suppl. 3, 273-274. Permission to publish these parts has been granted by S. Karger AG, Basel, Switzerland.








