Introduction
As the world population continues to grow, the demand for livestock and livestock products also rises, resulting in further increases in large-scale intensive livestock production to meet this increased demand. Accompanying this intensification in many countries is a rise in the use of antibiotics in the production system. This is particularly the case for emerging economies, particularly large animal-producing countries. For most Organisation for Economic Co-operation and Development (OECD) countries, however, the use of antimicrobials in livestock production is falling, as the traditional livestock production systems evolve and alternative approaches to disease management are adopted.
The growing resistance of microbes to the commonly used antimicrobials is a serious concern for human and animal health and, thus, for policy-makers in many countries. Moreover, it also raises important questions in relation to food safety, food security, trade and market access for livestock and livestock products. Globally, antibiotics are widely used in livestock production for a range of purposes, with the bulk used in the high-density intensive livestock production systems. The global antimicrobial use (AMU) in livestock can be divided into therapeutic, metaphylactic, prophylactic, and growth promotion. Antibiotic use is strictly under veterinary prescription in most OECD countries, but in many parts of the world veterinary drugs are available over the counter in pharmaceutical stores. In low-income countries, weaknesses in the legislative and veterinary infrastructure often present a challenge to the regulation of the access to veterinary drugs. The World Organisation for Animal Health (OIE) has worked to strengthen veterinary services and more recently to encourage global reporting of antimicrobial use in animals (http://www.oie.int/scientific-expertise/veterinary-products/antimicrobials).
Therapeutic use refers to the use of antibiotics to treat clinically diseased animals; whereas metaphylactic use involves treatment of entire groups of animals when some individuals in the group are diseased to avoid further spread of the infection. Prophylactic use is generally defined as preventive antibiotic use to avoid clinical problems (e.g. at a certain stage in the production cycle). Antimicrobial growth promotion means regular inclusion of subtherapeutic doses of antibiotics in feed with the aim of improving the feed conversion and growth rate of the animals. Antibiotics are often used as a regular and systematic input in intensive livestock production, as the productivity and financial benefits are perceived to outweigh the costs. They therefore have important implications for output, which, in turn, affects commodity markets and trade in livestock and livestock products.
The use of antibiotics in animal production has important implications not only for animal health and welfare, but also for food safety and food security at the global level. While comprehensive information and data on the productivity impact of antibiotics are sparse, data collection is improving as more resources are being employed in many countries to monitor AMU, as well as the growth and health impacts of this use. While antimicrobials are an important input in disease management in some modern livestock production systems, their use inevitably results in selection for antimicrobial resistance (AMR), which raises serious concerns that need to be addressed. In addition to the excessive AMU, other drivers, such as antimicrobial waste from farms or manufacturers, may also contribute to the rise in AMR. Besides AMU, other factors, such as the use of heavy metals (such as zinc oxide given to prevent weaning diarrhoea in piglets), may result in co-selection of resistance traits. In practice, an increasing number of studies have shown that AMU in humans (Charbonneau et al., Reference Charbonneau, Parienti and Thibon2006; Costelloe et al., Reference Costelloe, Metcalfe and Lovering2010; Sun, Klein & Laxminarayan, 2012) is the main driver for AMR in human bacteria, whereas AMU in animals (Burow et al., 2013; Hammerum et al., Reference Hammerum, Larsen and Andersen2014; Simoneit, Burow & Tenhagen, Reference Simoneit, Burow and Tenhagen2015; Chantziaras et al., Reference Chantziaras, Boyen and Callens2014) is the main driver for the development of AMR in animal bacteria. Yet there is also evidence for spill over of resistance from animals to humans and vice versa (Cabello, Reference Cabello2006; Crombé et al., Reference Crombé, Argudín and Vanderhaeghen2013; Liu et al., Reference Liu, Wang and Walsh2016; Madec et al., Reference Madec, Haenni and Nordmann2017).
Limiting the use of antimicrobials in livestock production is a challenge due to different regulatory systems, definitional issues, measurement methods, surveillance and monitoring challenges. Moreover, there is growing debate on the perceived short-term private benefits of AMU, primarily to livestock producers, versus the longer-term social costs of AMR on human health, the environment, animal health, and food production. At the global level, estimates have been made of the potential economic costs associated with the rise in AMR in the medium to long run (Laxminarayan, Van Boeckel & Teillant, 2015; Adeyi et al., Reference Adeyi, Baris and Jonas2017). Most of these estimates only relate to the impact on human health, with little empirical evidence of the impact on livestock production and, consequently on food supplies. Nonetheless, there are increasing numbers of reports on therapy failures in animal diseases linked to growing resistance levels.
This chapter reviews the current situation in terms of antibiotic use in modern livestock production. The core issues related to the impact of antibiotics in treating disease outbreaks and the productivity affects are explored. Moreover, the problems faced in measuring AMU and AMR continue to be rather contentious due to the lack of a harmonized global approach. The complexity of the transmission pathways between animals, the environment and humans remains a challenge to the better understanding of the means and speed of transmission of resistant pathogens and how long these pathogens remain viable in the environment. The final section identifies some pragmatic interventions that have been successfully adopted at the farm level to reduce AMU.
Current state of knowledge on antimicrobial use and antimicrobial resistance in livestock production and the food-chain
AMU and AMR in livestock production
Addressing the issue of the use of antimicrobials in meat production is complex because they are used to achieve both a health and a productivity benefit in livestock-producing farms. The multipurpose objectives of AMU in agriculture include therapeutic, metaphylactic, prophylactic use, and use for growth promotion. Of these, antimicrobial growth promoters (AGP) are clearly nontherapeutic while prophylactic and metaphylactic use falls somewhere in between nontherapeutic and therapeutic use. Some animal categories in intensive livestock production are particularly susceptible to infections, and although routine antibiotic treatment of such animal groups should be classified as prophylactic (or metaphylactic) use, it is often regarded as therapeutic, demonstrating the challenges when using these definitions in policy-making.
In some management systems, post-weaning diarrhoea in piglets has been regarded as inevitable without routine treatment with antibiotics, but improved management has proven that this disease can be prevented. Some animal production systems have a turnover rate that presents a challenge for prophylactic tools such as vaccines because of the time needed to develop an effective immune response. Metaphylactic use of ionophore antibiotics to prevent coccidiosis has been routinely applied in broiler production in large parts of the world. New vaccines and management optimization has allowed for a substantial reduction of this use in many countries. However, although ionophores are mainly used to prevent a parasitic infection (coccidiosis) they also prevent necrotic enteritis in poultry (a multifactorial disease induced by the presence of the bacterium Clostridium perfringens) and both of these diseases must be managed for a successful reduction of ionophore usage.
While the principal role of AMU in food animals should be therapeutic, in reality use has been substantially driven by the objective of improving farm productivity and income. Evaluating the impacts of antibiotics on animal productivity is difficult due to the relatively limited number of studies on the different food animal species. High antibiotic use is often related to poor management or health failures on the farm. The key question is whether the use of antibiotics could be replaced by better husbandry, management standards and production systems, and at what cost? Historically, the use of antibiotics in livestock production has been closely correlated to the size of the livestock population and the intensity of the production system in a country. Highly intensive animal production systems have tended to use more antibiotics than less intensive systems. However, during recent decades the sector has seen the rapid development of intensive production systems with higher biosecurity measures, improved husbandry and management, which together have led to a reduction in AMU in many countries (Postma et al., Reference Postma, Backhans and Collineau2016; Laanen et al., Reference Laanen, Maes and Hendriksen2014).
A 2012 study noted that over two fifths of all feedlot cattle and over four fifths of hogs in the USA were given antibiotics in their feed rations (Landers et al., Reference Landers, Cohen and Wittum2012). Another study estimated that food and agriculture production accounts for the bulk of antimicrobials consumed in the USA, estimated at over 70% of total consumption (Laxminarayan, Van Boeckel & Teillant, Reference Laxminarayan, Van Boeckel and Teillant2015). The study also estimated the global volume of antibiotics consumed in agriculture at 63 000 tonnes in 2010, and noted that this would rise to 106 000 tonnes by 2030 if no changes are made in the use of antibiotics. The authors attributed two thirds of this increase to a rise in the number of food animals and one third to more intensive livestock production systems. It also noted that four countries, namely China, the United States, India and Brazil, account for almost 50% of total global consumption, and that this would remain unchanged in the coming decade.
The study concluded that the consumption of antibiotics is closely related to the livestock population, with the highest consumption in countries which have a high concentration of industrial pig, poultry and cattle enterprises. These projections assume that no changes are introduced in the way antibiotics are used in animal production in the near future. However, several European countries have recently shown that very substantial decreases in use can be achieved in a short period of time without negative effects on animal health and production as long as they are accompanied by improved management and biosecurity practices. Also, the emergence of private initiatives and labels such as “No antibiotics ever” may have led to a decrease in antibiotic use in the US broiler chicken industry and a fall in the total consumption of antimicrobials. In several large animal-producing Asian countries important policy changes have recently been made regarding the use of antimicrobial growth promoters. Therefore, it is reasonable to expect that the predictions referred to above may be unduly pessimistic.
Aquaculture is one of the fastest growing food production sectors, and is regarded as an important part of the solution to global food insecurity. However, similar problems with infectious diseases and overuse of antibiotics, as seen in intensive terrestrial animal production systems, have been experienced in aquaculture. Prophylactic use of antimicrobials is common in many regions (Cabello, Reference Cabello2006; Done, Venkatesan & Halden, 2015). However, in aquaculture there has been some success in reducing antimicrobial use by the use of preventive measures such as vaccination; for example, the aeromonas vaccine in salmon production (Gulla et al., Reference Gulla, Duodu and Nilsen2016). Figure 5.1 summarizes the pathways of transmission of resistant bacteria in the environment.

Figure 5.1 Summary of the pathways of transmission of resistant bacteria between animals, humans and the environment
Note: The above image depicts the pathways of transmission of resistant bacteria between animals, humans and the environment. Such as; dissemination through water sanitation systems (1), the application of manure to fields with cultivated crops (2), which then leads to antibiotic-resistant bacteria developing on plants (3). The uptake of resistant bacteria through the food-chain (4) or within the meat products harbouring resistant bacteria (5). Water distribution systems can also spread resistant bacteria (6). Wildlife, insects and other bugs are also carriers of resistant bacteria (7). Lastly, tourism, migrations and trade (8) are drivers of spreading resistant bacteria across borders.
There is growing alarm at the rise in AMR and the potential consequences for food production, animal and human health. The use, overuse and misuse of antibiotics drives an increase in AMR and gives rise to serious technical difficulties when treating animal diseases. In food animal production, the rise in AMR not only increases the risk of animal mortality, but the inability to treat resistant infections also reduces animal performance, thus reducing the economic returns in agriculture and the food system, and potentially higher food prices for consumers. Current research indicates that livestock production accounts for more than two thirds of global antibiotics consumption. However, with the implementation of the WHO’s Global Action Plan on Antimicrobial Resistance (World Health Organization, Reference Organization2015), and the greater global awareness of the risks associated with increasing AMR, the use of antibiotics is likely to decline in the coming years. A more prudent approach to antibiotics consumption is necessary to slow the pace at which resistance develops.
Productivity gains appear to be declining
Prevention and management of animal diseases are critical in modern livestock production. An outbreak of an infectious disease on the farm not only reduces productivity and output, but also increases the costs of treating the animals. In modern and sustainable livestock production the focus should be on disease prevention. Only if this fails should antimicrobials be used. The productivity impact arising from AMU varies substantially by species and stage of growth of each species. And, as the productivity gain from AMU varies with health status and management, cost–benefit estimates are needed that take into account long-term costs of AMR to producers.
Box 5.2 Examples of responses to antibiotic reduction
The Netherlands and Belgium have recently reduced their antibiotic use in animal production substantially and demonstrated that this can have an almost immediate effect on lowering the levels of resistance in animal production (Dorado-García et al., Reference Dorado-García, Mevius and Jacobs2016; Callens et al., Reference Callens, Cargnel and Sarrazin2017). However, for some resistance traits, the process is more complex. If resistance involves a “cost” for the bacteria, i.e. slows their growth, or is located on a genetic element that is easily lost, removal of the selective pressure exerted by AMU will lead to loss of the resistance. If the resistance trait does not impair bacterial growth, or is linked to other genes that are needed in the current microenvironment, resistant bacteria will not be at a disadvantage when the selective pressure is removed and resistance may remain at high levels. This illustrates that although resistance selection is not an irreversible process it must be slowed down immediately and urgent intervention is called for.
Earlier studies on the production impacts of antibiotics given in the feed as growth promoters indicate productivity gains ranging from 1% to double digits, depending on factors such as nutrition, breeding, housing, sanitation, as well as husbandry and management practices. However, recent studies have concluded that the productivity benefits from the routine use of low levels of antibiotics in the feed have declined due in part to the adoption of modern production and management practices (Laxminarayan, Van Boeckel & Teillant, 2015). Hence, poor management systems have benefited from the use of AGPs but they should have no place in modern animal production as AMR is promoted by their use.
However, several factors can influence the performance including the species (pigs, poultry, cattle), age of the animal, nutrition, breed, as well as the production system and management practices. There is evidence to suggest that AGPs have no effect when fed to germ-free animals (Swedish Ministry of Agriculture, 1997). The intestinal characteristics of germ-free animals resemble the effects reported from AGP use. It has been proposed that most of the effect of AGPs is due to suppression of intestinal microbes that induce host immune responses that are detrimental to efficient growth (Broom, Reference Broom2017). In management systems with good hygiene and improved animal health, production performance is already optimized and the net benefit of AGPs is very doubtful.
Sweden was the first OECD country (in 1986) to ban the use of antibiotics as a growth promoter in animal feed. This was followed by several other countries and the EU banned the use of antibiotic growth promoters in animal feed for all EU Member States in 2006 (Regulation 1831/2003/EC). In contrast to some expectations, this ban has not resulted in a substantial decline in food animal production in Europe. While the first ban in Sweden led to some initial animal health problems that had to be addressed by improved management and disease prevention (Swedish Ministry of Agriculture, 1997), lessons learnt from the Swedish experience helped other EU/EEA countries to cope with the subsequent union-wide ban that was applied more gradually. In Denmark and the Netherlands a shift towards therapeutic AMU was also observed after the ban of antimicrobial growth promoters. However, this increase turned out to be only temporary and was even non-existent in Norway (Bos et al., Reference Bos, Taverne and van Geijlswijk2013; Grave et al., Reference Grave, Kaldhusdal and Kruse2004; 2006).
In the USA, the use of medically important antibiotics for animal growth promotion has been banned only since 2017. Several other OECD countries which have banned the use of antimicrobials for growth promotion in the last decade include Mexico, New Zealand and the Republic of Korea, while other countries such as Indonesia, Viet Nam, and China have recommended the gradual removal of antibiotics as a growth stimulant.
The Netherlands has implemented clear reduction targets and a range of measures such as a ban on in-feed mixing of antimicrobials, herd level monitoring of use, increased awareness building, and strict regulations on the farmer–veterinarian relationship. This has resulted in a 56% drop in consumption of antibiotics in agriculture between 2007 and 2012, without any serious adverse effects on animal welfare or on the profitability of the farms (Speksnijder et al., Reference Speksnijder, Mevius and Bruschke2015). Countries such as Belgium, France, Germany and the UK have implemented initiatives, including the setting of reduction targets, which also show promising reductions in antibiotic use.
Given the risk related to AMU and resistance selection, several European countries, including Denmark, Sweden, Belgium and the Netherlands, have introduced strict limits on the consumption of antibiotics on livestock farms. This has contributed to a significant fall in antibiotics usage in these countries without a substantial negative impact on production.
These initiatives show that substantial reductions in AMU are possible and that these initiatives should be focused on alternative disease prevention actions (e.g. improving biosecurity and animal husbandry). Limiting the increase in AMR requires a focus on both the demand for antibiotics and the supply of antibiotics for animal use. It is important that, first, the need for antimicrobials is reduced by focusing on disease prevention and improved production. In a second stage, access to necessary antibiotics to treat infectious diseases should be maintained, while at the same time eliminating the overuse and misuse of antibiotics in animal production.
Measuring AMU and AMR in animal production
There are enormous global challenges in measuring AMU in animal production, due to lack of resources, expertise and understanding of the adverse consequences of increasing levels of AMR. These difficulties have limited the availability of reliable and comparable data across species and across countries. As a consequence, international organizations have encouraged the collection of AMU data to manage and minimize the further development of AMR (World Health Organization, Reference Organization2015; World Organisation for Animal Health, 2016). The newly proposed EU Regulation on veterinary medicinal products regulates the collection of data on AMU in Member States and requires that such data should be comparable, compiled on an EU level, and published annually. The OIE, supported by the FAO and WHO within the Tripartite Collaboration on AMR, has taken the lead in building a global database for antimicrobial agents intended for use in animals (OIE, 2018).
While good progress has been made in recent years, the lack of comprehensive data has limited the development of alternative interventions to antibiotics in animal production. Comparable data are needed for benchmarking and assessing interventions to reduce AMU. EU legislation only allows AMU based on veterinary prescription, but sales of antibiotics are not regulated in most parts of the world. Within the EU, some Member States allow veterinary sales of antibiotics while others forbid veterinarians to make a profit from supplying medicines. Data on AMU may be held by various actors, such as the pharmaceutical industry, pharmacies and veterinary clinics. However, the difficulties lie not only in collecting data from multiple sources, but in assessing the actual use in regards to the number of treated animals of different species. Even when prescription data are available, these do not always provide enough detail to account for the exact number of daily doses, mainly due to large differences in body weight between animals of different age categories and different dosages for different routes (and formulae) of administration (Collineau et al., Reference Collineau, Belloc and Stärk2016). The European Surveillance of Veterinary Antimicrobial Consumption project aims to develop a system for collection of data per animal species and to establish technical units of measurement (EMA, n.d.).
Although efforts are made to collect and assess data on AMU in many parts of the world, challenges remain elsewhere. In systems where farmers buy feed without knowing the exact contents, and where antibiotic substances are only listed as “additives” on feed labels, much AMU may go unnoticed. In most parts of South-East Asia, Africa and South America, intensive production systems in general, and aquaculture in particular, there is a lack of specific information on AMU, but it is suspected to be quite substantial (Krishnasamy et al., Reference Krishnasamy, Otte and Silbergeld2015).
When it comes to AMR, the challenges are different. Most countries with ongoing or planned monitoring of AMU already have surveillance for AMR in place, but lack of laboratory capacity is one of the major problems globally. In regions where resources are lacking, available data are scarce, sporadic and usually non-validated. Even in regions where animal producers can afford diagnostics and these are available, data on AMR may be sporadic and difficult to compare when reliant on clinical samples alone. While such samples are valuable, they cannot replace systematic monitoring of AMR in indicator bacteria. This is needed for comparison between production categories, animal species, regions and over time, as a basis for interventions and benchmarking. As an example, in Europe, this type of AMR monitoring of indicator bacteria is undertaken by the European Food Safety Authority (EFSA) and the ECDC, resulting in an annual report on the presence of AMR in zoonotic and commensal bacteria originating from food-producing animals and animal products (EFSA/ECDC, 2018). Passive surveillance of AMR, based on clinical samples alone, will provide some insight into the current clinical problems and certain animal health threats due to AMR, but is less useful than active surveillance for monitoring trends and making comparisons between settings.
Box 5.4 Surveillance of antibiotic consumption and sales data
The US Department of Agriculture (USDA), in close collaboration with the US Food and Drug Administration, has also initiated projects that aim to assess AMU at the farm level, but data collected at the national level are not yet available. In Canada, information on AMU in animals is provided by the Canadian Animal Health Institute on a voluntary basis, based on sales data from companies, while mandatory reporting of sales data and collection of more detailed data on antimicrobial consumption have been proposed in Australia. The European Surveillance of Veterinary Antimicrobial Consumption collects information on how antimicrobials are used in animals across the European Union (EMA, n.d.). The ECDC, in conjunction with the European Medicines Agency (EMA) and the European Food Safety Authority (EFSA), also undertakes joint analysis of the consumption of antimicrobials and the occurrence of antimicrobial resistance in bacteria from humans and food-producing animals (ECDC/EFSA/EMA, 2017). Several countries have initiated sector-driven initiatives on measuring AMU. The recently established AACTINGFootnote 1 network has compiled all currently available systems for measuring AMU in animals at herd level and has identified at least 24 farm-level data collection systems from 15 countries in Europe and Canada.
1 Network on quantification of veterinary antimicrobial usage at herd level and analysis, communication and benchmarking to improve responsible usage (http://www.aacting.org).
Risk assessment aspects of AMR
Risk assessment forms the basis for planned interventions in animal production (risk management). This is not always straightforward. While the association between AMU and AMR is indisputable, it is often difficult to quantify as there are so many factors, such as dose and duration of treatment, route of administration, co- and cross-resistance selection effects, all influencing the direct association between the use of one specific antimicrobial and the rise of one specific type of resistance. However, there is a general agreement that AMU must be reduced in livestock production and, hence, risk assessment could focus on AMU. In order to appreciate the risk of AMR (and AMU), producers must have knowledge and awareness. The levels of these vary and, therefore, the risk profile of producers will differ according to the production system, species, age of the producer, geography, cultural norms and behaviour, as well as the potential economic aspects.
The structure of the production system plays an important role in determining the behaviour of producers with respect to the threats posed by AMR. Producers in the early stages of the animal life-cycle may regard the risks of not using antibiotics as more important than consumer perceptions while producers in the later stages are more dependent on consumer confidence and more directly affected by withdrawal periods and subsequent losses due to treatments. The relationship between knowledge, attitude and behaviour is complex, as illustrated by studies on farmers’ implementation of biosecurity measures (Racicot et al., Reference Racicot, Venne and Durivage2012; Laanen et al., Reference Laanen, Maes and Hendriksen2014; Kristensen et al., Reference Kristensen and Jakobsen2011). As reduction of on-farm AMU is dependent on disease prevention measures, these results are relevant for the issue of AMU reduction.
Most antibiotics are used both in humans and in animals
Research has shown that over 20 of the 27 different classes of antibiotics are used in both animals and humans. There is growing concern in relation to livestock production over the use of last-resort antibiotics for humans, such as colistin, as these are increasingly required for use in humans as AMR spreads. Many drugs that had been discarded for human use due to toxicity issues have been used in animals as growth promoters or as prophylaxis or therapy for enteric infections, and now are coming back as last-resort drugs in human medicine. The earliest example is avoparcin, which was banned as an AGP when selection for vancomycin resistance due to cross-resistance was reported (Swedish Ministry of Agriculture, 1997). Other AGPs that were previously regarded as irrelevant for human medicine but that have been discussed as potential candidates for last-resort drugs are avilamycin and flavomycin.
Resistance to colistin, a polymyxin substance widely used in pig and poultry production, was previously reported exclusively due to chromosomal mutations. In 2015, Chinese investigations into increased prevalence of colistin-resistant Escherichia coli from pigs revealed a resistance gene located on a plasmid (Liu et al., Reference Liu, Wang and Walsh2016). Hence, following the discovery of this transferable colistin resistance, the European Medicines Agency published targets for reduction of colistin use in animals in EU Member States, as well as a reclassification of colistin as a medicine “reserved for treating infections in animals for which no effective alternative treatments exist”. Countries such as China and Brazil have taken targeted measures to reduce the use of colistin. This example demonstrates the gradual transition from regarding AMU in humans and animals as separate, to a realization that this is indeed a One Health issue.
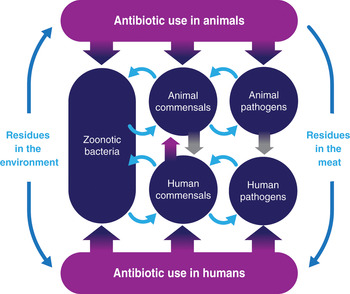
Transmission of AMR between livestock, the environment and humans
In the past there were populations that were extremely isolated, but globalization means that all parts of the world are now interconnected. Humans and animals (domestic animals as well as wildlife) continuously interact, both with each other and with the specific environment or ecosystem they inhabit. Moreover, in many parts of the world antimicrobials are used (in humans and animals), all potentially selecting for AMR.
Therefore, it is clear that the growth of AMR cannot be addressed by simply acting on one element. What happens in human medicine has an impact on the environment and the bacterial flora in animals. Similarly, what happens in veterinary medicine influences the bacterial flora in humans. This becomes even more obvious in ecosystems where intensive farming (of animals and crops) is combined with a dense population, providing the ideal circumstances for a dynamic exchange of bacteria and resistance genes. Figure 5.2 shows the potential routes for the exchange of resistant bacteria between animals and humans, and vice versa. It is important to emphasize that the exchange of resistant traits may go in both directions, from animals to humans as well as from humans to animals.

Figure 5.2 Different routes for exchange of resistant bacteria or genes from animals to humans and vice versa.
In the exchange between animals and humans, three types of transfer mechanisms can be distinguished. First, resistant traits (bacteria or genes) may be transferred between animals and humans through direct contact. It has long been established that farmers and farm workers have higher levels of resistant bacteria than people who do not live close to livestock. Similarly, hospitals can act as a hot spot for AMR, exposing both humans and animals that live nearby.
Companion animals should not be overlooked in the debate concerning transmission of resistance because of their close contact with humans. It is not surprising that an increasing amount of scientific literature describes resistance transmission between companion animals and humans (Pomba et al., Reference Pomba, Rantala and Greko2017).
Resistant traits can also reach humans through the consumption of food that contains resistant bacteria or genes. The most obvious form of foodborne transmission seems to be from the consumption of meat, milk or eggs. Yet if these animal products are heat treated (e.g. cooked or pasteurized), and the required hygienic measures are applied in the kitchen, there should be very little or no transfer of (resistant) bacteria. The consumption of raw products is more risky. Foodborne transmission may also occur as a result of the consumption of vegetables grown in soil fertilized with manure of animal origin, or irrigated with contaminated water. Finally, resistant traits can be spread via waste material contaminating the environment. Water is a particularly efficient and quick route of transmission.
Box 5.5 Animal to human transfer of antibiotic-resistant strains
Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) is frequently found in pigs, and people in contact with pigs, but also in other livestock species (Crombé et al., Reference Crombé, Argudín and Vanderhaeghen2013). Human carriers are typically people in contact with pigs, e.g. farmers, farm workers, veterinarians and slaughterhouse staff. Another example is the identification of shared reservoirs of extended spectrum beta-lactamase Enterobacteriaceae genes between humans and animals (Madec et al., Reference Madec, Haenni and Nordmann2017). Therefore, exposure to animals is regarded as a risk factor and indirect transmission is not unlikely.
Other interventions to reduce antimicrobial use in food animal production
There are numerous other ways to reduce AMU. Some are generically oriented towards disease prevention, such as the improvement of water quality and biosecurity. Others are designed to address specific pathogens, such as specific-pathogen-free (SPF) programmes. Some concern overall management or culture (e.g. benchmarking of veterinarians to raise awareness to differences in culture and traditions when prescribing antimicrobial agents), while others are of a biological nature, such as probiotics or prebiotics, or the use of genetically enhanced breeds that are less susceptible to certain diseases.
In a recent study (Postma et al., Reference Postma, Stärk and Sjölund2015), European veterinarian practitioners active in pig production were asked what they consider to be the most valid alternatives for AMU in pig production, taking into account expected effectiveness, feasibility and return on investment of the measures. Results indicated that practitioners believe the most promising alternatives to AMU are, in order of priority: improved biosecurity, increased and improved vaccination, use of zinc, improved feed quality and improved diagnostics.
In recent years, several studies have found that improved biosecurity may result in reduced AMU, without jeopardizing production results. In a study in breeder–finisher pig herds in Belgium, it was found that herds with higher internal biosecurity scores had lower antimicrobial treatment incidences, suggesting that improved biosecurity might help in reducing the amount of antimicrobials used (Laanen et al., Reference Laanen, Persoons and Ribbens2013). In a French study in breeder–finisher herds, biosecurity measures such as disinfection of the loading area, gilt quarantine and adaptation, farm structure/working lines and all in/all out practices were found to be significantly associated with lower AMU (Lannou et al., Reference Lannou2012). In a recent study in four European countries, it was shown that a higher weaning age, a week system of five weeks or more and the external biosecurity level were significantly associated with a lower antimicrobial treatment incidence (Postma et al., Reference Postma, Backhans and Collineau2016). This finding was confirmed in a study of the profile of top farmers. In this study, the level of internal biosecurity was positively associated with a better control of infectious diseases and a lower need for antimicrobials (Collineau et al., Reference Collineau, Backhans and Dewulf2017a).
In Denmark, measures were implemented by farmers and their veterinarians that managed to reduce their annual antimicrobial consumption by 10% or more following the introduction of the “Yellow Card system”. It was reported, among other parameters, that cleaning procedures, adequate action regarding diseased animals (e.g. an earlier decision to euthanize) and all in/all out were mentioned by farmers and veterinarians as means to reduce AMU (Dupont et al., Reference Dupont, Diness and Fertner2017). Another study concluded that improved biosecurity, especially the presence of a hygiene lock, and pest control by a professional, were related to lower probabilities of farms being infected with extended spectrum beta-lactamase E. coli (Dohmen et al., Reference Dohmen, Dorado-García and Bonten2017).
Farmers often perceive improvements in biosecurity as difficult to achieve and not cost-effective, mainly because they lack information on their associated costs and, especially, revenues (Fraser et al., Reference Fraser, Williams, Powell and Cook2010; Laanen et al., Reference Laanen, Maes and Hendriksen2014). One study made an inventory of the application of biosecurity measures in 77 breeder–finisher herds in France. It showed that the difference in standardized profit margins between farms with high biosecurity compared with those with lower levels of biosecurity were estimated at around €200 per sow per year (Corrégé et al., Reference Corrégé, Fourchon and Le Brun2012).
An intervention study in Belgium found that improving pig herd management and biosecurity status, in combination with antimicrobial stewardship, helped reduce AMU from birth till slaughter by 52%, and in sows by 32% (Postma et al., Reference Postma, Vanderhaeghen and Sarrazin2017). In this study, the management and biosecurity interventions were generally relatively simple for farmers to implement. They included changing the working habits and routines of the farmer (e.g. changing of needles, hand and personal hygiene, and analysis of water quality). Interventions incurring higher costs and/or more pronounced changes, such as introducing a new hygiene lock to change clothes/boots and wash hands, were implemented less frequently. A key recommendation was for a good and early registration of disease symptoms in order to be able to take proper and timely control measures (e.g. biosecurity, vaccination and climate change), and to create awareness of the importance of the principle that “prevention is better than cure”.
An important success factor from the above study was the order of action: “Check, Improve and Reduce”. It suggested that herd counselling should always start with a thorough evaluation of herd management, biosecurity and health status, followed by tailored advice with specific suggestions for improvement. In this process it was important that an adviser/coach helped the farmer by explaining what he/she could improve, and what the risk would be when certain practices were not performed correctly. In addition, follow-up and feedback on the agreed and executed improvements is of high importance to retain levels of motivation. Only after implementation of these improvements, may changes and reductions in AMU be proposed. Using this approach, farmers can keep control over the health situation and are less reluctant to change certain antimicrobial treatment procedures. An economic evaluation based on the results of the study has shown that, including labour costs of all persons involved (including the coach, veterinarian and farmer), the participating herds achieved an average financial gain or overall benefit of €2.67 per finisher pig per year from partaking in this “team effort” approach (Rojo-Gimeno et al., Reference Rojo-Gimeno, Postma and Dewulf2016). In a comparable study performed in four European Union countries, an economic evaluation of suggested interventions in, among others, biosecurity resulted in a median change in net farm profits among Belgian and French farms estimated at €4.46 and €1.23 per sow per year, respectively (Collineau, Rojo-Gimeno & Léger, 2017b). A comparable type of intervention study performed in Belgium on 15 broiler farms, based on improved biosecurity, recorded an average reduction of 29% in AMU (Gelaude et al., Reference Gelaude, Schlepers and Verlinden2014).
In other animal species, studies about the association between biosecurity and AMU are scarce. However, the results obtained in pig production may be applicable to other farm animals. Experiences from the introduction of high biosecurity standards in Swedish broiler production to reduce the risk of Salmonella have demonstrated the close association between improved biosecurity and reduced AMU. Improvements in the level of biosecurity should at least be at the basis of any effort to reduce AMU at herd or flock level.
Besides the above described effects of improved biosecurity, other methods such as improved vaccination, use of feed and water additives and an improved feed regimen are also available. For example, essential oils, prebiotics (feed ingredients with beneficial effects on the gut microbiota) and probiotics (microorganisms with beneficial health effects) have been proposed for managing post-weaning diarrhoea in piglets (Gresse et al., Reference Gresse and Chaucheyras-Durand2017) and various probiotics have been developed to control necrotic enteritis in broiler chickens (Caly et al., Reference Caly, D’Inca and Auclair2015). Feed additives such as probiotics, prebiotics, organic acids and hyper-immune egg yolk antibodies have also been used to enhance the growth of broiler chickens (Gadde et al., Reference Gadde, Kim and Oh2017). However, despite a wide range of new potential alternatives to antibiotics, including vaccines, other immunomodulators, bacteriophages, lysins, hydrolases, antimicrobial peptides, plant extracts, quorum sensing inhibitors, biofilm inhibitors, bacterial virulence inhibitors, enzymes, pre-, pro- and synbiotics it has been concluded that antibiotic resistance and tolerance in bacteria are natural evolutionary consequences and in the foreseeable future prudent use of antibiotics is the best and fastest way to limit the growth of AMR (Cheng et al., Reference Cheng, Hao and Xie2014; Sang & Blecha, Reference Sang and Blecha2015).
Conclusions
The global use of antibiotics in animal production has been excessive and contributed to the selection of antibiotic resistance affecting both human and animal health. The realization that even low doses of antimicrobials, such as are used for growth promotion in animals and seen in agricultural waste, exerts a selective pressure for increasing AMR among bacteria in the environment, animals and humans has sparked a range of global activities to counteract these effects.
In recent years, however, huge progress has been made in the field of improved animal management. In addition, new tools for disease prevention and control are being developed. To ensure a global implementation of these tools for better animal management and more prudent use of antibiotics in animal production, significant efforts will be required in several areas. These include increasing awareness of the risks associated with AMR, improving training and education on the use of antibiotics, enhancing external and internal biosecurity measures, and improving the overall husbandry and management practices on many animal farms.
Implementation of these measures indicates already that the use of antimicrobials in animal production can be substantially reduced in the future without a negative impact on production and animal health and welfare. This reduction will also result in the checking, and eventually even reversal, of resistance selection which will have further benefits for animal health and production as well as human health, global food safety and food security.