Management Implications
Diverse communities of submersed aquatic vegetation are ecologically beneficial to aquatic ecosystems; however, non-native plants commonly disrupt these communities and their associated benefits. Vallisneria species are recognized as desirable plants for aquatic resource managers, but recent documentation of multiple non-native Vallisneria taxa in the United States has prompted concerns in management of aquatic systems. The primary objective was to evaluate the response of two native eelgrass taxa (Vallisneria americana and Vallisneria neotropicalis) and two non-native taxa (Vallisneria australis and hybrid Vallisneria spiralis × Vallisneria denseserrulata) to common aquatic herbicide treatments in a small-scale mesocosm study. This research also provided efficacy data for new aquatic invasive taxa. Managers may control native and invasive Vallisneria taxa with endothall or combinations of endothall and diquat, endothall and florpyrauxifen-benzyl, and florpyrauxifen-benzyl and flumioxazin. Florpyrauxifen-benzyl applied alone does not provide nominal above- or belowground biomass control compared with some of the other treatments tested. Fluridone reduced native V. americana above- and belowground biomass but is less likely to suppress V. australis, V. neotropicalis, or V. spiralis × V. denseserrulata. Control of invasive Hydrilla verticillata (hydrilla) with minimal damage to native Vallisneria was also observed with florpyrauxifen-benzyl treatments. These observations may be of particular interest to managers looking to control H. verticillata growing intermingled with native Vallisneria taxa, especially in restoration sites.
Introduction
Diverse, native submersed aquatic vegetation (SAV) assemblages are essential for aquatic ecosystems as they provide fish and wildlife habitat and sediment stabilization, reduce wave action, and improve water quality (Dodd et al. Reference Dodd, Harms and Schad2021; Gettys and Haller Reference Gettys and Haller2013; Korschgen et al. Reference Korschgen, George, Green and Weller1987; Moore et al. Reference Moore, Shields and Jarvis2010; Owens et al. Reference Owens, Smart and Dick2008). However, native aquatic plant communities are sensitive to invasion by non-native plants that reduce biodiversity and habitat quality as well as negatively impact human uses of aquatic systems such as hydropower, flood control, irrigation, and recreation (Langeland Reference Langeland1996; Madsen and Sand-Jensen Reference Madsen and Sand-Jensen1991; Smart et al. Reference Smart, Barko and McFarland1994; Zhang and Boyle Reference Zhang and Boyle2010). Aquatic ecosystem restoration efforts often include re-vegetation plantings of native plants (Canfield and Hoyer Reference Canfield and Hoyer1992; Gettys and Haller Reference Gettys and Haller2013). In the United States, American eelgrass (Vallisneria americana Michx.) is arguably the most common and sought after submersed plant species used in restoration projects due to ease of propagation; resistance to invasive species pressure; tolerance of wave action; growth in a variety of soil and water characteristics; and ecosystem services provided, including stabilizing water and soil quality and serving as a food source or host to food sources for invertebrate and vertebrate species (Gettys and Haller Reference Gettys and Haller2013; Henry Reference Henry2017; Korschgen and Green Reference Korschgen and Green1988; Moore et al. Reference Moore, Shields and Jarvis2010).
Vallisneria is a genus with global distribution; however, V. americana is native to North America, primarily found in eastern North America, with some distribution in Europe and Asia as well (Korschgen and Green Reference Korschgen and Green1988; Les et al. Reference Les, Jacobs, Tippery, Chen, Moody and Wilstermann2008; Martin and Mort Reference Martin and Mort2023; Mesterházy et al. Reference Mesterházy, Somogyi, Efremov and Verloove2021). Vallisneria taxa are herbaceous and monocotyledonous and form rosettes at the hydrosoil surface (Godfrey and Wooten Reference Godfrey and Wooten1979). They are dioecious, with female flowers in long sessile stalks at the water surface and male flowers on short stalks near the basal rosette that detach and float free to the water surface (Les et al. Reference Les, Jacobs, Tippery, Chen, Moody and Wilstermann2008; Martin and Mort Reference Martin and Mort2023). Vallisneria also reproduces asexually via stolons, which produce clonal daughter plants and some species, such as V. americana, also reproduce via subterranean turions. Unlike most aquatic macrophytes, which are canopy forming, Vallisneria is a meadow-forming plant with biomass more evenly distributed in the water column (Best et al. Reference Best, Kiker and Boyd2004; Haller and Sutton Reference Haller and Sutton1975; Hauxwell et al. Reference Hauxwell, Frazer and Osenberg2007).
Recently, genetic divergence between U.S. native populations of V. americana was recognized, which suggested the resurrection of Vallisneria neotropicalis Vict., previously consolidated with V. americana (Gorham et al. Reference Gorham, Seyoum, Furman, Darnell, Reynolds and Tringali2021; Les et al. Reference Les, Jacobs, Tippery, Chen, Moody and Wilstermann2008; Martin and Mort Reference Martin and Mort2023). These taxa were formerly considered separate ecotypes of V. americana, despite considerable phenological differences, including annual versus perennial habits of overwintering and the lack of turion production in southern ecotypes, thought to be a function of geography or environmental factors (Godfrey and Wooten Reference Godfrey and Wooten1979; Korschgen and Green Reference Korschgen and Green1988; McFarland and Schafer Reference McFarland and Schafer2008; Smart et al. Reference Smart, Dick and Snow2005). Anecdotal evidence indicates there is a geographic delineation separating V. americana and V. neotropicalis, but the exact distribution is not currently understood and requires further investigation.
The presence of non-native Vallisneria species, cultivars, and hybrids has also been recently documented in the United States, primarily Vallisneria spiralis L. and Vallisneria spiralis L. × Vallisneria denseserrulata Makino in the southeastern United States and Vallisneria australis S.W.L. Jacobs & Les in California (CDFA 2021; Gorham et al. Reference Gorham, Seyoum, Furman, Darnell, Reynolds and Tringali2021; Martin and Mort Reference Martin and Mort2023). Given the prevalence of different Vallisneria species in the aquarium and water garden communities, it is possible some of these new infestations have arisen from accidental releases. Vallisneria australis and V. neotropicalis have been identified as new invasions in Europe, and the hybrid V. spiralis L. × V. denseserrulata has been documented in Japan (Mesterházy et al. Reference Mesterházy, Somogyi, Efremov and Verloove2021; Wasekura et al. Reference Wasekura, Horie, Fujii and Maki2016). Hybrids between V. australis and one of the U.S. native species (V. americana or V. neotropicalis) are commercially available in the aquarium trade mistakenly labeled as V. americana (Martin and Mort Reference Martin and Mort2023). Unfortunately, some restoration efforts have unknowingly planted non-native Vallisneria taxa, as plants collected from Crystal River, FL, were identified as V. spiralis L. × V. denseserrulata following a restoration project (Martin and Mort Reference Martin and Mort2023). However, we cannot yet determine the contribution of spread that restoration efforts may have played compared with aquarium-based releases.
Differentiation between cryptic species and hybrids via plant morphology alone is often difficult with closely related taxa, especially Vallisneria. Hybridization between macrophytes is likely more common than currently recognized and has been observed in Myriophyllum and Nymphoides (Harms et al. Reference Harms, Thum, Gettys, Markovich, French, Simantel and Richardson2021; Parks et al. Reference Parks, McNair, Hausler, Tyning and Thum2016; Tavalire et al. Reference Tavalire, Bugbee, LaRue and Thum2012). Multiple Vallisneria taxa have been documented to coexist in waterbodies, so selective management actions would be desirable to suppress invasive populations while mitigating adverse effects to native populations (Gorham et al. Reference Gorham, Seyoum, Furman, Darnell, Reynolds and Tringali2021).
To combat invasions by other species and restore SAV communities, aquatic resource managers have been motivated to identify and cultivate Vallisneria populations with growth characteristics favorable for restoration efforts. This includes establishing or reestablishing self-sustaining populations that are fast growing; tolerant to chemical and nonchemical management activity; and resistant to invasion, competition, and environmental disturbance. Unfortunately, these characteristics are also common in many weedy species. Vallisneria may also slow or prevent invasions by introduced plant species, especially in low-nutrient sediments, where it has been shown to outcompete hydrilla [Hydrilla verticillata (L. f.) Royle] in mesocosm trials (Owens et al. Reference Owens, Smart and Dick2008; Van et al. Reference Van, Wheeler and Center1999). Chadwell and Engelhardt (Reference Chadwell and Engelhardt2008) observed reduced H. verticillata establishment via fragmentation alone in field trays with V. americana, but in plots where H. verticillata subterranean turions were not removed, V. americana did not prevent colonization. The competitive pressure posed by exotic macrophytes such as H. verticillata and Eurasian watermilfoil (Myriophyllum spicatum L.) has bolstered the need for selective management options of ecologically important species such as Vallisneria (Beets et al. Reference Beets, Heilman and Netherland2019; Mudge Reference Mudge2013; Netherland et al. Reference Netherland, Getsinger and Skogerboe1997).
Effective and selective control of aquatic invasive plants is commonly achieved using registered herbicides and is dependent on a concentration and exposure time (CET) that is plant and herbicide specific. CET requirements have been widely studied primarily in H. verticillata and M. spicatum. The required exposure times are not always feasible in water bodies where increased water exchange occurs. In these systems, increasing application rate is not always desired, as this can reduce selectivity and increase costs due to required herbicide volumes (Nault et al. Reference Nault, Netherland, Mikulyuk, Skogerboe, Asplund, Hauxwell and Toshner2014; Netherland Reference Netherland2015; Netherland and Getsinger Reference Netherland and Getsinger1995; Netherland et al. Reference Netherland, Green and Getsinger1991, Reference Netherland, Getsinger and Skogerboe1997). Netherland (Reference Netherland2015) observed equivalent efficacy on monoecious H. verticillata between intermittent and continuous exposure to fluridone in mesocosm experiments. These intermittent exposures represent the CET requirements separated by nontreated periods, and these separation periods may need to be adjusted based on mode of action (Darnell Reference Darnell2022). The concept of intermittent exposures builds on the practice of injecting fluridone over long periods of time. Results have indicated that pausing application during a dilution event would not negatively impact efficacy, and subsequent applications may build on the initial exposure period (Darnell Reference Darnell2022; Netherland Reference Netherland2015).
Vallisneria, presumed to be V. americana has shown sensitivity to fluridone at concentrations of 1.5 to 20 µg L−1 in mesocosms; however, field studies indicate recovery from initial phytotoxicity often occurs and increased Vallisneria frequency can occur (Mudge Reference Mudge2013; Netherland et al. Reference Netherland, Getsinger and Skogerboe1997; Smith and Pullman Reference Smith and Pullman1997; Valley et al. Reference Valley, Crowell, Welling and Proulx2006). Fluridone concentrations below 12 µg L−1 with 35 to 60 d of exposure are generally employed to control H. verticillata and M. spicatum while providing selectivity (Gettys and Leon Reference Gettys and Leon2021; Netherland and Getsinger Reference Netherland and Getsinger1995; Netherland et al. Reference Netherland, Getsinger and Turner1993). Endothall applications of 0.5 to 3.4 mg L−1 with 24 h to 3 wk of exposure have provided control of various Vallisneria taxa in mesocosm and field studies (Dugdale et al. Reference Dugdale, Islam, Hunt, Liu, Butler and Clements2022; Mudge Reference Mudge2013; Skogerboe and Getsinger Reference Skogerboe and Getsinger2002). Mudge (Reference Mudge2013) reported suppression of suspected V. americana with diquat at 100 µg L−1 and excellent control with combinations of endothall and flumioxazin at 500 µg L−1 + 50 µg L−1. Combinations of endothall and diquat have proven effective on H. verticillata, with indication of additive effects when the two herbicides are combined (Chiconela and Haller Reference Chiconela and Haller2013; Pennington et al. Reference Pennington, Skogerboe and Getsinger2001; Skogerboe et al. Reference Skogerboe, Pennington and Aguillard2004). Vallisneria has shown limited sensitivity to florpyrauxifen-benzyl at 3 to 48 µg L−1 for 6 h to 15 d, although this may be dependent on plant age and size (Beets et al. Reference Beets, Heilman and Netherland2019; Dodd et al. Reference Dodd, Mudge and Schad2022; Sperry et al. Reference Sperry, Leary, Jones and Ferrell2021). There is a lack of knowledge concerning efficacy of combinations of florpyrauxifen-benzyl and other aquatic registered herbicides.
Previous studies have largely focused on selectivity for what were thought to be one or two native ecotypes of V. americana, but there is a lack information concerning differential herbicide sensitivity in Vallisneria taxa (Beets et al. Reference Beets, Heilman and Netherland2019; Mudge Reference Mudge2013; Netherland and Glomski Reference Netherland and Glomski2014; Skogerboe and Getsinger Reference Skogerboe and Getsinger2002). Additionally, these studies only differentiated northern and southern Vallisneria, or narrowleaf and broadleaf, assuming these plants to be V. americana. This is likely no longer a valid assumption in future studies without confirmation through genetic testing. Hydrilla verticillata has historically been identified as a major threat to native Vallisneria populations and has a large body of research for comparison of efficacious management options. As such, H. verticillata was included for comparison of Vallisneria control with a well-researched species. The objective of this research was to evaluate the response of Vallisneria taxa to a variety of aquatic herbicide CETs used in H. verticillata management and determine their utility in management of invasive Vallisneria taxa.
Materials and Methods
Mesocosm experiments were conducted and repeated under greenhouse conditions in the summer of 2023 (Trial 1 in June and Trial 2 in July) at North Carolina State University in Raleigh, NC (35.810278°N, 78.721714°W) to evaluate the response of Vallisneria taxa to common aquatic herbicide treatments. Plant material for the four Vallisneria taxa was sourced from the following sites: V. americana from the Connecticut River (41.48333°N, 72.50656°W), V. neotropicalis from Lake Gaston, NC (36.49792°N, 77.84371°W), V. australis from Lake Mattamuskeet, NC (35.52013°N, 76.1088°W), and V. spiralis × V. denseserrulata from Wheeler Lake, AL (34.62456°N, 86.98359°W). Monoecious H. verticillata was originally sourced from an impoundment in Granville County, NC (36.136993°N, 78.794979°W). Genetic confirmation of Vallisneria was performed using ITS sequencing (Les et al. Reference Les, Jacobs, Tippery, Chen, Moody and Wilstermann2008) at Montana State University. Single rosettes of each Vallisneria population were planted in 0.52-L pots containing topsoil (Timberline Soil Top Soil, Oldcastle® Lawn & Garden, Atlanta, GA) with slow-release fertilizer (Osmocote® Smart Release 15-9-12, Scotts, Marysville, OH) at a rate of 3 g L−1 soil and covered with a sand cap to reduce nutrient leaching into the water column. Water used in this study was conditioned tap water (API Tap Water Conditioner®, Mars Fishcare North America, Chalfont, PA) with a pH of 7.8 to 8.2. A single sprouted apical stem of H. verticillata was planted in a 0.09-L cup with the same amended topsoil to serve as a bioindicator for treatment efficacy. Plants were established for four wk before study initiation in 16-L mesocosms.
Following the establishment period, one pot of each taxon was placed in a 16-L mesocosm containing 12 L of conditioned tap water. Each mesocosm was considered an experimental unit and was treated with one of 11 applications containing diquat, endothall, florpyrauxifen-benzyl, flumioxazin, fluridone, or a combination of these herbicides (Table 1). Nontreated controls and a pretreatment biomass harvest control were included in the experiment as references. Following predetermined herbicide exposure periods, plants were placed in new mesocosms containing nontreated water for a 6-wk recovery period. Treatments were applied via syringe using herbicide stock solutions. The experiment was set up as a randomized complete block design with four replications. Mesocosms were maintained under 50% shade to reduce light stress, minimize algal growth, and regulate water temperature. Mean water temperature was 26.7 C and pH was 8.1 at time of treatment. Mesocosms were maintained at a consistent water level via addition of conditioned tap water.
Table 1. Herbicides evaluated in a greenhouse trial examining control of four Vallisneria taxa and Hydrilla verticillata in Raleigh, NC, in 2023.
Six weeks after treatment (WAT), above- and belowground biomass was harvested, placed in a forced-air dryer for 72 h at 65 C, and weighed. Fluridone treatments were harvested 6 wk after the end of their exposure period due to long exposure requirements of fluridone. Likewise, an additional set of nontreated controls was included for each fluridone harvest time. Biomass data were normalized using their respective nontreated controls to calculate percent biomass reduction using the following equation:
Biomass reduction was subjected to mixed-model ANOVA for each herbicide treatment, with trial and block as random effects and taxa as a fixed effect. Where appropriate, means were separated using a Tukey’s honest significant difference (HSD) in JMP Pro 17 (JMP Pro v. 17, SAS Institute, Cary, NC).
Results and Discussion
Trial and block factors were not significant when included in separate mixed-model ANOVAs for each herbicide, so results between trials were pooled. Vallisneria americana was highly sensitive to most herbicide treatments, resulting in increased control compared with the other Vallisneria taxa tested. Diquat alone for 12 h at 370 µg L−1 resulted in 84% biomass reduction of V. americana at 6 WAT (Table 2). Biomass reduction from this treatment was similar in V. australis and V. spiralis × V. denseserrulata (59% and 63% respectively) but was significantly lower in V. neotropicalis (11%). Visual injury was observed at 1 to 2 WAT in sensitive taxa. Reductions in belowground biomass did not significantly differ between the four tested taxa when exposed to diquat alone despite a wide range of biomass reductions (Table 3).
Table 2. Aboveground biomass reduction of four Vallisneria taxa 6 wk after in-water herbicide application (6 wk after end of exposure period for fluridone).
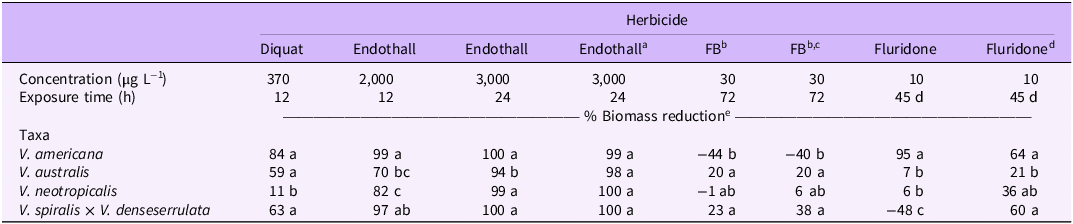
a Intermittent exposure (8 h + 40-h rest [3×]).
b Florpyrauxifen-benzyl.
c Intermittent exposure (24 h + 6-d rest [3×]).
d Intermittent exposure (15 d + 6-d rest [3×]).
e Mean responses within a column followed by the same letter do not differ according to Fisher’s protected LSD (P ≤ 0.05). Negative values indicate plant growth.
Table 3. Belowground biomass reduction of four Vallisneria taxa 6 wk after in-water herbicide application (6 wk after end of exposure period for fluridone).

a Intermittent exposure (8 h + 40-h rest [3×]).
b Florpyrauxifen-benzyl.
c Intermittent exposure (24 h + 6-d rest [3×]).
d Intermittent exposure (15 d + 6-d rest [3×]).
e Mean responses within a column followed by the same letter do not differ according to Fisher’s protected LSD (P ≤ 0.05). Negative values indicate plant growth.
Endothall alone for 12 h at 2,000 µg L−1 resulted in 99% aboveground biomass reduction of Vallisneria americana and 97% reduction of V. spiralis × V. denseserrulata biomass at 6 WAT (Table 2). Aboveground biomass reduction was significantly lower for V. australis (70%) and V. neotropicalis (82%) than for the other two taxa. Visual injury was observed in all taxa within 1 WAT. Belowground biomass reduction was significantly lower in V. australis (25%) than the other tested taxa (Table 3). One V. americana replicate in this treatment produced a winter bud/turion despite a substantial amount of injury from this contact herbicide. Endothall alone (3,000 µg L−1) under a 24-h continuous exposure resulted in 99% to 100% aboveground biomass reduction of V. americana, V. spiralis × V. denseserrulata, and V. neotropicalis, but biomass reduction was slightly lower in V. australis (94%). A similar trend was observed in belowground biomass, but there was no significant difference in the reduction among the tested taxa. When the 24-h exposure of endothall was split into three 8-h exposures and a 40-h rest between applications, there was no significant difference in above- or belowground biomass reduction between the Vallisneria taxa, and biomass reduction was comparable to continuous endothall exposure at the same CET.
Florpyrauxifen-benzyl alone for 72 h at 30 µg L−1 resulted in 20% and 23% aboveground biomass reduction for V. australis and V. spiralis × V. denseserrulata, respectively, while growth was observed in V. americana (−44%) and no impact to V. neotropicalis was observed at 6 WAT (Table 2). This CET resulted in minimal (−11% to 33%) impact to belowground biomass, and no significant differences between taxa were observed (Table 3). Similar trends were observed when the 72-h exposure period was broken up into three 24-h exposure periods. Aboveground biomass of V. australis and V. spiralis × V. denseserrulata was reduced 20% and 38%, respectively, with growth (−40%) of V. americana and no effect on V. neotropicalis. Minimal impacts on belowground biomass (−14% to 24%) were observed in the intermittent applications of florpyrauxifen-benzyl. No injury to Vallisneria was observed when florpyrauxifen-benzyl was applied alone. The observed growth in aboveground biomass may be a result of hormesis, or the augmented growth due to sublethal auxin-mimic exposure, which has been observed in Canadian waterweed (Elodea canadensis Michx.) and Brazilian waterweed (Egeria densa Planch.) when exposed to florpyrauxifen-benzyl (Cedergreen et al. Reference Cedergreen, Streibig, Kudsk, Mathiassen and Duke2007; Howell et al. Reference Howell, Hofstra, Heilman and Richardson2022; Mudge et al. Reference Mudge, Sartain, Sperry and Getsinger2021).
Fluridone applied at 10 µg L−1 with a 45-d exposure reduced aboveground biomass of Vallisneria americana by 95%, V. australis by 7%, and V. neotropicalis by 6% (Table 2). Growth and a lack of visual injury were observed in V. spiralis × V. denseserrulata with this treatment (−48% biomass reduction). This trend was also observed for belowground biomass of all taxa (Table 3). Interestingly, when fluridone was split into three 15-d applications with a 6-d rest period, aboveground biomass reduction on V. spiralis × V. denseserrulata was 60%, which was similar to the aboveground biomass reduction on V. americana and significantly higher than the efficacy on V. australis. Intermittent applications of fluridone resulted in a 10% reduction in V. australis belowground biomass and 35% for V. neotropicalis belowground biomass. Chlorosis, a common symptom of fluridone applications (Netherland and Getsinger Reference Netherland and Getsinger1995; Netherland et al. Reference Netherland, Getsinger and Skogerboe1997; Sprecher et al. Reference Sprecher, Netherland and Stewart1998) was observed in V. australis leaves treated with fluridone; however, this symptom did not persist, which is reflected in the lack of biomass reduction at 6 wk after the end of the exposure period.
A 12-h exposure of endothall (2,000 µg L−1) plus diquat (370 µg L−1) reduced V. americana aboveground biomass by 98% and V. spiralis × V. denseserrulata by 100% at 6 WAT (Table 4). Aboveground biomass reductions from this herbicide combination were lower in V. australis (11%) and V. neotropicalis (62%). A similar trend was observed in belowground biomass, with reductions in belowground biomass of 41% and 100% for V. australis and V. americana, respectively (Table 5). The combination of flumioxazin at 300 µg L−1 and florpyrauxifen-benzyl at 30 µg L−1 for a 48-h exposure resulted in 97%, 98%, and 99% aboveground biomass reduction of V. spiralis × V. denseserrulata, V. australis, and V. americana, respectively. Efficacy of this combination treatment against aboveground biomass remained high for V. neotropicalis (91%). There were no significant differences in belowground biomass reduction between taxa when treated with this herbicide combination (79% to 99%). Combinations of 2,000 µg L−1 endothall and 30 µg L−1 florpyrauxifen-benzyl were highly efficacious against all Vallisneria, with 100% aboveground biomass reduction of V. americana and V. spiralis × V. denseserrulata and 99.9% in V. neotropicalis. Aboveground biomass reduction was significantly lower in V. australis (94%), but still considered efficacious. Similar to the previous herbicide combination, there were no differences in belowground biomass reduction across plant species (77% to 100%).
Table 4. Aboveground biomass reduction of four Vallisneria taxa 6 wk after in-water herbicide applications.

a Herbicides were applied simultaneously at indicated rates.
b Florpyrauxifen-benzyl.
c Mean responses within a column followed by the same letter do not differ according to Fisher’s protected LSD (P ≤ 0.05).
Table 5. Belowground biomass reduction of four Vallisneria taxa 6 wk after in-water herbicide applications.

a Herbicides were applied simultaneously at indicated rates.
b Florpyrauxifen-benzyl.
c Mean responses within a column followed by the same letter do not differ according to Fisher’s protected LSD (P ≤ 0.05).
Hydrilla verticillata was included as a bioindicator in each treatment, as these treatments were selected from mesocosm trials targeting monoecious and dioecious H. verticillata (Darnell Reference Darnell2022; JPB, unpublished data). Rapid symptomology was observed in H. verticillata treated with all herbicides except fluridone and the 12-h exposure of endothall. At 6 WAT, H. verticillata aboveground biomass was reduced 80% to 100% except for the 12-h exposure of endothall (Table 6). This treatment only resulted in a 7% reduction in aboveground biomass. While an 83% reduction in belowground biomass was observed, this was likely due to several replicates in this treatment detaching from the sediment and persisting as fragments. Reductions in belowground biomass did not significantly differ between treatments, with several treatments resulting in 100% belowground biomass reduction.
Table 6. Biomass reduction of Hydrilla verticillata 6 wk after in-water herbicide application (6 wk after end of exposure period for fluridone).

a Mean responses within a column followed by the same letter do not differ according to Fisher’s protected LSD (P ≤ 0.05).
These results are promising for long-term H. verticillata control, as reductions in belowground biomass can be indicative of low regrowth potential via root crown or subterranean turions (Haller et al. Reference Haller, Miller and Garrad1976; Nawrocki et al. Reference Nawrocki, Richardson and Hoyle2016; Netherland et al. Reference Netherland, Getsinger and Skogerboe1997; Steward Reference Steward1980). The high level of efficacy demonstrated by the intermittent applications of endothall, florpyrauxifen-benzyl, and fluridone also further validates preliminary studies for intermittent applications. Monoecious H. verticillata overwinters as subterranean turions, so early-season management is often desirable before biomass has peaked and has proven highly efficacious, and small plants such as those utilized in this study can be representative of plants at this growth stage in the field (Langeland and Pesacreta Reference Langeland and Pesacreta1986; Nawrocki et al. Reference Nawrocki, Richardson and Hoyle2016). While not the main objective of the study, we find it important to highlight that H. verticillata bioindicator plants were well managed utilizing florpyrauxifen-benzyl treatments, with biomass reductions of 96% while native V. americana remained relatively unharmed (Table 6). Fluridone applications provided similar selectivity between H. verticillata and V. neotropicalis (Tables 2 and 3). Hydrilla verticillata can outcompete native species such as V. americana and V. neotropicalis. Therefore, identification of selective management options is important, even if they are not effective against the exotic Vallisneria taxa (Beets et al. Reference Beets, Heilman and Netherland2019; Chadwell and Engelhardt Reference Chadwell and Engelhardt2008; Haller and Sutton Reference Haller and Sutton1975).
There is a currently a lack of knowledge about management strategies for the newly documented invasive hybrid species V. spiralis × V. denseserrulata, and the populations of Vallisneria australis that have been identified in the United States. The results of this research can provide an initial understanding of potentially efficacious management actions. Additionally, comparisons with historical datasets are complicated and may not be accurate, because previous research may not have accurately identified the Vallisneria species being studied without genetic confirmation of the tested species (Gorham et al. Reference Gorham, Seyoum, Furman, Darnell, Reynolds and Tringali2021; Martin and Mort Reference Martin and Mort2023).
Native Vallisneria of various previously classified ecotypes have shown sensitivity to endothall at concentrations of 0.5 mg L−1 and higher, thus validating the findings of this research (Mudge Reference Mudge2013; Skogerboe and Getsinger Reference Skogerboe and Getsinger2001, Reference Skogerboe and Getsinger2002). These previous studies also indicated recovery from viable root crowns and turions, so treatment timing may play an important role in recovery, and further investigation into direct effects on Vallisneria turions is warranted to improve strategies for effective selective management of these native species. Similarly, Turnage and Madsen (Reference Turnage and Madsen2015) observed expansion of Vallisneria in Minnesota lakes following diquat applications, and Mudge (Reference Mudge2013) observed minimal biomass reduction in Vallisneria exposed to diquat.
The observation of minimal impact on native Vallisneria with florpyrauxifen-benzyl is corroborated by previous mesocosm and field studies, indicating that florpyrauxifen-benzyl alone is not an effective control method of Vallisneria but does provide a selective management option for H. verticillata when Vallisneria is a non-target species (Beets et al. Reference Beets, Heilman and Netherland2019; Dodd et al. Reference Dodd, Mudge and Schad2022; Sperry et al. Reference Sperry, Leary, Jones and Ferrell2021). Vallisneria sensitivity to fluridone has been variable in previous mesocosm and field studies, which may be partially explained by differentiation between V. americana and V. neotropicalis or other Vallisneria taxa, as seen in this study (Getsinger et al. Reference Getsinger, Madsen, Koschnick, Netherland, Stewart, Honnell, Staddon and Owens2001, Reference Getsinger, Poovey, James, Stewart, Grodowitz, Maceina and Newman2002; Nelson et al. Reference Nelson, Shearer and Netherland1998; Netherland et al. Reference Netherland, Getsinger and Skogerboe1997; Poovey et al. Reference Poovey, Skogerboe and Getsinger2004). Several divergences in efficacy compared with previous studies were observed. Dugdale et al. (Reference Dugdale, Hunt, Clements and Butler2012) observed a 90% reduction in V. australis treated with diquat, while diquat efficacy was minimal in this study (Tables 2 and 3). Previous studies have indicated low efficacy of flumioxazin when applied alone and in combination with endothall or diquat (Mudge Reference Mudge2013; Mudge et al. Reference Mudge, Haller, Netherland and Kowalsky2010). However, combinations with flumioxazin have not been fully investigated at higher doses, especially in combination with florpyrauxifen-benzyl.
With the resurrection of the distinction between V. americana and V. neotropicalis, future studies must confirm which species are being utilized, and new evaluations are warranted to properly correlate selectivity with the species present. This study presents new evidence that previous differences in efficacy between V. americana populations may in fact have been differences in response between these two species, especially when comparing northern and southern populations in the United States. While the observed differences in herbicide response are indicative of variation between species, it is possible that the observed differences are limited to the tested populations. Further investigation and replication are needed to see whether there is a noticeable within-taxa variation to herbicide response in Vallisneria.
Previous field and mesocosm studies have indicated that Vallisneria australis is sensitive to but not completely controlled by similar concentrations and exposure times that were effective in reducing above- and belowground biomass in this study (Clements et al. Reference Clements, Butler, Hunt, Liu and Dugdale2018; Dugdale et al. Reference Dugdale, Islam, Hunt, Liu, Butler, Clements and Netherland2019, Reference Dugdale, Islam, Hunt, Liu, Butler and Clements2022). Dugdale et al. (Reference Dugdale, Islam, Hunt, Liu, Butler, Clements and Netherland2019) also observed improved endothall efficacy on Vallisneria australis in flowing mesocosms compared with quiescent mesocosms. There is a general lack of information concerning the efficacy of other herbicides on V. australis to further corroborate our findings. However, the findings of this research with V. australis, especially those surrounding florpyrauxifen-benzyl, flumioxazin, and fluridone, are similar to the results observed with other species. Further investigation into the effects of water flow on herbicide efficacy can greatly improve management strategies, especially in lotic systems such as the San Joaquin River delta in California, where invasions of V. australis have been identified (CDFA 2021).
Female flowers were observed in all Vallisneria taxa included in this study. However, no male flowers were observed in the hybrid V. spiralis × V. denseserrulata or V. australis. Male flowers have not been observed in the hybrid Vallisneria in other studies, indicating propagation may primarily be occurring via asexual reproduction (Gorham et al. Reference Gorham, Seyoum, Furman, Darnell, Reynolds and Tringali2021; Wasekura et al. Reference Wasekura, Horie, Fujii and Maki2016). Considerable genetic plasticity has been observed in V. australis, and its documented hybridization with native Vallisneria indicates these female flowers are not sterile or male plants are also present in the United States (Martin and Mort Reference Martin and Mort2023). The possibility of hybridization between these invasive and native taxa should be investigated further, and additional field monitoring is needed to confirm the lack of male plants in the United States, considering the issues that have arisen with hybridization between native and invasive species such as Myriophyllum spicatum L. × Myriophyllum sibiricum Kom. and Nymphoides cristata (Roxb.) Kuntze × Nymphoides aquatica (J.F. Gmel.) Kuntze (Harms et al. Reference Harms, Thum, Gettys, Markovich, French, Simantel and Richardson2021; Parks et al. Reference Parks, McNair, Hausler, Tyning and Thum2016).
In conclusion, these small-scale mesocosm experiments provide an initial understanding of herbicide response in two new invasive aquatic species, as well as two native species that were previously understood to be the same species. No herbicide treatments tested definitively provided selectivity between invasive and native Vallisneria taxa. However, H. verticillata was selectively managed while minimal damage to native Vallisneria was observed for several well-established treatments, and these results further document the efficacy of intermittent exposures. Future research should scale up the size and study length in experimental design (i.e., larger mesocosms or small field plots and evaluation periods longer than 6 wk) to provide a more operational understanding of selective management practices. Given the observed resilience of V. australis and the hybrid V. spiralis × V. denseserrulata, rapid endeavors should be made to improve understanding of efficacious management options as well as phenological and growth patterns to improve timing of management efforts. Turion production was not consistent throughout the trial for statistical analysis and was only observed in V. americana, thus future studies are needed to determine what factors lead to turion formation, including temperature, season, and herbicide application.
There are several key concepts future research endeavors should seek to investigate to improve management of a spreading problematic invasive plant. (1) Identify potential hybridization of non-native Vallisneria taxa with native Vallisneria taxa, as well as other Vallisneria taxa that may be present in the United States, given the genus’s prevalence in the aquarium and water garden trade. (2) Implement improvements in genetic confirmation of Vallisneria populations before treatment to inform herbicide treatment decisions and improve potential selectivity, as plants in this genus demonstrate similar morphology. (3) Improve the understanding of the ecological and anthropological impacts these non-native Vallisneria taxa have on aquatic ecosystems. (4) Identify phenological and growth differences between Vallisneria taxa to better inform management actions and the potential spread of these plants. (5) Identify new herbicide use patterns and potential new herbicide chemistries and use integrated pest management strategies to provide selective management options for Vallisneria taxa.
Acknowledgments
The authors would like to thank Kara Foley, Andrew Howell, Michael Punt, Delaney Davenport, and Logan Wilson for their assistance with plant propagation, treatment, and harvesting. The manuscript was reviewed in accordance with U.S. Army Engineer Research and Development Center policy and approved for publication. Citation of trade names does not constitute endorsement or approval of the use of such commercial products. The content of this work does not necessarily reflect the position or policy of the U.S. government and no official endorsement should be inferred. The U.S. Department of Agriculture is an equal opportunity employer and provider.
Funding statement
Funding for this research was provided by the U.S. Army Corps of Engineers Environmental Research and Development Center Aquatic Plant Control Research Program and the Michael D. Netherland Graduate Student Scholarship through the Aquatic Plant Management Society.
Competing interests
The authors declare no conflicts of interest.









