INTRODUCTION
Diphtheria, historically one of the most feared diseases of childhood, is now uncommon in the UK due to national immunization since the 1940s (Fig. 1). Since 1990, UK diphtheria vaccination coverage at age 2 years has exceeded 90%, rising to 94% from the beginning of the 21st century, close to the World Health Organization (WHO) 95% target. Diphtheria vaccine is made from inactivated diphtheria toxin and protects individuals from the effects of toxin-producing corynebacteria. Three Corynebacterium spp. can potentially produce diphtheria toxin; C. diphtheriae (associated with epidemic diphtheria and spread from person-to-person via respiratory droplets and close contact), C. ulcerans and C. pseudotuberculosis (both less common globally and traditionally associated with farm animal contact and dairy products). The classic and most severe presentation of diphtheria is a respiratory disease with a swollen ‘bull neck’ and strongly adherent pseudomembrane, which obstructs the airways. Patients with less severe respiratory disease can present with a sore throat. Diphtheria can also cause cutaneous infection, characterized by ‘rolled edge’ ulcers, which are more common in tropical areas of the world.
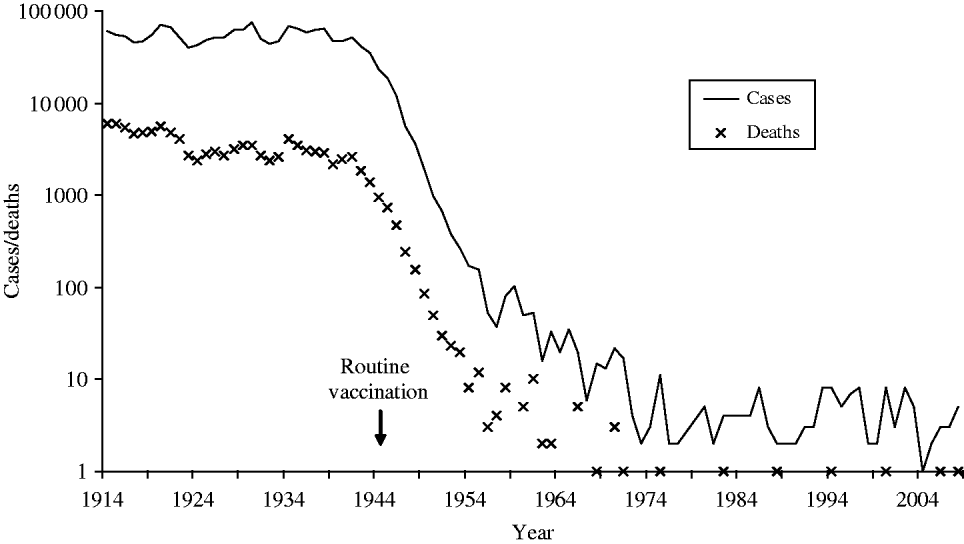
Fig. 1. Diphtheria notifications and deaths in England and Wales 1914–2008 (notifications up to 1985, laboratory-confirmed cases 1986–2008).
Diphtheria vaccine is currently scheduled in the UK as shown in Table 1. An accelerated schedule (2, 3, 4 months) for infant immunization replaced an extended schedule [2] (3, 4½–5, 8½–11 months) in the early 1990s [3], and the low-dose diphtheria component was added to the school-leaver dose of tetanus toxoid (Td) in 1994 (Td/IPV since 2004) [4]. A total of five doses of a diphtheria-containing vaccine at appropriate intervals are considered to give satisfactory long-term protection in most circumstances.
Table 1. UK vaccination schedule for diphtheria-containing vaccines
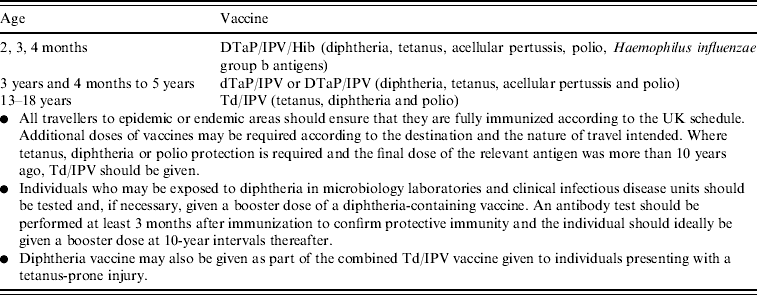
Source: Green Book 2006 [Reference Salisbury, Ramsay and Noakes1].
UK guidelines for the control of diphtheria, and laboratory diagnosis of infections caused by Corynebacterium diphtheriae and C. ulcerans were published in 1999 [Reference Bonnet and Begg5]. A clinical case of respiratory diphtheria requires rapid administration of diphtheria antitoxin (a concentrated immunoglobulin preparation prepared from horse serum, that neutralizes circulating toxin), as well as antibiotics to clear the bacterial infection. Antibiotics of choice are erythromycin, azithromycin, clarithromycin, or penicillin, all of which are active in vitro against C. diphtheriae and C. ulcerans. Administration of diphtheria vaccine is recommended during convalescence because diphtheria infection does not always confer immunity.
This paper summarizes all cases of diphtheria and other related infections caused by toxigenic corynebacteria that have been reported in the UK during the last 23 years, highlighting key trends and characteristics of the disease, as well as its changing epidemiology.
METHODS
Information concerning diphtheria cases in the years 1986–2008 was obtained from the following routine sources:
• Statutory notifications to the Office for National Statistics up to 1996, which then transferred to the Communicable Disease Surveillance Centre, now the Health Protection Agency (HPA) Centre for Infections (CfI).
• Death registrations to the Office for National Statistics.
• Laboratory reports from the WHO Collaborating Centre for Diphtheria and Streptococcal Infections, Respiratory and Systemic Infections Department.
• Case follow-up information from the HPA CfI Immunization, Hepatitis and Blood Safety Department.
In addition a literature search was carried out across Medline, EMBASE and Scopus databases during July–September 2008 in order to identify any additional cases not reported through routine surveillance. The search strategies covered titles and abstracts of English-language publications from 1985 to 2008 and comprised key-word combinations of ‘diphtheria’, ‘Corynebacterium and diphtheriae’, ‘Corynebacterium and ulcerans’, ‘Corynebacterium and pseudotuberculosis’.
In the UK, toxigenicity testing of suspect isolates from local laboratories is carried out by the WHO Collaborating Centre for Diphtheria and Streptococcal Infections, Respiratory and Systemic Infections Department (RSID) at the HPA in London. Historically, the Elek test has been used since 1940 to assess toxin production; prior to 1991, toxigenicity was also assessed by an in vivo subcutaneous test, and since 1993 PCR has been used to detect the presence of the toxin gene. However, the gold standard phenotypic test is the Elek test [Reference Efstratiou and Maple6]. Case follow-up is prompted by either a report of a toxigenic Corynebacterium isolate from the RSID, a direct communication with a consultant in communicable disease control or a clinician involved in the management of a suspected case, or notification of a suspected case of diphtheria to the local authority. Follow-up of all toxigenic isolates (cases and carriers) of Corynebacterium spp. has been standardized since 1995 using a questionnaire to ascertain the patient's clinical and immunization history, travel history and contact with travellers, exposure to raw dairy produce and domestic animals (C. ulcerans only) and management of the case and contacts. Information about contact with companion animals (cats/dogs) for C. ulcerans cases has been included on follow-up forms since 2003.
Here, a confirmed case is defined according to the Diphtheria Surveillance Network (DIPNET, www.dipnet.org) case definition (see Appendix) whereby a toxigenic isolate of C. diphtheriae, C. ulcerans or C. pseudotuberculosis has been isolated from the patient with an appropriate clinical presentation. An asymptomatic carrier is defined as having a toxigenic isolate with no symptoms. In this paper cases are further grouped according to the severity of their disease, the most severe presentation being classic respiratory diphtheria with pseudomembrane.
Statistical analyses involved χ2 tests using Stata statistical software, release 8.0 (StataCorp, USA).
Patients were assigned to four groups according to their vaccination status:
• Fully immunized for age [have received all scheduled vaccinations appropriate for their age (and vaccination schedule of their time), if they have a history of recent travel to an endemic area or work in a laboratory handling diphtheria this includes receipt of appropriate booster immunizations].
• Partially immunized (have received some scheduled vaccinations but not all appropriate for their age, or have not received appropriate booster vaccinations for travel/occupation).
• Vaccination history not known or not reported.
• Unimmunized (if no history was available, patients born prior to 1940 were assumed to be unimmunized).
RESULTS
During the 23-year period 1986–2008, there were 125 toxigenic Corynebacterium isolates; C. diphtheriae (62), C. ulcerans (62) and C. pseudotuberculosis (1). The data for C. diphtheriae and C. ulcerans, clinical presentation and immunization status are summarized in Table 2 (data for C. pseudotuberculosis are described later). The analysis includes two cases of toxigenic C. ulcerans which had not been routinely reported but were detected through the literature search [Reference Leek7, Reference de Carpentier8].
Table 2. Toxigenic C. diphtheriae and C. ulcerans isolates by clinical presentation and immunization status

* One patient from each of these groups also had cutaneous lesions but has been assigned to this group since this is the most serious presentation.
† Observation of tonsillar exudate, although not a solid membrane, could indicate the early stages of membrane formation.
‡ Toxigenic organism isolated from both sites.
§ May have been swabbed due to another illness or may be a contact (with no symptoms) of a confirmed case.
Fifteen cases of classic respiratory diphtheria with pseudomembrane were reported between 1986 and 2008, none of whom were fully vaccinated (Table 2). The most frequent presentation among UK cases is respiratory disease; typically a sore throat in a fully or partially immunized individual. Twenty-nine patients presented with cutaneous lesions, six of whom also had respiratory symptoms (including two with a pseudomembrane). One patient with toxigenic C. diphtheriae infection presented with bacterial endocarditis. Vaccination history was frequently unavailable in the follow-up notes for C. ulcerans cases (particularly earlier cases), and for asymptomatic carriers of C. diphtheriae. However, based on all cases with available data, the protective effect of vaccination could be demonstrated since none of the 39 fully vaccinated cases were recorded as presenting with classic respiratory diphtheria with pseudomembrane, whereas 14 of the 43 unvaccinated/incompletely vaccinated cases presented with these symptoms (P<0·001).
The following analysis excludes the asymptomatic patients listed in Table 2, these patients are described separately later. Between one and nine symptomatic cases of diphtheria were recorded each year in the UK between 1986 and 2008 (Fig. 2); an average of four cases per year. The yearly incidence rates ranged from 0·0141 (in 1986) to 0·0017 (in 2004) cases per 100 000 population. In the last 10 years C. ulcerans, rather than C. diphtheriae, has been the predominant cause of diphtheria in the UK. Forty per cent of the C. diphtheriae cases were reported from the London region, whereas the C. ulcerans cases were distributed more evenly across the country. The predominant toxigenic C. diphtheriae biotype in the UK during 1986–2008 was var. mitis (81% of cases), followed by var. gravis (17%) and var. intermedius (one case only).
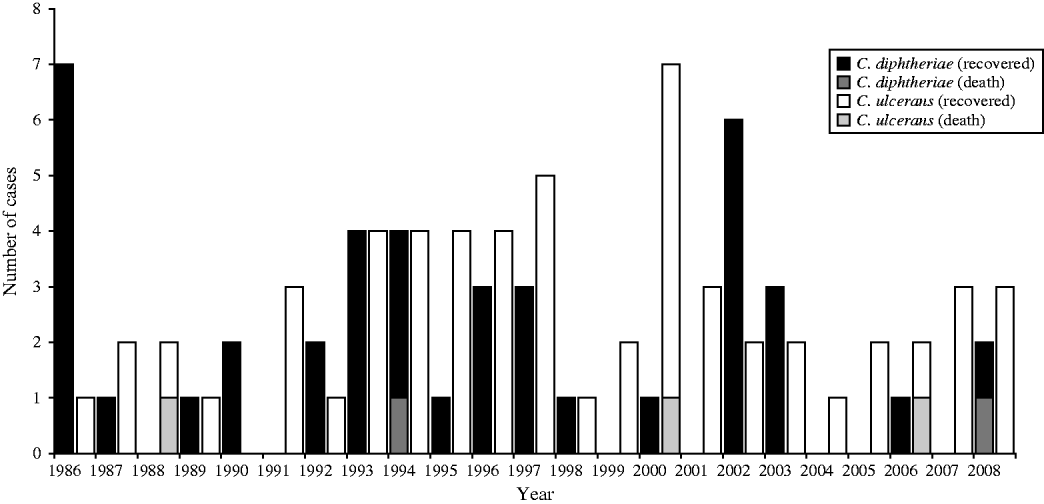
Fig. 2. Toxigenic cases of diphtheria and outcome in the UK, 1986–2008.
The majority of C. ulcerans cases (76%) were female whereas the sex distribution was even for C. diphtheriae (Fig. 3). In addition C. ulcerans cases were generally older than C. diphtheriae cases with mean and median age for C. ulcerans cases of 38 years compared to 15 years (mean) and 21·5 years (median) for C. diphtheriae cases.
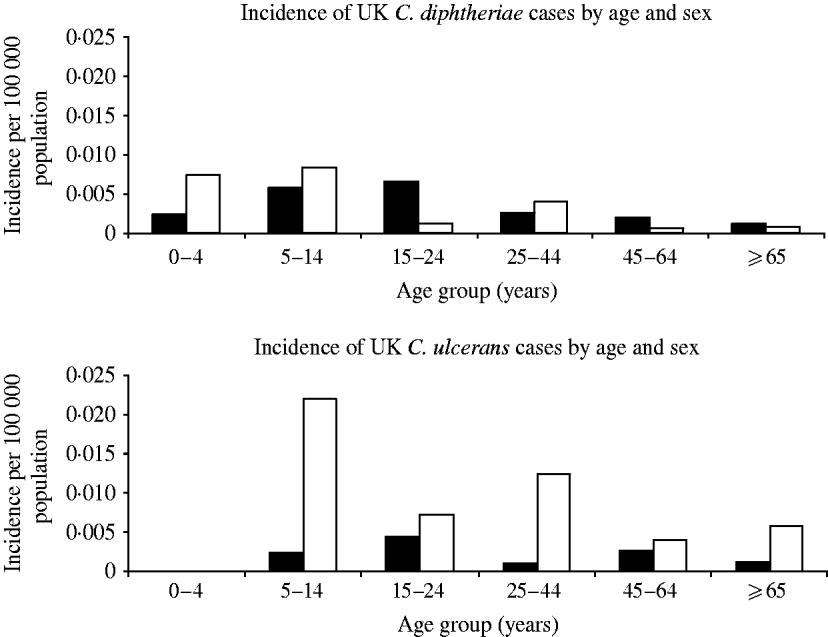
Fig. 3. Distribution of diphtheria cases by organism, age and sex. ▪, Males; □, females.
C. diphtheriae risk factors
The main risk factor for acquisition of toxigenic C. diphtheriae was travel to the Indian sub-continent, Africa or South East Asia (Table 3). Only eight cases had no history of travel or contact with a traveller recorded, three of which were laboratory-acquired infections. All three cases of laboratory-acquired diphtheria were due to toxigenic C. diphtheriae and occurred in separate incidents. The first in 1987 concerned a senior registrar in medical microbiology who had handled a non-toxigenic isolate of C. diphtheriae and a toxigenic control strain. The patient was known to have received childhood immunizations and presented with a severe sore throat with white exudate on both tonsillar beds. The second case in 1997 concerned an experienced medical laboratory scientific officer (MLSO) who became infected with a toxigenic strain of C. diphtheriae while handling a sample distributed by the National External Quality Assessment Scheme for microbiology in a non-containment facility [20]. The MLSO was known to have received childhood immunizations and developed severe tonsillitis. The third case in 2003 also occurred in a laboratory worker handling liquid samples of a toxigenic strain on an open bench in a microbiology laboratory.
Table 3. Origin of infection for toxigenic cases C. diphtheriae 1986–2008 in the UK
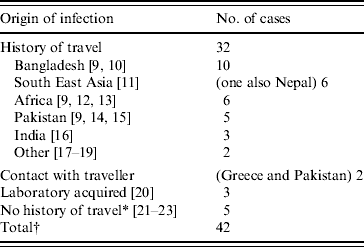
* One case report describes contact with a family member who had travelled to Africa, returning approximately 1 month before the child became ill [23]. This contact was swabbed and tested negative for C. diphtheriae, although this does not exclude the possibility of earlier carriage of the organism.
† This total excludes the 20 asymptomatic infections which are described later.
C. ulcerans risk factors
Only eight C. ulcerans cases had a history of travel abroad within the 3 months prior to the onset of their infection. Seven of 59 (12%) C. ulcerans cases were recorded as having consumed raw milk or dairy products, one of these also had contact with cattle. One further case, who had previous contact with a range of animals, was also recorded as having had contact with cattle. All 13 cases reported between 2003 and 2008 had made contact with domestic pets (cats and dogs) [24–27]. In recent years domestic cats and dogs in contact with five UK cases were swabbed but in only one case was C. ulcerans isolated, that case was from dogs that the patient had been in contact with; the strain was identical to that found in the patient [Reference Hogg28].
Case management
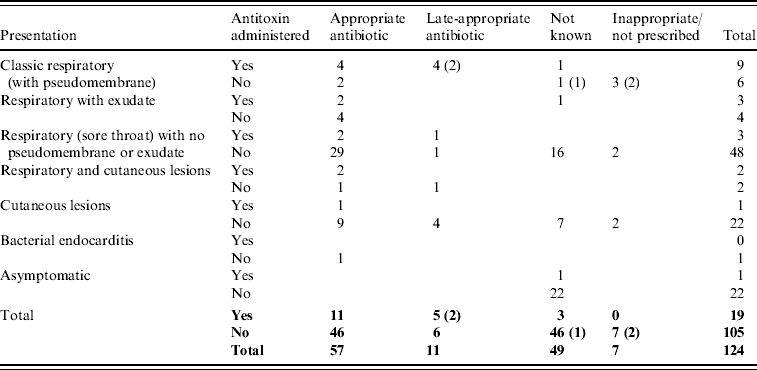
Six of 15 classic respiratory presentations with pseudomembrane did not receive diphtheria antitoxin (Table 4). Antitoxin was not administered to three of these cases because the disease was not recognized in time [Reference Leek7, Reference de Carpentier8, 23], for the other three an explanation was not available. Most cases (77% of those with treatment known) were prescribed appropriate antibiotics (erythromycin, azithromycin, clarithromycin, or penicillin) although the precise timing of administration was not available. In total, only 18/96 cases that recovered (8%) are recorded as receiving diphtheria vaccine during convalescence.
Table 4. Treatment prescribed to toxigenic C. diphtheriae and C. ulcerans cases by presentation (fatal cases indicated in parentheses)
Deaths
There were five fatal cases (two C. diphtheriae, three C. ulcerans) between 1986 and 2008, a case-fatality rate in patients with respiratory symptoms of 6%. The deaths all occurred in unvaccinated patients. Clinical presentations and treatments are detailed in Table 4; in each of the fatal cases the disease was not immediately recognized as diphtheria and there were consequent delays in administration of appropriate treatment. The fatality rate in patients with classic respiratory diphtheria (including all fatal cases) was 33%. The death of a school-aged child in 2008, due to C. diphtheriae infection, was only diagnosed at post-mortem [23]. The presentation was consistent with laryngeal diphtheria, not recognized at the time of treatment. The other C. diphtheriae fatality occurred in 1994; a 14-year-old male patient, recently returned from Pakistan, who presented with pharyngitis, a unilateral pharyngeal swelling, bull neck and respiratory distress [14]. A pseudomembrane was visible during attempts to drain what was initially considered to be quinsy. In view of the patient's respiratory distress he was intubated and ventilated but subsequently developed complete heart block and renal failure. Antitoxin and high-dose intravenous penicillin were administered, but this was delayed due to late diagnosis. The three deaths from C. ulcerans were all in elderly (>70 years) females. The first (in 1988) presented with sore throat, painful cough and difficulty breathing, and died the same day without receiving antitoxin [Reference Leek7]. She had stridor, and yellowish mucus covering the fauces and palate. At autopsy the entire respiratory tree from the upper part of the larynx to the small bronchi was found to be covered by a thick yellowish membrane. The second fatal case (in 2000) was admitted to hospital with a pharyngeal membrane and died of pneumonia 10 days after admission (no antitoxin was administered) [29]. The most recent fatality from C. ulcerans (in 2006) was hospitalized with a 2-day history of malaise, sore throat and a change in the sound of her voice. On the day of admission she had difficulty breathing and said that she felt her throat was closing. A preliminary diagnosis of angio-oedema was made, related to her recent treatment with an angiotensin-II receptor antagonist, and the patient was treated accordingly. However, her condition deteriorated and a diagnosis of diphtheria was made when a greyish-white membrane was observed across the pharynx during a tracheostomy. She received diphtheria antitoxin (4 days after onset of first symptoms) and antibiotics but died from her infection [27].
Transmission and carriage
Only one cluster of symptomatic cases, comprising four unimmunized family members, was identified during the study period. This cluster of cases caused by C. diphtheriae var. mitis occurred in 1986, in a family of recent immigrants from Bangladesh. The 14-month-old index case and 6-year-old sibling had both classic respiratory and cutaneous diphtheria. Another 3-year-old sibling had classic respiratory diphtheria, and a 9-year-old sibling had respiratory diphtheria. Their 47-year-old father was found to be an asymptomatic carrier. Extensive investigation of almost 250 contacts identified no further cases. A total of 20 asymptomatic carriers of toxigenic C. diphtheriae were recorded between 1986 and 2008; eight were fully immunized, one was unimmunized, and for the remaining 11 the immunization histories were unknown. The carriers fell into three main groups:
• Contacts of an index case, thought to have acquired infection through contact with an index case in the UK (three index cases, eight carriers).
• Fellow travellers of a case or carrier; may have acquired the infection abroad from the same source as the index case or carrier, or through contact with the index case (three index cases, four fellow traveller carriers).
• Patients that had recently returned from travel abroad and were seeking medical attention for an unrelated condition (n=7, four of whom were siblings from the same family).
In addition, an asymptomatic carrier of toxigenic C. diphtheriae was identified when screened as a contact of a patient infected with a non-toxigenic C. diphtheriae strain; contact-tracing would not usually be carried out in response to non-toxigenic infections.
Of the 16 unrelated cutaneous cases four (25%) had infected contacts, while two (10%) of the 20 unrelated isolations of C. diphtheriae from the throat had infected contacts (patients with both respiratory and cutaneous diphtheria excluded), the difference in these percentages was not significant (P=0·37).
Two unrelated C. ulcerans cases each had an infected asymptomatic contact. In 1996 toxigenic C. ulcerans was isolated from a 20-year-old male who presented with a sore throat, and also from his asymptomatic 18-year-old sibling. They both lived in a rural area but had no contact with cattle or raw dairy produce, no data regarding domestic pets were recorded. In 1998 toxigenic C. ulcerans was isolated from a 35-year-old male who presented with respiratory diphtheria with a pseudomembrane. The organism was also isolated from his asymptomatic 11-year-old son. Five household dogs were swabbed but C. ulcerans was not isolated. Details concerning the third asymptomatic C. ulcerans case in Table 2 are not available. The absence of any apparent source of infection for the first two incidents raised the possibility of person-to-person transmission.
Management of contacts was generally undertaken with advice from the Centre for Infections and, from 1999, with reference to UK published guidance [Reference Bonnet and Begg5] and hence was consistent with respect to swabbing of contacts, offering diphtheria vaccine, and prescribing prophylactic antibiotics (macrolides) where necessary.
C. pseudotuberculosis
In addition to the C. diphtheriae and C. ulcerans cases described above, one case of toxigenic C. pseudotuberculosis was reported in 2008. This was the only reported isolation of toxigenic C. pseudotuberculosis from a human in the UK during the study period. The organism was isolated from the aortic root vegetation of an injecting drug user with endocarditis. C. pseudotuberculosis is typically associated with contact with cattle, sheep and goats [Reference Dorella30]; however, this patient had no history of animal contact and no possible source of infection was identified.
DISCUSSION
Diphtheria vaccine is highly effective, and good immunization coverage in the UK has resulted in very few cases of diphtheria being reported over the last 23 years. Although infection has been reported in vaccinated or partially vaccinated individuals, severe or fatal cases have been limited to the unvaccinated, and this analysis demonstrates the protective effect of vaccination. The main risk factor for C. diphtheriae infection remains travel, or contact with someone who has recently travelled, to an endemic area; asymptomatic carriers of C. diphtheriae can pose a threat to unimmunized individuals [Reference Lumio31]. Individuals intending to travel abroad (particularly to the Indian sub-continent, South East Asia or Africa) should ensure they have received all childhood immunizations as well as booster vaccinations appropriate for their destination [Reference Salisbury, Ramsay and Noakes1, Reference Cameron32]. These data also highlight the importance of ensuring those with potential occupational exposure to toxigenic organisms follow UK recommendations and are fully protected by vaccination [Reference Salisbury, Ramsay and Noakes1], and strongly emphasize the importance of microbiology laboratory workers undertaking procedures in containment facilities [Reference Efstratiou and George33].
The epidemiology of diphtheria in the UK appears to be changing with the majority of toxigenic isolates in recent years associated more often with C. ulcerans than C. diphtheriae. Travel does not appear to be a major risk factor for C. ulcerans.
C. ulcerans is a veterinary pathogen and infection in humans was traditionally associated with the consumption of raw milk or dairy products (cow and goat) [Reference Barrett34–Reference Hart36]. The last outbreak of milk-borne diphtheria reported in the UK was in 1943 (prior to the introduction of the national immunization programme) [Reference Barrett34]. However, many cases reported in this paper had no association with raw milk products or farming communities suggesting another source. Although there is no direct evidence of person-to-person transmission for C. ulcerans, this route of transmission was considered following events in the USA and UK in the mid 1990s. In 1997 the US Center for Disease Control and Prevention reported a case of membranous pharyngitis caused by toxigenic C. ulcerans in which it recommended people exposed to the index case should be treated along similar lines to cases exposed to toxigenic C. diphtheriae, because it was considered there was inadequate information about human-to-human transmission [37]. The 1999 UK guidelines for the control of diphtheria were also changed to include the recommendation that anyone who has been in close contact with a case of diphtheria caused by toxigenic C. diphtheriae or C. ulcerans (whatever the clinical presentation) in the previous 7 days should be considered as potentially at risk [Reference Bonnet and Begg5]. This was based on the US recommendation and the report of two asymptomatic C. ulcerans contacts in the UK in 1996 and 1998. However, domestic cats and dogs have recently been proposed as potential sources of human infection [Reference De Zoysa38, Reference Lartigue39]. Identical strains were reported from a UK patient and dogs that the patient had been in contact with [Reference Hogg28]; further studies in this area would be of benefit in order to elucidate the transmission route. The reason for the bias in C. ulcerans cases towards females is unclear although it may be related to the greater tendency for females to consult a general practitioner [40], or could be related to pet ownership habits if domestic animals are indeed a reservoir of C. ulcerans. However, it is thought that about half of households in the UK own pets [Reference McNicholas41]. It is important to note that these analyses are based on small numbers of cases so this discussion is only speculative.
Maintaining high immunization coverage across the UK is essential given the occurrence of sporadic, and apparently indigenous, C. ulcerans cases; clinicians could use routine consultations as opportunities to check the immunization status of elderly patients who may not have received diphtheria immunizations during childhood, and of adult patients born before 1980 who would not have been offered a routine booster dose of diphtheria at school-leaving age (introduced in 1995).
Despite being clinically indicated, several of the cases reported in this paper did not receive antitoxin treatment. In some this was due to the delay in diagnosis; antitoxin has been shown to be ineffective if administered after the second day of diphtheritic symptoms [Reference Logina and Donaghy42]. In the UK, antitoxin can only be obtained from one of nine issuing centres, coordinated by the HPA, CfI [Reference Salisbury, Ramsay and Noakes1]. Antitoxin is given on clinical diagnosis but, as it is an animal blood product, treatment can have severe side-effects so the benefits and risks need careful consideration. Most patients were prescribed appropriate antibiotics although the information available was not always detailed so only limited conclusions can be drawn. The low percentage of patients recorded as receiving a diphtheria vaccine booster during convalescence may be due to this section of the follow-up questionnaire being under-completed if vaccine is generally given after the questionnaire has been returned, or it might highlight a gap in convalescent care.
Cutaneous infection has previously been reported to be more contagious than respiratory diphtheria [Reference Belsey and LeBlanc43–Reference Koopman and Campbell45]; although the data reported in this paper appear to support this, the numbers are too small to adequately test this hypothesis. As well as infection of contacts of cutaneous diphtheria cases, toxigenic organisms were isolated from the throats of six patients with cutaneous infection, suggesting autoinfection.
The presentation of bacterial endocarditis due to toxigenic C. diphtheriae is unusual, and is more commonly reported as due to non-toxigenic [Reference Love46–Reference Tiley48] rather than toxigenic strains [Reference Pennie, Malik and Wilcox49, Reference Lolekha50]. As the fatal cases demonstrated, even severe diphtheria can be unrecognized, or the diagnosis delayed, as most clinicians are unfamiliar with the disease. Case ascertainment may be particularly high in the UK due to the expertise and interest of the London-based WHO Collaborating Centre for Diphtheria & Streptococcal Infections. In addition, some UK laboratories routinely screen all throat swabs for corynebacteria, and hence detect mild and atypical infections.
In the UK, although diphtheria is a statutory notifiable disease, where reporting is supposed to be on clinical suspicion, the majority of notifications relate to non-toxigenic strains [51]. The Centre for Infections can offer advice regarding case management and the reference laboratory provides full species confirmation and toxigenicity testing (full details are provided on the HPA website [52]). The toxin test is the most important component of microbiological diagnosis and it is of concern that in one paper reporting a fatal C. ulcerans case the toxin result was not described and it was not reported to the HPA (although the strain was assumed to be toxigenic because of the pathology described) [Reference Leek7]. For those cases reported to the HPA CfI, the quality of follow-up data was variable and comprised a combination of notes, microbiology reports, and fully/partially completed follow-up forms. Data for recent years was generally more complete due to the use of a standardized follow-up form. It is important to continue to improve the quality and completeness of the surveillance data, particularly for a rare disease such as diphtheria where analyses are based on small numbers of cases. We also recommend the use of literature-searching for unreported cases as good practice in investigations for other rare diseases which may not always be routinely reported. Vaccination histories, particularly for immigrants and elderly patients are often difficult to obtain and hence were sometimes based on assumptions relating to the country of origin and the age of the patient. Despite these limitations the data available has allowed analysis of recent trends and presentations which should be of interest to vaccine policy makers, public health specialists and clinicians encountering a case in the future.
APPENDIX
EU Case Definition for National Diphtheria Surveillance
Community Decision of 19 March 2002 (under 2119/98/EC).
Modified version (by A. Efstratiou, N. Crowcroft, J. White, on behalf of DIPNET, November 2002).
Clinical description
Clinical picture compatible with diphtheria, i.e. an upper respiratory tract illness characterized by sore throat, low grade fever, and an adherent membrane of the tonsils, pharynx or nose or non-respiratory diphtheria; cutaneous, conjunctival, otic and genital lesions.
Laboratory criteria for diagnosis
Isolation of diphtheria toxin-producing corynebacteria from a clinical specimen.
Case classification
Possible: Not applicable.
Probable case: A clinically compatible case that is not laboratory confirmed and does not have an epidemiological link to a laboratory-confirmed case.
Confirmed case: A clinically compatible case that is laboratory confirmed with the isolation of a toxigenic strain of C. diphtheriae, C. ulcerans, or C. pseudotuberculosis or has an epidemiological link to a laboratory-confirmed case.
Confirmed case (other): Non-respiratory/cutaneous diphtheria cases with isolation of toxigenic strains, or cases not meeting the specified clinical criteria but with isolation of toxigenic strains (e.g. mild respiratory diphtheria, or respiratory diphtheria with absence of membrane).
Asymptomatic carriers: Asymptomatic carriers (any anatomical site) with toxigenic strains.
Cases with non-toxigenic C. diphtheriae, C. ulcerans or C. pseudotuberculosis should not be reported.
ACKNOWLEDGEMENTS
The authors thank the clinicians, microbiologists, and consultants in communicable disease control in Health Protection Units who completed follow-up questionnaires. Thanks are also due to Sheila O'Malley from the Health Protection Agency library for assistance with the literature search.
DECLARATION OF INTEREST
None.









