Introduction
Idiopathic normal pressure hydrocephalus (iNPH) is a neurological disorder hallmarked by ventriculomegaly from abnormal build-up of intracranial cerebrospinal fluid (CSF) due to imbalances in CSF formation and clearance. It primarily presents with a combination of core symptoms, including gradually worsening urinary incontinence, symmetric gait disturbance, and cognitive impairment (i.e., Hakim’s triad; McGirt et al., Reference McGirt, Woodworth, Coon, Thomas, Williams and Rigamonti2005; Williams et al., Reference Williams, Nagel, Luciano, Relkin, Zwimpfer, Katzen, Holubkov, Moghekar, Wisoff, McKhann, Golomb, Edwards and Hamilton2019). Treatment primarily involves shunt placement to redistribute excess CSF elsewhere in the body for reabsorption (McGirt et al., Reference McGirt, Woodworth, Coon, Thomas, Williams and Rigamonti2005; Williams & Malm, Reference Williams and Malm2016; Williams et al., Reference Williams, Nagel, Luciano, Relkin, Zwimpfer, Katzen, Holubkov, Moghekar, Wisoff, McKhann, Golomb, Edwards and Hamilton2019).
Alzheimer’s disease (AD) is among the most common co-occurring pathologies in individuals with iNPH (Bech-Azeddine et al., Reference Bech-Azeddine, Høgh, Juhler, Gjerris and Waldemar2007; Cabral et al., Reference Cabral, Beach, Vedders, Sue, Jacobson, Myers and Sabbagh2011; Malm et al., Reference Malm, Graff-Radford, Ishikawa, Kristensen, Leinonen, Mori, Owler, Tullberg, Williams and Relkin2013). Several CSF biomarkers, imaging, and neurocognitive studies have shown that the presence or absence of AD pathology may be associated with post-shunt outcomes (Hong et al., Reference Hong, Kim, Jeong, Kim, Hwang, Lee, Lee and Na2018; Kazui et al., Reference Kazui, Kanemoto, Yoshiyama, Kishima, Suzuki, Sato, Suehiro, Azuma, Yoshimine and Tanaka2016; Niermeyer et al., Reference Niermeyer, Gaudet, Malloy, Piryatinsky, Salloway, Klinge and Lee2020; Pomeraniec et al., Reference Pomeraniec, Bond, Lopes and Jane2016; Yasar et al., Reference Yasar, Jusue-Torres, Lu, Robison, Patel, Crain, Carson, Hoffberger, Batra, Sankey, Moghekar and Rigamonti2017). However, results are inconsistent, with some linking AD pathology to relatively poorer outcomes, and others reporting comparable postsurgical improvements regardless of AD status. Thus, it is challenging to determine the degree of AD pathology that, if present, would mitigate the potential benefits of shunting.
Apart from inconsistent results, studies differ in the proteinaceous biomarkers used to quantify baseline AD pathology, with some relying on biomarkers that are less specific to AD or less sensitive to its clinical progression. For example, some studies operationalized AD status using neuritic plaque counts (e.g., Pomeraniec et al., Reference Pomeraniec, Bond, Lopes and Jane2016; Yasar et al., Reference Yasar, Jusue-Torres, Lu, Robison, Patel, Crain, Carson, Hoffberger, Batra, Sankey, Moghekar and Rigamonti2017) or baseline levels of total tau/amyloid-beta (Aβ) in CSF, which are weakly correlated AD clinical progression and sensitive to non-neurodegenerative factors (Blennow & Zetterberg, Reference Blennow and Zetterberg2015; Nelson et al., Reference Nelson, Alafuzoff, Bigio, Bouras, Braak, Cairns, Castellani, Crain, Davies, Del Tredici, Duyckaerts, Frosch, Haroutunian, Hof, Hulette, Hyman, Iwatsubo, Jellinger, Jicha and Beach2012). The ratio of phosphorylated tau (p-tau) to Aβ in CSF, used by Hong et al. (Reference Hong, Kim, Jeong, Kim, Hwang, Lee, Lee and Na2018) is one such protein marker that is both specific to neurodegeneration and highly correlated with AD clinical progression (Blennow et al., Reference Blennow, Vanmechelen and Hampel2001; Hampel et al., Reference Hampel, Blennow, Shaw, Hoessler, Zetterberg and Trojanowski2010; Kandimalla et al., Reference Kandimalla, Prabhakar, Wani, Kaushal, Gupta, Sharma, Grover, Bhardwaj, Jain and Gill2013). However, Hong et al. (Reference Hong, Kim, Jeong, Kim, Hwang, Lee, Lee and Na2018) were unable to determine if baseline p-tau/Aβ predicted shunt outcomes due to the relatively small number of CSF assays available in their sample.
Previous studies also vary in terms of methodology for evaluating cognitive outcomes; some solely relied on subjective report (Pomeraniec et al., Reference Pomeraniec, Bond, Lopes and Jane2016), whereas others utilized brief measures of global cognitive functioning (Hong et al., Reference Hong, Kim, Jeong, Kim, Hwang, Lee, Lee and Na2018; Yasar et al., Reference Yasar, Jusue-Torres, Lu, Robison, Patel, Crain, Carson, Hoffberger, Batra, Sankey, Moghekar and Rigamonti2017). One study utilized a multi-domain cognitive assessment (Kazui et al., Reference Kazui, Kanemoto, Yoshiyama, Kishima, Suzuki, Sato, Suehiro, Azuma, Yoshimine and Tanaka2016); however, it did not measure all cognitive domains. Further, this study assessed executive function, a cognitive domain known to be impacted by iNPH (Peterson et al. Reference Peterson, Savulich, Jackson, Killikelly, Pickard and Sahakian2016) using the Frontal Assessment Battery, an instrument sensitive to gross executive dysfunction, but with potential ceiling effects inherent in its design as a bedside screener (Dubois et al., Reference Dubois, Slachevsky, Litvan and Pillon2000; Hurtado-Pomares et al., Reference Hurtado-Pomares, Carmen Terol-Cantero, Sánchez-Pérez, Peral-Gómez, Valera-Gran and Navarrete-Muñoz2018; Ilardi et al., Reference Ilardi, Chieffi, Scuotto, Gamboz, Galeone, Sannino, Garofalo, La Marra, Ronga and Iavarone2022). Finally, most longitudinal studies of these individuals have relied on a single postoperative time point (vs. multiple time points) for comparison with patients’ baselines, ranging from several months to years (Hong et al., Reference Hong, Kim, Jeong, Kim, Hwang, Lee, Lee and Na2018; Kazui et al., Reference Kazui, Kanemoto, Yoshiyama, Kishima, Suzuki, Sato, Suehiro, Azuma, Yoshimine and Tanaka2016; Pomeraniec et al., Reference Pomeraniec, Bond, Lopes and Jane2016; Yasar et al., Reference Yasar, Jusue-Torres, Lu, Robison, Patel, Crain, Carson, Hoffberger, Batra, Sankey, Moghekar and Rigamonti2017).
In an effort to address some of the above-mentioned limitations, this study aimed to use a mixed factorial design (i.e., between- and within-subject comparisons) to explore if having comorbid AD (iNPH + AD) versus iNPH without comorbid AD affects post-shunt outcomes over time. Specifically, using continuous variables, this study evaluated changes in gait, incontinence, cognitive functioning, and activities of daily living (ADLs) from preoperative baseline to 3, 12, and 24–60 months after placement of a ventriculoperitoneal (VP) shunt (Aim 1). Additionally, by dichotomizing outcomes (i.e., improved/not improved) 3 months after VP shunt placement, this study aimed to identify if certain presurgical variables (e.g., extent of AD pathology, functional independence, age at shunting, neurocognitive functioning, first iNPH symptom) can predict reductions in one or more of the core iNPH symptoms (Aim 2).
In addition to exploring iNPH and iNPH + AD outcomes across multiple time points, this study measured cognitive outcomes using a comprehensive multi-domain neuropsychological assessment battery. This was done to improve on the understanding of postoperative cognitive outcomes relative to methods used in some earlier studies in this area. Lastly, similar to Hong et al. (Reference Hong, Kim, Jeong, Kim, Hwang, Lee, Lee and Na2018), this study will utilize p-tau and Aβ from presurgical CSF samples when determining if the extent of baseline AD pathology influences iNPH symptom outcome after VP shunt placement.
Hypotheses
Hypothesis 1: We hypothesized that both groups (iNPH and iNPH + AD) would display reductions in at least one core iNPH symptom 3-months after surgery. We also expected the iNPH group would remain relatively stable over time, whereas iNPH + AD individuals would show subsequent downward trends in cognitive functioning.
Hypothesis 2: Despite having been more exploratory in nature, we expected that some presurgical variables will yield predictive value in determining 3-month postoperative symptom reduction.
Method
Participants
This study utilized archival data from patients and their caregivers collected as part of an iNPH outcome study at Butler Hospital’s Normal Pressure Hydrocephalus clinic (IRB Study Title: Normal pressure hydrocephalus: Advancing diagnosis and treatment). The study was approved by the Butler Hospital Institutional Review Board and conducted in accordance with the Helsinki Declaration. All participants provided informed consent. Participants were recruited consecutively from 2009 to 2015. Figure 1 depicts the timeline of procedures for participants; inclusion and exclusion criteria for the originating iNPH outcome study are included in the accompanying caption.

Figure 1. Sequence of date collection for iNPH outcome study participants.
Note. Figure 1 provides a visualization of data collection for study participants over the course of the Idiopathic Normal Pressure Hydrocephalus (iNPH) outcome study conducted Butler Hospital from 2009 to 2015. Inclusion criteria for the originating iNPH outcome study were referral to the hospital clinic with at least one core iNPH symptom and supportive radiological findings (i.e., ventricular dilation disproportionate to atrophy present). iNPH diagnoses and shunt eligibility were determined by a neurologist (SS) and neurosurgeon (PK) who are experts in iNPH, based on clinical symptomatology, radiological findings, and evidence of symptom reduction following a high-volume lumbar puncture (i.e., walking speed/stride length, motor speed/dexterity, and/or cognitive functioning). Initial iNPH symptoms and time since symptom onset were recorded based on patients’ and informants’ reports. Exclusion criteria included: history of substance abuse within the past year; history of acute neurological events (e.g., large vessel stroke, neoplasm in the brain, etc.); neurologist’s/neurosurgeon’s diagnosis of secondary hydrocephalus (i.e., due to an unrelated neurological condition); previous shunt insertion; or inability to comply with the formal assessment schedule. Those who deteriorated or showed no improvement after shunt placement underwent workup (i.e., computerized tomography studies, shuntogram to rule-out shunt malfunction/obstruction), followed by shunt-valve adjustment to improve drainage. 63 individuals consented to participate, of whom 33 underwent shunt placement and at least one follow-up visit postoperatively. Two individuals had follow-up visits at outside institutions and their data was therefore not included in subsequent longitudinal analyses. Gait, incontinence, upper motor dexterity, and cognition were routinely evaluated beginning at patient's baseline evaluations for study eligibility. For those who qualified for the study, all baseline measures were repeated 3 months, 12 months, and every subsequent year following ventriculoperitoneal (VP) shunt placement. Participants underwent a High-volume Lumbar Puncture (HVLP) as part of determining candidacy for shunt placement, which included assessment of post-HVLP changes in gait, incontinence, and upper motor dexterity/speed. Alzheimer's Page 40 of 47 Under review at JINS - Do not cite - Do not distribute Journal of the International Neuropsychological S For Peer Review disease (AD) biomarker data was also obtained during study enrollment in the form of cerebral spinal fluid (CSF) samples (from HVLP) to be assayed by a third-party lab (i.e., levels of total tau, phosphorylated tau, and beta-amyloid (Aβ)) and/or standardized uptake value ratios of Aβ (SUVR) on positron emission tomography (PET) scans.
Full NP Battery = Neuropsychological testing, including the Mini Mental Status Exam (MMSE), Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), Trail Making Test (TMT) A & B, Color-Word Interference Test (CWIT) and Digit Span Backward. Informant measures = Frontal Systems Behavior Rating Scale (FrSBe) and Lawton-Brody ADL Questionnaire. Upper motor dexterity = Serial Doting Task and Line Tracing Task.
In compiling iNPH outcome study data for this archival analysis, inclusion criteria consisted of completion of a baseline cognitive and neurological evaluation and at least one follow-up evaluation (i.e., postoperatively) at the clinic. Of the 63 individuals who consented to take part in the iNPH outcome study, 33 underwent VP shunt placement and at least one follow-up visit. Data were excluded (n = 2) due to baseline neuropsychological assessments being completed at another institution using a different test battery. Case data from the remaining 31 individuals were included in the analyses. All 31 individuals (iNPH = 20; iNPH + AD = 11) completed their 3-month follow-up visit. 26 individuals (iNPH = 16; iNPH + AD = 10) completed a 1-year follow-up, and 16 individuals (iNPH = 8; iNPH + AD = 8) were seen for follow-up 2–5 years after surgery. When comparing baseline demographic, cognitive, or pathological (e.g., AD biomarkers) variables in those with only one follow-up (i.e., 3-months) to those with 1-year and/or 2–5-year follow-up data, groups differed only in that those with Year 1 or Years 2–5 follow-up data had more years of education (U(1) = 177.5, p = 0.021) than those lost to follow-up. Postsurgical follow-ups were tracked by research coordinators who phoned patients and/or their caregivers to remind them of and schedule upcoming appointments. Two deaths were confirmed during the study period. The cause of higher lost-to-follow-up rate among the iNPH individuals (relative to iNPH + AD) is unclear. One potential explanation is that caregivers of those with iNPH + AD are more involved in managing participants’ schedules, making them more likely to return clinic phone calls and/or less likely to miss subsequent follow-up visits.
Based on a power analysis conducted through G * Power (Faul et al., Reference Faul, Erdfelder, Lang and Buchner2007, Reference Faul, Erdfelder, Buchner and Lang2009) the current sample size (n = 31) provided enough statistical power (>0.8) to detect small to medium effect sizes using an error probability of 0.05.
Measures
AD biomarkers
Comorbid AD status was determined by the presence of at least one positive AD biomarker (e.g., amyloid positron emission tomography (PET) or CSF markers). PET standardized uptake value ratios of Aβ (SUVR) of > 1.1 were considered positive for AD (Clark et al., Reference Clark, Schneider, Bedell, Beach, Bilker, Mintun, Pontecorvo, Hefti, Carpenter, Flitter, Krautkramer, Kung, Coleman, Doraiswamy, Fleisher, Sabbagh, Sadowsky, Reiman and Reiman2011). CSF results were deemed positive for AD if total tau to amyloid ratio was >1.0. One CSF sample was compromised in transit and one sample yielded invalid results during assay at the lab. All participants had data from at least one biomarker (i.e., CSF or PET) available. Seven iNPH + AD individuals had PET data available and seven iNPH individuals had PET data available. CSF data were available for all 20 iNPH individuals and 9 out of 11 iNPH + AD individuals. 12 individuals had both PET and CSF data available (iNPH = 7; iNPH + AD = 5). In cases of discrepant CSF and PET results (i.e., one suggestive of AD and the other not suggestive of AD), deference was given to the patient’s PET scan results.
Gait
Gait was measured both by average number of steps and completion time when walking a 10-meter distance. Videos of patients were coded by a trained research assistant (CW). Interrater reliability of this research assistant (CW) with the established coding standard on a training set of 10 videos was excellent (ICC range for steps and time forward and backward = 0.90–0.97).
Incontinence
Level of incontinence was determined by a neurologist’s rating on a 6-point Likert scale where 1 = “Normal,” 2 = “Urgency without Incontinence,” 3 = “Infrequent Incontinence without a Pad,” 4 = “Infrequent Incontinence with a Pad,” 5 = “Bladder Incontinence,” and 6 = “Bladder and Bowel Incontinence.”
Cognition
All patients underwent comprehensive neuropsychological evaluations during preoperative and postoperative workups. Excluding tests of executive functions and upper motor dexterity, only one measure from each cognitive domain (e.g., attention, processing speed, visuoconstruction, fluency, wordlist memory, episodic memory) was included for analysis. In cases where multiple measures assessing the same domain were available, preference was given to those with score distributions most closely resembling a Gaussian distribution in the test’s standardization sample.
Global cognitive functioning
The Mini-Mental Status Examination (MMSE; Folstein et al., Reference Folstein, Folstein and McHugh1975) was used as an indicator of global cognitive functioning in order to maintain comparability with previous studies. The total index score from the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Randolph, Reference Randolph1998) was included as another measure of global cognition. Participants were administered differing RBANS forms (Form A, B, D, C) at follow-up clinic visits in a randomized order to reduce practice effects.
Executive functions
The Trail Making Test Part B efficiency score (TMT-Be) proposed by Correia et al. (Reference Correia, Ahern, Rabinowitz, Farrer, Smith Watts, Salloway, Malloy and Deoni2015) was used, as this method increases the range of scores among more impaired individuals and has been shown useful in tracking changes in multitasking/set-shifting over time (Smith Watts et al., Reference Smith Watts, Ahern, Jones, Farrer and Correia2019). Z-scores from the third trial (i.e., inhibition trial) of both the Stroop (Golden & Freshwater, Reference Golden and Freshwater2002) and Delis–Kaplan Executive Function System (D-KEFS; Delis et al., Reference Delis, Kaplan and Kramer2001) Color-Word Interference Tests (CWIT) were included as measures of timed inhibitory control. This study also included Z-scores of longest digit span backward (LDS-B) from the Digit Span subtest of the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV; Wechsler, Reference Wechsler2008) to measure auditory working memory. The post-onset Executive Dysfunction subscale from the informant-rated Frontal Systems Behavior Scale (FrSBe-ED; Grace et al., Reference Grace, Grace and Malloy2001) was included as well, as prior research by Niermeyer et al. (Reference Niermeyer, Gaudet, Malloy, Piryatinsky, Salloway, Klinge and Lee2020) using this sample identified significant elevations in this scale among those with iNPH + AD relative to those with iNPH alone.
Upper motor dexterity
The Line Tracing Test (LTT) and the Serial Dotting Test (SDT) were used to measure patients’ fine motor dexterity (Schomerus et al., Reference Schomerus, Weissenborn, Hamster, Rückert and Hecker1999; Weissenborn et al., Reference Weissenborn, Ennen, Schomerus, Rückert and Hecker2001). We used a combined completion time and error score for the LTT for data analysis proposed by Rossetti et al. (Reference Rossetti, Piryatinsky, Ahmed, Klinge, Relkin, Salloway, Ravdin, Brenner, Malloy, Levin, Broggi, Gavett, Maniscalco and Katzen2016), as well as total completion times for the SDT. A research assistant (CW) scored patients’ LTTs following training and after interrater reliability was established. The research assistant’s interrater reliability with a set of 12 LTTs independently scored by two of the researchers (DG & IP) was excellent (ICC = 0.99).
Other domains
Auditory attention was measured by patients’ longest span correct on the Digit Span subtest from the RBANS (Randolph, Reference Randolph1998). Processing speed was measured by Trail Making Test Part A (TMT-A; Reitan & Wolfson, Reference Reitan and Wolfson1993) completion time. Select subtests from the RBANS (Randolph, Reference Randolph1998) were used: Semantic Fluency (fluency); Figure Copy (visuoconstruction); List Learning, Recall and Recognition (wordlist memory); and Story Learning and Recall (episodic memory). The Zarit Caregiver Burden Scale (Zarit et al., Reference Zarit, Reever and Bach-Peterson1980) was used to measure baseline levels of caregiver burden.
ADL functioning
Functional status was determined by informant ratings on the Lawton–Brody ADL Questionnaire (Lawton–Brody; Lawton & Brody, Reference Lawton and Brody1969), which captures functioning in instrumental (e.g., bill-paying, shopping, food preparation, medication management) and personal (e.g., bathing, dressing, grooming, feeding, toileting) ADLs. Each of the Lawton–Brody items was scored using a trichotomous scoring system; responses indicating the highest level of functional independence were assigned two points, whereas responses indicating the lowest level of independence were assigned zero points. Any response falling between these two poles was assigned 1 point. Questions that were omitted were automatically assigned a score of 1; however, in cases where four or more questions were omitted, scores were not assigned to personal ADL or instrumental ADL totals.
Operationalizing symptom improvement 3 months after shunt placement
To examine our second hypothesis of predicting shunt response after 3 months, each of the three core iNPH symptoms was dichotomized based on an individual’s status 3 months after surgery relative to their respective baseline. Improved gait 3 months after surgery was defined as a decrease of >1.5 standard deviations (of the baseline of the total sample) in either average number of steps or completion time when walking a 10-meter distance. Improvement in incontinence was defined as a one-point decrease in neurologists’ ratings on the 6-point Likert scale of bladder incontinence. Improvement in cognitive functioning was defined as patients fulfilling one of the following criteria: (1) increases in RBANS total Standard Scores of >19 points (i.e., Z-score of 1.3) consistent with the reliable change values provided in the RBANS manual (Randolph, Reference Randolph1998); or (2) increases of >1.5 standard deviations on at least two measures of frontal executive functions. For tests converted into a demographically corrected Z-score (e.g., CWIT, LDS-B, FrSBe-ED), improvement was defined as a Z-score change of 1.5 the direction consistent with better performance. Improvement on the TMT-Be was defined as a decrease (i.e., improvement) >1.5 standard deviation of the control group described by Correia et al. (Reference Correia, Ahern, Rabinowitz, Farrer, Smith Watts, Salloway, Malloy and Deoni2015).
Statistical analysis & data treatment
All analyses were performed using version 27 of SPSS for Windows. Due to the non-normal distributions of certain variables, nonparametric testing (i.e., Mann–Whitney U test) was used for comparing baseline descriptive statistics among the groups, though similar results were obtained with parametric testing as well.
Missing data were imputed using linear regression modeling. A decision to use imputed data was made for each variable at each time point. Data was not imputed if 40% or more of participants were missing values for said variable at a given time point. Variables were also not imputed in cases when doing so would require utilization of an already imputed value. Participant data from 24–60 months was not imputed. Table 1 provides additional information regarding missing variables and the number imputed using linear regression modeling.
Table 1. Number of missing variables at baseline, 3 months, and 12 months before and after imputation

iNPH = normal pressure hydrocephalus without comorbid Alzheimer’s disease; iNPH + AD = normal pressure hydrocephalus with comorbid Alzheimer’s disease; CWIT = Color-Word Interference Test; FrSBE-ED = Frontal Systems Behavior Rating Scale Executive Dysfunction Subscale; Lawton-B IADLs = Lawton–Brody instrumental activities of daily living; Lawton-B PADLs = Lawton–Brody personal activities of daily living; LTT = line tracing task; LDS-F = longest digit span forward; LDS-B = longest digit span backward; MMSE = mini mental status exam; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; SDT = serial doting task; TMT-A = Trail Making Test Part A; TMT-Be = Trail Making Test Part B efficiency score.
[n of missing cells preimputation]/[n of missing cells after imputation]; n/a indicates no missing values.
Hypothesis 1: We used a mixed effect model to determine the interaction and main effects of time since VP shunt placement (time) and AD comorbidity (iNPH + AD, iNPH) on shunt outcomes (functional status, gait, incontinence, and cognition). To adjust for the risk of a Type-I error, a Bonferroni-corrected significance value adjusting for the number of executive functioning measures was used to reevaluate the significance of any interaction effects observed in this domain (i.e., p values = 0.0125).
Hypothesis 2: A series of binary logistic regressions were performed to identify potential baseline predictors of a positive shunt response at 3 months. Using the procedures outlined above, changes in gait, incontinence and cognition 3 months after shunt placement were dichotomized as either improved or not improved to serve as the dependent variables for logistic regression modeling. Baseline p-tau/Aβ CSF ratios, total Lawton–Brody scores, age, initial iNPH symptoms, and MMSE and RBANS total scores were selected as potential a priori predictors of reduction in iNPH symptoms 3 months after shunt placement. While modeling each outcome, nonsignificant variables were removed incrementally based on their respective p values (i.e., removing higher p values to improve the predictive model).
Results
Descriptive, clinical, and AD biomarker characteristics of the iNPH + AD (n = 11) and iNPH (n = 20) groups are provided in Table 2. Both groups were similar in terms of gender distributions (iNPH %Men = 45; iNPH + AD %Men = 64), age (iNPH = 74.2 ± 6.0; iNPH + AD = 77.2 ± 8.1), years of education (iNPH = 13 ± 2.6; iNPH + AD = 13.8 ± 2.7), as well as duration of neurological symptoms and levels of caregiver burden. As expected, iNPH + AD individuals had significantly higher baseline levels of Aβ SUVRs (p < 0.001) on PET and p-tau/Aβ in CSF (p = 0.02). See Table 2 for more information.
Table 2. Baseline descriptive statistics for patients with normal pressure hydrocephalus with and without Alzheimer’s disease

iNPH = normal pressure hydrocephalus without comorbid Alzheimer’s disease; iNPH + AD = normal pressure hydrocephalus with comorbid Alzheimer’s disease; Ab-42 = 42 amino acid form of amyloid-beta; Ab-42 SUVR = standardized uptake value ratio of amyloid-beta on positron emission tomography imaging; CSF = cerebrospinal fluid; P-Tau = phosphorylated tau protein.
* Higher scores indicative of poorer outcome.
† p values for continuous variables based on results of an independent samples Mann–Whitney U test.
Main and interaction effects of time & AD comorbidity on shunt outcome
Means and standard deviations of iNPH and iNPH + AD individuals on measure of gait, incontinence, cognition, dexterity, and ADL functioning across time points (baseline, 3 months, 12 months, 24–60 months) are presented in Table 3. Results of the mixed effect model described below are also presented in Table 4. Figures 2, 3, and 4 provide visuals of the results of the mixed effect model.
Table 3. Means and SDs of iNPH patients with and without comorbid Alzheimer’s disease over time

iNPH = normal pressure hydrocephalus without comorbid Alzheimer’s disease; iNPH + AD = normal pressure hydrocephalus with comorbid Alzheimer’s disease; CWIT = Color-Word Interference Test; FrSBE-ED = Frontal Systems Behavior Rating Scale Executive Dysfunction Subscale; Lawton-B IADLs = Lawton–Brody instrumental activities of daily living; Lawton-B PADLs = Lawton–Brody personal activities of daily living; LTT = line tracing task; LDS-F = longest digit span forward; LDS-B = longest digit span backward; MMSE = mini mental status exam; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; SDT = serial doting task; TMT-A = Trail Making Test Part A; TMT-Be = Trail Making Test Part B efficiency score.
* Higher scores = worse performance.
† Significant baseline differences between iNPH and iNPH + AD groups.
Table 4. Main & interaction effects of time since shunt placement & Alzheimer’s disease comorbidity on shunt outcome

CWIT = Color-Word Interference Test; FrSBE-ED = Frontal Systems Behavior Rating Scale Executive Dysfunction Subscale; Lawton-B IADLs = Lawton–Brody instrumental activities of daily living; Lawton-B PADLs = Lawton–Brody personal activities of daily living; LTT = line tracing task; LDS-F = longest digit span forward; LDS-B = longest digit span backward; MMSE = mini mental status exam; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; SDT = serial doting task; TMT-A = Trail Making Test Part A; TMT-Be = Trail Making Test Part B efficiency score.

Figure 2. iNPH & iNPH + AD scores on gait, incontinence, motor dexterity, and ADL measures.
Note. iNPH = normal pressure hydrocephalus without comorbid Alzheimer’s disease; iNPH + AD = normal pressure hydrocephalus with comorbid Alzheimer’s disease. Instrumental ADLs = Lawton–Brody instrumental activities of daily living; personal ADLs = Lawton–Brody personal activities of daily living; LTT = line tracing task; SDT = serial doting task.

Figure 3. iNPH & iNPH + AD scores on MMSE & RBANS measures.
Note. iNPH = normal pressure hydrocephalus without comorbid Alzheimer’s disease; iNPH + AD = normal pressure hydrocephalus with comorbid Alzheimer’s disease. LDS-F = longest digit span forward; MMSE = mini mental status exam; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status.
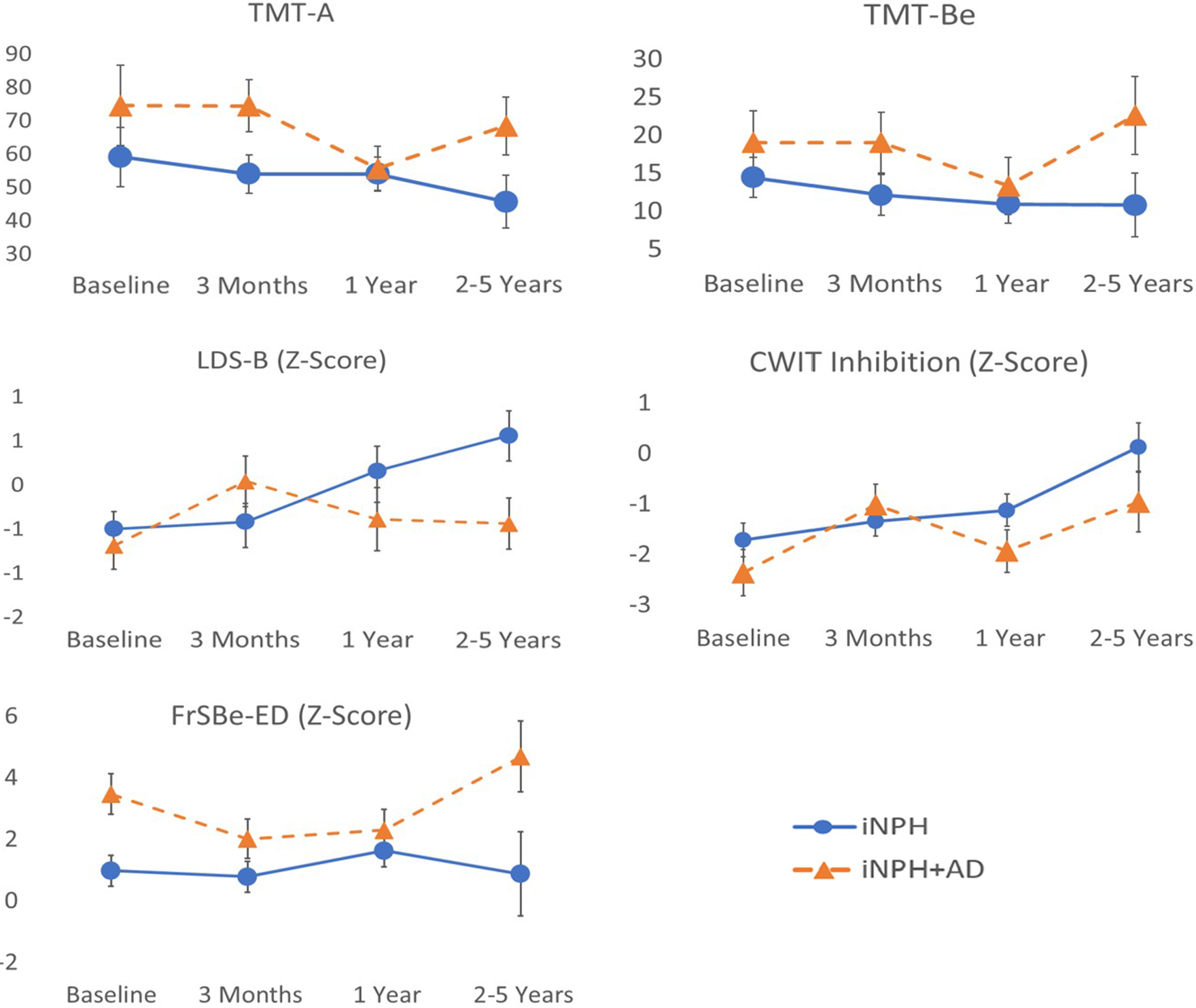
Figure 4. iNPH & iNPH + AD scores on processing speed & executive functioning measures. Note. iNPH = normal pressure hydrocephalus without comorbid Alzheimer’s disease; iNPH + AD = normal pressure hydrocephalus with comorbid Alzheimer’s disease. CWIT = Color-Word Interference Test; FrSBE-ED = Frontal Systems Behavior Rating Scale Executive Dysfunction Subscale; LDS-B = longest digit span backward; TMT-A = Trail Making Test Part A; TMT-Be = Trail Making Test Part B efficiency score.
Gait
There was a significant main effect of time since shunt placement on time to walk a 10-meter distance (F(3,91) = 5.6, p = 0.001), while there was no significant main effect of AD comorbidity or interaction effect between these variables. Relative to patients’ baselines, both groups showed relative improvement in walking speed at 3 months and 12 months, with a slight regression toward baseline levels at 24–60 months.
Incontinence
There was a significant main effect of time on Bladder Scale (F(3,77) = 5.3, p = 0.002), while there was no significant main effect of AD. No interaction effect was found. Relative to baseline ratings of incontinence, both groups showed improvements in bladder control at 3 months and 12 months, with a trend toward baseline levels of incontinence at 24–60 months.
Global cognitive functioning
There was a significant main effect of AD comorbidity on MMSE scores (F(1,93) = 7.07, p = 0.009); the iNPH + AD scored lower than the iNPH group at all time points. There was a significant main effect of time since shunt placement on RBANS total Z-scores (F(3,96) = 3.4, p = 0.021); relative to their respective baselines, both groups displayed higher total scores on the RBANS at 3 months, 12 months, and 24–60 months. There were no significant interaction effects.
Executive functions
There were no significant main effects of time or AD on the LDS-B. There was a significant interaction effect between time and AD comorbidity (F(3,94) = 3.8, p = 0.012). Those in the iNPH group scored slightly higher than iNPH + AD individuals at baseline. At 3-month follow-up, individuals in the iNPH group showed stable LDS-B relative to their baseline, whereas iNPH + AD individuals showed considerable improvements from their baseline. However, LDS-B scores of those in the iNPH group improved at 12 months and improved further at 24–60 months whereas iNPH + AD individuals declined at 12 months and at 24–60 months to the point where they approached near baseline levels.
There was a significant main effect of time on the CWIT inhibition trial (F(3,91) = 6.8, p < 0.001), but no main effect of AD. There was a significant interaction effect between time and AD (F(3,91) = 4.0, p = 0.010). Both iNPH + AD and iNPH groups improved at 3 months relative to their respective baselines. While scores in the iNPH group continued to steady show improvements at subsequent follow-ups, scores in the iNPH + AD group were more variable (i.e., decrease to near baseline levels at 12 months with return to previous peak at 24–60 months) with minimal gains beyond those observed 3 months postoperatively.
There were significant main effects of time since shunt placement (F(3,78) = 4.3, p = 0.008) and AD comorbidity (F(1,78) = 6.18, p = 0.015) on the FrSBe-ED subscale, as well as a significant interaction effect between these variables (F(3,78) = 3.5, p = 0.019). The iNPH + AD scored higher (i.e., worse) than the iNPH group at baseline. iNPH individuals showed minimal changes from baseline at 3 months and 12 months, whereas those in the iNPH + AD group improved at 3 months (i.e., lower score) and maintained these improvements at 12 months. At the 24–60-month follow-up, iNPH individuals showed lower FrSBe-ED scores (i.e., less executive dysfunction) compared to preceding time points, whereas those in the iNPH + AD group showed the opposite trend with higher-than-baseline FrSBe-ED scores (i.e., more executive dysfunction symptoms than at baseline).
There were no significant main effects of time and AD or an interaction effect on the TMT-Be.
After applying Bonferroni correction procedures, interaction effects between time and AD comorbidity remained significant on the CWIT and LDS-B.
Upper motor dexterity
Three was no main effect of time on upper motor dexterity measures, but there was a significant effect of AD comorbidity on SDT completion times (F(1,96) = 7.7, p = 0.007) and LTT combined time and error scores (F(1,96) = 7.3, p = 0.008); those with iNPH + AD performed worse than iNPH individuals. No interaction effect was found.
Other cognitive domains
On tasks from the RBANS, there was a significant main effect of time on List Learning (F(3,96) = 5.8, p = 0.001) seemingly as a result of both groups displaying lower scores 12 months after surgery relative to performance across all other time points. Further analysis of this variable revealed a lower median List Learning score and greater standard deviation for the entire sample at 12 months relative to all other time points. As such, this finding may reflect a type-I error possibly due to random variability within the sample or external factors (e.g., environmental, examiner), rather than reflecting clinically meaningful change over time. There was a significant effect of AD comorbidity on Semantic Fluency (F(1,96) = 6.4, p = 0.013) and List Recognition (F(1,95) = 5.3, p = 0.024) in that those with iNPH + AD performed worse than iNPH individuals. There were no significant main effects of time or AD comorbidity or interaction effects on the TMT-A, LDS-F, Figure Copy, List Recall, or Story Memory and Recall tasks.
ADL functioning
There were no main or interaction effects on measures of personal or instrumental ADLs.
Baseline predictors of iNPH symptom reduction after 3 months
The proportion of patients in each group satisfying criteria for improvement in gait, incontinence, and cognition 3 months after shunt placement are shown in Table 5. Groups were not significantly different at 3 months in most areas with the exception of a higher proportion of iNPH + AD individuals (46%) showing improvements in executive functioning relative to the iNPH (10%) group (p = 0.024).
Table 5. Rates of symptom improvement 3 months after shunt placement

iNPH = normal pressure hydrocephalus without comorbid Alzheimer’s disease; iNPH + AD = normal pressure hydrocephalus with comorbid Alzheimer’s disease; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status.
* Improvement in iNPH symptoms is not mutually exclusive. Improved gait was defined as a decrease of >1.5 standard deviations (of the total sample’s baseline) in either average number of steps or completion time when walking a 10-meter distance. Improved incontinence was defined as a one-point decrease in neurologists’ ratings on the 6-point Likert scale of bladder incontinence. Improved cognitive functioning was defined as patients either: (1) increases in RBANS total Standard Scores of >19 points (i.e., Z-score of 1.3) consistent with the reliable change values provided in the RBANS manual (Randolph, Reference Randolph1998); or (2) increases of >1.5 standard deviations on at least two measures of frontal executive functions.
Results of the models predicting improvements in each of the three iNPH symptoms are shown in Table 6.
Table 6. Baseline factors predicting symptom reduction 3 months after shunt placement

OR = odds ratio; MMSE = Mini Mental Status Examination; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status.
Improved gait
Logistic regression using participants’ baseline p-tau/Aβ CSF ratio, total ADL scores, ages, initial iNPH symptoms, and MMSE and RBANS total scores did not predict improvements in gait at 3 months.
Improved bladder control
Higher MMSE scores (b = 0.83; p = 0.009; OR = 2.29) and lower RBANS total Z-scores (b = -1.56; p = 0.035; OR = 0.21) at baseline predicted improvements in incontinence 3 months after shunt placement. Baseline p-tau/Aβ CSF ratios, total ADL scores, age, and initial iNPH symptom were not predictive.
Improved cognition
Age at baseline was inversely associated with improvements in cognitive functioning (b = -0.17; p = 0.04; OR = 0.85), whereas baseline p-tau/Aβ CSF ratios, total ADL scores, initial iNPH symptom, MMSE, and RBANS total scores were not significantly associated with postoperative improvements in cognition.
Discussion
This study aimed to compare postoperative outcomes among iNPH patients with and without AD using a multi-domain neurocognitive battery, and determine if certain baseline features could predict reductions in iNPH symptoms 3 months after shunt placement. Results revealed comparable improvements between groups in gait, bladder control, and global cognition, whereas neither group showed any significant changes in ADLs postoperatively. These findings support the potential benefits of shunt placement regardless of AD comorbidity, and are consistent with other longitudinal studies of iNPH outcomes (Grasso et al., Reference Grasso, Torregrossa, Leone, Frisella and Landi2019; Illán-Gala et al., Reference Illán-Gala, Pérez-Lucas, Martín-Montes, Máñez-Miró, Arpa and Ruiz-Ares2017; Pujari et al., Reference Pujari, Kharkar, Metellus, Shuck, Williams and Rigamonti2008). However, analyses of individual cognitive domains revealed divergent postoperative trajectories as a function of AD comorbidity on measures of executive functions including working memory and timed inhibitory control. Specifically, those with iNPH either improved or remained stable over time, whereas those with iNPH + AD showed more variability over time and at times performed near their presurgical baselines, with only marginal improvements beyond those observed at 3 months. Considering that AD pathology inevitably advances beyond medial temporal structures (see Weintraub et al., Reference Weintraub, Wicklund and Salmon2012), the observed divergence in cognitive outcomes theoretically could reflect expected progression of AD pathology to prefrontal networks subserving executive functions in those with iNPH + AD. These findings of differing postoperative cognitive outcomes are consistent with Hong et al. (Reference Hong, Kim, Jeong, Kim, Hwang, Lee, Lee and Na2018) and Pomeraniec et al. (Reference Pomeraniec, Bond, Lopes and Jane2016), both of whom reported that comorbid AD contributes to poorer postsurgical outcomes. Interestingly, the findings regarding differing cognitive outcomes conflict with those of Kazui et al. (Reference Kazui, Kanemoto, Yoshiyama, Kishima, Suzuki, Sato, Suehiro, Azuma, Yoshimine and Tanaka2016), who found comparable levels of postsurgical improvement among iNPH patients with and without AD. That said, these discrepancies may owe to differences in neuropsychological tests, methods for assaying AD pathology, sample size, and/or follow-up durations between Kazui et al. (Reference Kazui, Kanemoto, Yoshiyama, Kishima, Suzuki, Sato, Suehiro, Azuma, Yoshimine and Tanaka2016) and our study. Ultimately, additional investigation of postoperative outcomes over multiple follow-ups is needed to further confirm if and how AD pathology influences shunt outcomes.
In terms of predicting shunt response 3 months after surgery, baseline p-tau/Aβ CSF ratios were not significantly associated with improvements in any of the core iNPH symptoms, contrasting findings from Hong et al. (Reference Hong, Kim, Jeong, Kim, Hwang, Lee, Lee and Na2018). Consistent with previous studies which identified age as a predictor of postoperative outcomes (Bugalho et al., Reference Bugalho, Alves, Ribeiro, Bugalho, Alves and Ribeiro2013; Illán-Gala et al., Reference Illán-Gala, Pérez-Lucas, Martín-Montes, Máñez-Miró, Arpa and Ruiz-Ares2017), this study found that older age predicted worse cognitive outcomes postoperatively. Likewise, results of the present study suggest that higher MMSE scores and lower RBANS total scores at baseline predict improvements in incontinence, a finding that aligns with previous reports showing postoperative iNPH symptom reduction is more likely when baseline cognitive impairment is relatively minimal (McGirt et al., Reference McGirt, Woodworth, Coon, Thomas, Williams and Rigamonti2005; Thomas et al., Reference Thomas, McGirt, Woodworth, Heidler, Rigamonti, Hillis and Williams2005). Qualitative interpretation of this predictive model suggests there may be an optimal point along the spectrum of cognitive dysfunction where improvements in bladder control are most likely to occur. Specifically, these findings suggest that patients are most likely to show improvements in incontinence when cognitive deficits are severe enough to impact performance on the RBANS, but not to the extent where performance on the MMSE, a coarser screening measure, is affected.
Strengths, limitations & future directions
The present study has notable methodological strengths. Importantly, a comprehensive battery of neuropsychological tests was used to evaluate cognitive outcomes at multiple intervals. Apart from adding greater nuance to our understanding of postoperative cognitive functioning, this approach demonstrates the relatively fleeting nature of postoperative gains in executive functions in iNPH + AD, which may have gone undetected in previous studies using a single postoperative time point. We also included a biomarker highly specific to AD pathology and progression in predictive analyses, which has been lacking in previous studies exploring shunt outcomes in iNPH + AD.
That said, there are several limitations, For instance, while our sample size was adequate for detecting small to medium effects in the mixed effects model, the attrition rate over longitudinal follow-ups may have precluded detection of relatively smaller differences in outcomes. Another weakness stems from the use of imputation to replace missing variables, which may have introduced additional error. We also cannot dismiss the possibility that relatively higher levels of education among those who remained in the study (vs. those lost to follow-up) influenced the results. Similarly, the asymmetric attrition rate observed between iNPH and iNPH + AD groups is another potential confound, as it may reflect latent group differences contributing to postoperative outcomes. Thus, it will be important to replicate this study in a larger sample where additional resources are dedicated to minimizing attrition rates.
Our study also did not include information regarding comorbid medical conditions or medications patients were taking, which may have influenced baseline measurements and subsequent outcomes. For instance, cardiac disease and associated cardiovascular conditions are prevalent in older adults and confer an increased risk of cognitive impairment (Abete et al., Reference Abete, Della-Morte, Gargiulo, Basile, Langellotto, Galizia, Testa, Canonico, Bonaduce and Cacciatore2014; Eggermont et al., Reference Eggermont, de Boer, Muller, Jaschke, Kamp and Scherder2012), which could have influenced our results. Likewise, we cannot wholly rule out potential iatrogenic confounds related to anticholinergics, benzodiazepines, neuroleptics, or analgesics (see Fox et al., Reference Fox, Smith, Maidment, Chan, Bua, Myint, Boustani, Kwok, Glover, Koopmans and Campbell2014; Wright et al., Reference Wright, Roumani, Boudreau, Newman, Ruby, Studenski, Shorr, Bauer, Simonsick, Hilmer and Hanlon2009). Thus, we recommend future studies consider measuring comorbid medical conditions and treatment related factors in a continuous manner (e.g., creating comorbidity or iatrogenic indices) to control for potentially confounding effects of these variables.
The generalizability of our findings may also be impacted by the limited sociodemographic information available and generally narrow age range and relatively high education level of our sample (i.e., greater than high school diploma on average). Thus, it will be important to replicate this study in a more racially/ethnically inclusive and educationally heterogeneous sample. Further, it may be useful for future studies to consider exploring how postsurgical outcomes may or may not differ in samples of highly educated and relatively less educated individuals.
It should also be noted that our predictive analyses were conducted using a series of a priori variables; however, this does not preclude the possibility of other variables predicting symptom reduction (e.g., mood/psychiatric functioning, social support, etc.). While our small sample size precludes such an endeavor, studies involving serial assessments in larger samples could better clarify how various cognitive, symptom, and demographic factors collectively influence shunt response.
Another area warranting further investigation is the potential roles of the extent of baseline ventriculomegaly and white matter vascular changes in mediating shunt outcomes in these groups. Similarly, while we did not find associations between baseline levels of AD pathology and postoperative symptom reduction, future studies should consider serial biomarker collection to investigate how changes in AD biomarkers influence postoperative clinical changes.
Lastly, there may be benefit to studying postoperative outcomes for iNPH when it presents in the context of other neurodegenerative conditions, as the findings obtained here are specific to individuals with iNPH + AD, and cannot be generalized to other co-occurring protienopathies. Some studies have examined outcomes of shunt placement in small samples of patients with Parkinson’s disease (e.g., Sakurai et al., Reference Sakurai, Tsunemi, Shimada, Kawamura, Nakajima, Miyajima and Hattori2022) and identified similar patterns of postoperative improvement and relatively poorer outcomes for those with the disease. Further longitudinal studies of postoperative trajectories of iNPH presenting with these and other concomitant neurodegenerative syndromes are nevertheless needed to fully inform the field of the risks and potential benefits of shunt placement.
Conclusions
Our findings suggest comorbid AD may be associated with differing trajectories of postoperative cognitive outcomes among iNPH patients. The groups showed similar outcomes in most areas up to 5 years following their surgery; however, patients with iNPH alone showed a stable pattern of improvement in aspects of executive functions, whereas those with comorbid AD failed to maintain initial gains in these areas. These findings may inform shunt placement for those with iNPH + AD, particularly when patients display prominent features of executive systems dysfunction preoperatively. Despite findings from the mixed effect model, baseline levels of AD pathology did not appear significantly predictive of a positive shunt response. This study instead identified that older age at baseline was associated with poorer cognitive outcomes, and that there may be an ideal point along the continuum of cognitive impairment where patients are most likely to experience improvements in bladder control postoperatively. Preoperative neuropsychological testing may therefore inform postoperative expectations and promote identification of patients who are most likely to experience reduction in primary iNPH symptoms following surgical intervention.
Acknowledgements
The authors are grateful to the patients and their families who participated in this longitudinal study. We are also grateful for the administrative and clinical support staff across Butler Hospital and Rhode Island Hospital for their care in working with these individuals and providing them the best care possible.
Author contributions
DG developed the research question, completed analysis, and drafted and finalized this manuscript, under the supervision of AL, with funding support from the Warren Alpert Medical School of Brown University Clinical Psychology Training Program. IP, PM, SS, and PK designed the original iNPH outcome study. IP, PM, and AL oversaw the neuropsychological evaluation and data collection, while SS and PK evaluated and monitored patients medically. CW, CG, and MN contributed to data coding, statistical analysis, and results interpretation. SC provided consultation on statistical approach and neuropsychological outcome variables. All authors reviewed and revised the manuscript and approved the final paper.
Funding statement
This study was supported by the Warren Alpert Medical School of Brown University Clinical Psychology Internship Training Program Research Grant.
Conflicts of interest
None.












