1. Introduction
Millitary deployment is often a stressful period and regularly leads to mental and social difficulties after homecoming Reference Fear, Jones, Murphy, Hull, Iversen and Coker[1]. Frequently occurring problems, besides symptoms of posttraumatic stress disorder and depression, are anger and aggression [Reference Reijnen, Rademaker, Vermetten and Geuze2, Reference Heesink, Rademaker, Vermetten, Geuze and Kleber3]. Emotional and behavioral manifestations of these problems can be very disabling for the individual as well as their surroundings Reference Anderson and Bushman[4]. Problems regarding anger and aggression occur in many psychiatric disorders, the current study therefore takes a transdiagnostic approach [Reference Newby, McKinnon, Kuyken, Gilbody and Dalgleish5, Reference Sukhodolsky, Smith, McCauley, Ibrahim and Piasecka6]. Anger and aggression problems have been linked to disturbed emotional processing Reference Davidson, Putnam and Larson[7]. Stimuli are more easily perceived as negative or threatening, which might lead to reactive or impulsive aggression.
An important brain area in emotional processing is the amygdala [Reference Etkin, Büchel and Gross8, Reference Janak and Tye9]. The amygdala consists of distinct subnuclei [Reference Janak and Tye9, Reference Hrybouski, Aghamohammadi-Sereshki, Madan, Shafer, Baron and Seres10]. The basolateral amygdala (BLA) plays a role in differentiating responses to stimuli currently evaluated to have biologically significant outcomes [Reference Murray11–Reference Balleine and Killcross13]. The BLA is reciprocally connected with a wide range of brain areas, including medial and orbitofrontal prefrontal cortex and has projections to the central medial amygdala (CMA). The CMA in turn projects to areas such as the hypothalamus and brainstem, including the periaqueductal gray, thereby activating appropriate physiological responses such as freezing Reference Hermans, Henckens, Roelofs and Fernández[14].
In patients diagnosed with Intermittent Explosive Disorder (IED) hyperactivity of the amygdala has been reported in response to angry faces Reference Coccaro, McCloskey, Fitzgerald and Phan[15, Reference McCloskey, Phan, Angstadt, Fettich, Keedy and Coccaro16]. Furthermore, the circuitry of the amygdala, including the orbitofrontal cortex and the anterior cingulate cortex, has been implicated in disorders characterized by aggressive behavior such as IED and borderline personality disorder Reference Davidson, Putnam and Larson[7, Reference Adams17, Reference Adams18].
Post-traumatic stress disorder (PTSD) is a common mental disorder after deployment and also associated with aggression Reference Jakupcak, Conybeare, Phelps, Hunt, Holmes and Felker[19, Reference Taft, Vogt, Marshall, Panuzio and Niles20]. Although in PTSD no evidence was found for amygdala dysfunction in relation to general, non-facial, emotional stimuli Reference van Rooij, Rademaker, Kennis, Vink, Kahn and Geuze[21], it was found that patients with PTSD who did not respond to therapy show heightened amygdala activation to such stimuli before treatment Reference van Rooij, Kennis, Vink and Geuze[22]. Furthermore, stronger activation in the dorsal anterior cingulate cortex (ACC) is implicated in the processing of negative emotional stimuli in PTSD Reference van Rooij, Rademaker, Kennis, Vink, Kahn and Geuze[21]. Increased attention to negative emotions has been related to dACC activity Reference Etkin, Egner and Kalisch[23] and might therefore be of interest in aggression as well.
Differences in the processing of emotional stimuli in anger and aggression are mostly tested using facial stimuli Reference Coccaro, McCloskey, Fitzgerald and Phan[15, Reference McCloskey, Phan, Angstadt, Fettich, Keedy and Coccaro16], but general non-facial negative emotional stimuli also elicit amygdala activation Reference Hariri, Tessitore, Mattay, Fera and Weinberger[24]. However, whether such stimuli also result in enhanced responses in the amygdala related to anger and aggression is not yet known. Therefore, it is important to investigate the neural response to emotional stimuli in anger and aggression.
Here, we investigate brain responses to general, non-facial, emotional stimuli, in military veterans with and without anger and aggression problems. To this aim, 28 military veterans with anger and aggression problems and 28 veterans without a psychiatric diagnosis (all males) rated 32 negative, 32 positive and 32 neutral pictures from the IAPS while being scanned with fMRI. We studied both brain activity and the connectivity of the amygdala and the dACC with other areas of the brain in relation to the task. Based on previous studies in patients with aggressive behavior, we hypothesize that amygdala and dACC activation will be higher in the impulsive aggression group during the viewing of negative emotional pictures, in comparison to the control group. We expected that the functioning of the amygdala and dACC connectivity is also disturbed in aggression.
2. Methods
2.1. Participants
In this study, 30 male veterans with anger and aggression problems were included (Aggression group). They were recruited via their psychologists/psychiatrists at the Military Mental Health Care Institute and via advertisements in the waiting room and newsletters for veterans. Additionally, 29 male control veterans without anger and aggression problems were also included. It was attempted to include participants in the control group such that this group did not differ on age, education and number of deployments. These participants were recruited by advertisements in magazines for veterans or had participated in previous studies. Inclusion criteria for the Aggression group were based on the four research criteria for impulsive aggression described by Coccaro (2012):
• verbal or physical aggression towards other people occurring at least twice weekly on average for one month; or three episodes of physical assault over a one year period;
• the degree of aggressiveness is grossly out of proportion;
• the aggressive behaviour is impulsive (not premeditated);
• the aggressive behaviour causes either distress in the individual or impairment in occupational or interpersonal functioning (Coccaro, 2012 Reference Coccaro[25]).
Inclusion criteria for the Control group were:
• no current DSM-IV diagnosis;
• no history of pathologic aggressive behaviour.
The Ethics Committee of the University Medical Center Utrecht, The Netherlands, approved this study and all participants signed an informed consent before participation after having received a complete written and verbal explanation of the study. This study was carried out in accordance with the Declaration of Helsinki.
2.2. Interview and questionnaires
The Dutch version of the International Neuropsychiatric Interview (MINI) was used in order to screen for the presence of comorbid psychiatric disorders Reference Van Vliet, Leroy and Van Megen[26]. The complete MINI was administered. In this interview the following current or life-time disorders were screened: depressive disorder, dysthymia, suicidal risk, (hypo)manic disorder, panic disorder, anxiety disorder, agoraphobia, social phobia, obsessive compulsive disorder, PTSD, alcohol or drug dependence and/or abuse, psychotic disorders, anorexia nervosa, bulimia nervosa, generalized anxiety disorder, antisocial personality disorder, somatization disorder, hypochondria, body dysmorphic disorder, pain disorder, attention deficit hyperactivity disorder (ADHD) and adjustment disorder. The interview was carried out by the research staff (psychologists with psychodiagnostic expertise).
To measure anger and aggression, the Dutch version of the State-Trait Anger Expression Inventory-revised (STAXI-2; Hovens, Rodenburg, & Lievaart, 2015, Spielberger, 1999 Reference Hovens, Rodenburg and Lievaart[27, Reference Spielberger28]) was used. The STAXI-2 consists of 57 items on a 4-point Likert scale and is divided into two subscales: State Anger and Trait Anger.
Furthermore, the Dutch translation of the Buss-Perry Aggression Questionnaire (AQ) Reference Meesters, Muris, Bosma, Schouten and Beuving[29, Reference Buss and Perry30] was administered. The AQ consists of 29 items on a 5-point Likert scale and is divided into 4 subscales: Physical Aggression, Verbal Aggression, Anger and Hostility.
2.3. Task
The task (Van Rooij et al., 2015; Vink, Derks, Hoogendam, Hillegers, & Kahn, 2014 Reference van Rooij, Rademaker, Kennis, Vink, Kahn and Geuze[21, Reference Vink, Derks, Hoogendam, Hillegers and Kahn31]) consisted of 96 pictures from the IAPS Reference Lang, Bradley and Cuthbert[32]. These pictures elicit general emotional experience Reference Lang, Bradley and Cuthbert[32]. The pictures were categorized as neutral, positive, or negative based on the IAPS rating. The pictures were presented for 2 seconds, after which an evaluation screen was presented. By pressing a button with the thumb of their right hand, participants could give their rating (positive, negative or neutral) of the picture within 2seconds. Immediately after giving the rating, a fixation cross appeared for the remaining trial duration. The task consisted of four blocks, each with 24 pictures in pseudo-randomized order (8 neutral, 8 positive, 8 negative). Between the blocks, a fixation cross was presented for 32 seconds. For a schematic overview of the task, see Fig. 1.
2.4. MRI acquisition
A 3.0-T whole-body magnetic resonance imaging scanner (Philips Medical System, The Netherlands) was used to acquire the functional images during the task, and a T1 weighted image for within-subject registration. An EPI-SENSE sequence scan acquired 322 functional images during the task, with the following parameters: repetition time (TR) = 1600ms; echo time (TE) = 23 ms; flip angle = 72.5°; 64 × 51 matrix; 4mm slice thickness; field of view (FOV) = 256 × 204mm. For within subject registration, a T1 weighted image was used (200 slices, TR = 10ms; TE = 3.8ms; flip angle = 8°; FOV = 240 × 240 × 160mm).
2.5. Preprocessing
Preprocessing and analyzing the data was done using SPM 12 (http://www.fil.ion.ucl.ac.uk/spm) and hiro3, a Matlab tool for visualizing and analyzing fMRI data Reference Gladwin, Vink and Mars[33]. Volumes were slice-time corrected to the middle slice and realigned to the first acquired volume. The data were spatially normalized to an MNI T1-weighted template. Smoothing was done using an 8-mm full-width-at-half-maximum (FWHM) Gaussian kernel.

Fig. 1 Outline of the task. Pictures from three categories (Neutral; left, Positive; middle, Negative; right) were presented in an intermixed order for two seconds. After that, an evaluation screen was shown until the participant pressed a button to indicate their evaluation or for a maximum of two seconds. Next, a fixation cross was presented for the remaining duration of the trial.
2.6. Data analyses
FMRI data were analyzed using a general linear model regression analysis. Trials were only included when the participants rated the picture congruent to the IAPS rating. For each participant, first-level analyses were performed with the predictors: Neutral stimulus (2 s boxcar), Positive stimulus (2 s boxcar), Negative stimulus (2 s boxcar), response (stick function) and motion parameters. The used contrasts were Negative minus Neutral and Positive minus Neutral stimulus presentation. Further, overall activation due to stimulus presentation was tested, contrasting all stimuli against the implicit baseline. A whole-brain corrected threshold was used such that the family-wise error rate was controlled at 5%; that is, the chance of any voxel showing a false positive was 5%.
In order to investigate differences in amygdala activation during the viewing of emotional pictures, an ROI analysis was performed. The CMA and BLA were defined based on the probabilistic cytoarchitectonic areas from the SPM anatomy toolbox Reference Eickhoff, Paus, Caspers, Grosbras, Evans and Zilles[34]. When the probability of a certain voxel was higher for the CMA than for the BLA, it was included in the CMA-map and vice versa. The dACC was defined based on the WFU Pick atlas, by using Brodmann's area 32. The same ROI's were used in psychophysiological interaction (PPI) analyses Reference O’Reilly, Woolrich, Behrens, Smith and Johansen-Berg[35]. In the first analyses, familywise error rate correction was again used. However, after failing to find effects strong enough to survive this correction, exploratory analyses were added in order to show weaker but possibly informative effects. The used threshold was P<.001, uncorrected, with an extent of k ≥ 20 voxels Reference Lieberman and Cunningham[36]. As has been pointed out before Reference Gladwin, Vink and Mars[33, Reference Lieberman and Cunningham36, Reference Eklund, Nichols and Knutsson37], this heuristic measure does not (and does not claim to) provide whole-brain corrected results. In order to at least provide an indication of the level of whole-brain significance, permutation tests were used to acquire the null-hypothesis distribution of cluster extents over the chosen threshold.
3. Results
3.1. Demographics
Demographic information is depicted in Table 1. The groups did not differ on age, education and number of deployments. The Aggression group scored significantly higher on all anger and aggression measures.
3.2. Behavioral data
Table 2 shows the results of the ANOVA. After analysing the behavioural data of the task, two participants in the Aggression group and 1 participant in the Control group were excluded because they rated too few trials congruently (i.e., according to the IAPS-rating) to include them in the analyses. The Aggression group rated significantly more pictures incongruently to the IAPS rating compared to the Control group for both the Positive (F(1,56) = 10.21, P < .01, partial η 2 = .16) and the Neutral picture categories (F(1,56) = 5.43, P < .05, partial η 2 = .09). The Aggression group rated the neutral pictures more often as negative (F(1,56) = 10.11, P < .01, partial η 2 = 16), and the positive pictures more often as neutral (F(1,56) = 5.93, P < .05, partial η 2 = .10) and negative (F(1,56) = 6.37, P < .05, partial η 2 = .11).
3.3. fMRI results
3.3.1. Task effect
Brain areas involved in emotional processing were activated by the task, in both the Negative minus Neutral contrast and the Positive minus Neutral contrast, see Table 3 and Fig. 2. For the Negative minus Neutral contrast and the Positive minus Neutral contrast, these regions included the amygdala, hippocampus and orbitofrontal cortex.
3.3.2. Whole brain group differences
The whole brain analyses revealed no differences between the two groups on the Negative minus Neutral contrast or the Positive minus Neutral contrast. However, whole brain group differences were found in activation due to stimulus presentation in general, regardless of the valence of the stimuli. Among other regions, stronger activation was found in the supplemental motor area, frontal cortex, inferior parietal cortex and the anterior cingulum. All differences are depicted in Table 4 and Fig. 3.
Table 1 Description of the Anger group and the Control group.

Table 2 Behavioral data of the task.
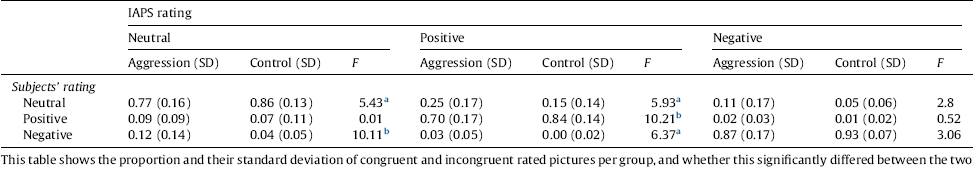
a Indicates significance at the .05 level.
b Indicates significance at the .01 level.
Table 3 Whole-brain activation within subjects during the negative and positive contrast.
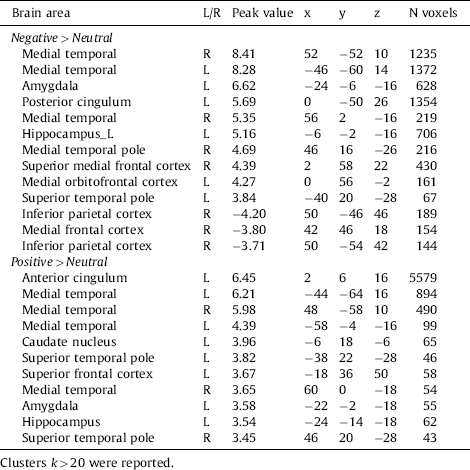
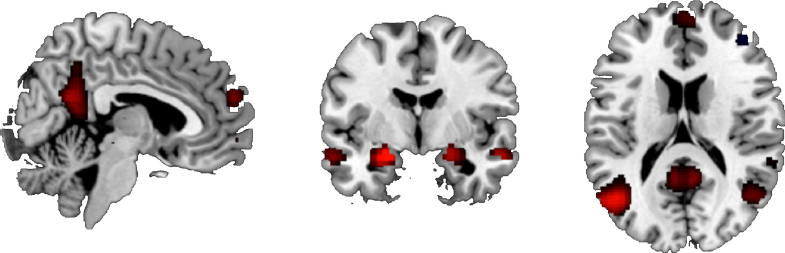
Fig. 2 Brain activation during the Negative minus Neutral contrast, showing the task effect. P < .05, FWE-corrected.
Table 4 Stronger brain activity during all stimuli in the Aggression group compared to the control group.
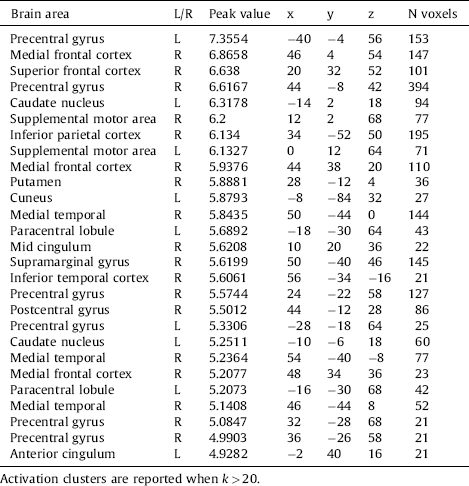

Fig. 3 Stronger brain activation in the Aggression group during the viewing of the pictures, regardless of category. P < .05, FWE-corrected.
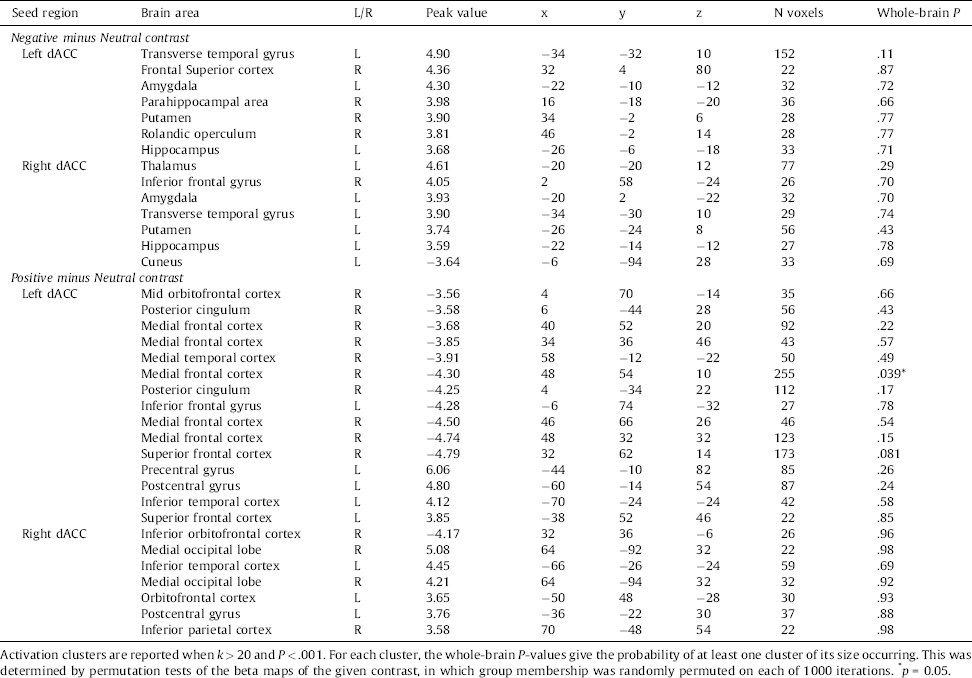
Table 5 Group differences in dACC coupling during negative versus neutral picture viewing.
3.3.3. ROI analyses
Activation of the basolateral and the centromedial amygdala and the dorsal ACC did not differ between the two groups (all P&s> .10, uncorrected).
3.3.4. PPI analyses
PPI analyses using the BLA and the CMA as seed regions, did not reveal differences in task-related changes in functional connectivity, both within and between the two groups, using a threshold of P < .001, uncorrected.
PPI analyses using the left and right dACC as seed regions, revealed stronger connectivity with the amygdala in the Aggression group compared to the control group during the Negative minus Neutral contrast, using a threshold of P < .001, uncorrected. The Positive minus Neutral contrast revealed diminished connectivity with the (orbito)frontal cortex in the Aggression group. All differences are shown in Table 5.
4. Discussion
In this study we examined whether veterans with anger and aggression problems show different brain activation and functional connectivity in response to general, non-facial emotional stimuli. To test this, positive, negative and neutral pictures were shown during an fMRI scan. It was found that the groups showed no differences either on the negative minus neutral contrast or the positive minus neutral contrast. However, a main effect of picture presentation was found, with stronger activation in motor areas and the parietal cortex evoked by stimuli in the Aggression group compared to the Control group.
These group differences in the parietal cortex point towards increased attention to the stimuli in general, regardless of their valence in the Aggression group, possibly due to the context in which every stimulus had the potential to be negative. Indeed, attentional problems have been reported in aggression and emotion regulation before Reference Jaworska, Berrigan, Fisher, Ahmed, Gray and Bradford[38, Reference Ochsner and Gross39]. In individuals reporting dysfunctional anger, differences in levels of oscillatory EEG activity were found that were interpreted as a chronic hypervigilant state Reference Jaworska, Berrigan, Fisher, Ahmed, Gray and Bradford[38], which may lead to an overreaction to non-harmful situations. The increased cue reactivity as found in the current study might also reflect a general heightened arousal level. In line with this possibility, in an earlier study we showed that military veterans with aggression had a heightened startle response Reference Heesink, Kleber, Häfner, van Bedaf, Eekhout and Geuze[40].
Furthermore, stronger activation in motor areas points was found in the Aggression group. This could be related to impulsivity and reduced inhibition Reference Bari and Robbins[41], which are strongly associated with aggression Reference Ramirez and Andreu[42]. The concept of impulsivity refers to the tendency to act quickly, without thinking or planning. Furthermore, individuals with higher trait anger show impaired response inhibition in a Go/NoGo task Reference Pawliczek, Derntl, Kellermann, Kohn, Gur and Habel[43], and individuals with higher trait aggression showed a combination of reduced orienting but enhanced preparation for action in a threat-anticipation task Reference Gladwin, Hashemi, van Ast and Roelofs[44]. The motor-related activation in the Aggression group might therefore be related to impulsiveness and preparation to respond quickly, prior to proper stimulus discrimination, although we do not have direct measures of this.
Using the dACC as a seed region, differences in functional connectivity between the two groups were found. During the viewing of negative pictures, the Anger group show stronger connectivity between the left amygdala and both the left and right dACC. This is similar to a previous finding in which participants with an anxiety disorder showed increased dACC-amygdala connectivity during the viewing of negative facial stimuli Reference Robinson, Krimsky, Lieberman, Allen, Vytal and Grillon[45]. Because the dACC is involved with responses to stimuli requiring control or adaptation Reference Bush, Luu and Posner[46], this effect might indicate a tendency to attend to negative stimuli and respond to them via up-regulation of their emotional processing Reference Robinson, Krimsky, Lieberman, Allen, Vytal and Grillon[45]. Furthermore, the diminished connectivity of the dACC with frontal areas as observed in the Aggression group, might point towards reduced attention to positive stimuli or their evaluation Reference Etkin, Egner and Kalisch[23, Reference Kanske, Heissler, Schönfelder, Bongers and Wessa47]. The hemispheric effect is hard to interpret, however, a systematic review reported that the left amygdala often shows stronger activation compared to the right amygdala Reference Baas, Aleman and Kahn[48]. Taken together, the connectivity results therefore suggest a negative bias in attentional processes that could skew the perception of situations as threatening. We note however that these results were not generally whole-brain significant. Future studies need to confirm the valildy of our findings and interpretations.
The behavioral data in the current study show that the participants in the Aggression group were more likely to rate the positive pictures as neutral or negative, and the neutral pictures more likely as negative. This is in line with the finding that people with anger regulation deficits show a hostile attribution bias. According to the hostile attribution bias, ambiguous situations are more easily interpreted as hostile Reference Wilkowski and Robinson[49]. In individuals with aggression problems it is often reported that they tend to interpret cues and situations as hostile Reference Schönenberg and Jusyte[50], from which dysfunctional behavior could follow. This tendency could be related to the findings showing abnormal connectivity discussed above: If individuals are highly sensitive to negative information but fail to pay attention to positive information, this would be expected to negatively bias their interpretations of situations.
The finding that amygdala activation did not differ between the two groups, is not in line with previous studies Reference Coccaro, McCloskey, Fitzgerald and Phan[15, Reference McCloskey, Phan, Angstadt, Fettich, Keedy and Coccaro16], possibly due to the use of different stimuli or the military versus non-military populations. In the previous studies, heightened amygdala activation was found in Intermittent Explosive Disorder in response to angry faces. Also in an anger-inducing experiment, stronger activation of the amygdala has been reported Reference Dougherty, Rauch, Deckersbach, Marci, Loh and Shin[51]. Furthermore, individuals scoring high on trait anger, show a stronger bias for angry faces Reference Van Honk, Tuiten, de Haan, Van den Hout and Stam[52]. These studies evoked negative emotions with different stimuli compared to the current study. In the current study, general, non-facial, emotional stimuli were used, and although the task did reveal amygdala reactivity, this reactivity did not differ between the two groups. Facial expressions are rather homogeneous in comparison to IAPS pictures, and might represent danger more consistently than non-facial stimuli Reference Hariri, Tessitore, Mattay, Fera and Weinberger[24]. Affective biases in individuals with dysregulated aggression might mainly be observed when social cues are presented. Thus, the current task might induce different emotions compared to previous studies, which do not distinguish the Aggression group from the control group.
A limitation of the current study is that the task we used does not actively require regulation of emotions. It remains unknown whether participants used strategies in order to regulate or suppress evoked emotions and whether this differed between the two groups. In future studies, it might be relevant to study emotion regulation instead of a passive viewing task. Furthermore, in this study only military veterans were compared; therefore, the effects of military training and deployment cannot be excluded. For example, brain activation of combat veterans with PTSD was only different compared to civilian controls and not compared to combat controls Reference van Rooij, Rademaker, Kennis, Vink, Kahn and Geuze[21]. The common military training and experience may have diminished symptom-related group differences. Also, the duration of the problems as well as the treatment and/or medication that the participants were receiving was not taken into account in the analyses. Furthermore, the current sample size is limited and only male veterans were included. The 8-mm smoothing kernel and the voxel size of 4 mm used in the current study might have limited detection of group differences in the PPI analyses using parcellations of the amygdala. However, in studies using similar analyses, differences were detected Reference Yoder, Porges and Decety[53, Reference Stock, Van den, Hortensius, Sinke, Goebel and de Gelder54], indicating that subdivisions of the amygdala are indeed sufficiently parcellated using this smoothing method. Another limitation is that the stimuli were presented in a pseudo-random order, thus participants could not predict the valence of the stimuli. This may have resulted in effects related to potentially threatening or negative stimuli, instead of reactions actual negative stimuli. In future studies, it would be interesting to compare trials on which participants can versus cannot predict the valence of the upcoming stimulus, providing potentially interesting comparisons involving processes such as vigilance, uncertainity and reactivity.
In conclusion, the findings in the current study indicate a valence-aspecific increase in arousal and impulsivity in veterans with impulsive aggression in response to non-facial emotional stimuli. Furthermore, effects on functional connectivity involving the dACC, amygdala and medial prefrontal cortex point towards attentional abnormalities involving positive and negative stimuli. Impulsive aggression may emerge from a combination of negative biases in attention and interpretation, the consequences of which are exacerbated by impulsivity. These findings may provide targets for interventions, for example neurostimulation or biofeedback methods to decrease impulsivity and hypervigilance.
Funding
This work has been financially supported by the Dutch Ministry of Defense.
Disclosure of interest
The authors declare that they have no competing interest.











Comments
No Comments have been published for this article.