Significant outcomes
Repeated subcutaneous treatment with ACTH (100 µg/0.1 ml/rat/day) for 14 days display a treatment-resistant depressive-like phenotype
The behavioural phenotype was reversed following administration with s-ketamine (15 mg/kg) and rapastinel (10 mg/kg), but was unaffected following treatment with imipramine (15 mg/kg)
In the plasma, the levels of corticosterone and ACTH were increased after 14 days of daily treatment with ACTH, independently of the treatment.
Limitations
The study was conducted only in male Wistar rats with only single doses of the drugs used, and no measurement of rapastinel following intraperitoneal administration was carried out.
ACTH and corticosterone was not measured following s-ketamine treatment.
The resistance to treatment was seen only to Imipramine – and no other monoaminergic antidepressants were investigated.
Introduction
Depression is a severe and debilitating disease that affects millions of people around the world and it is considered the leading cause of disability worldwide (WHO, 2018). Currently available antidepressants mainly target brain monoaminergic neurotransmission, and they require repeated treatment for at least 2–4 weeks to induce effects that are clinically significant (Hindmarch, Reference Hindmarch2002; Browne & Lucki, Reference Browne and Lucki2013). Despite that many treatment options are available, 15–30% of depressed individuals do not respond to pharmacological treatment even after months of continuous drug administration, which makes resistance to treatment one of the main problems associated with major depression (Kessler et al., Reference Kessler, Berglund, Demler, Jin, Koretz, Merikangas, Rush, Walters and Wang2003; Trevino et al., Reference Trevino, McClintock, McDonald Fischer, Vora and Husain2014; Zorumski et al., Reference Zorumski, Nagele, Mennerick and Conway2015). These aspects increase the individual’s suffering before efficacy is evident, and may also contribute to reduced patient adherence to the treatment and impair achievement of optimal results regarding remission rates (Hindmarch, Reference Hindmarch2002; Browne & Lucki, Reference Browne and Lucki2013). Therefore, the need for new and better drugs for the treatment of depression is urgent. In this context, animal models that help to identify drugs that can potentially overcome resistance to treatment and induce a rapid antidepressant response are of special importance.
Ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist originally developed as an anaesthetic drug, has revealed potential avenues for the development of new and better antidepressant drugs (Machado-Vieira et al., Reference Machado-Vieira, Salvadore, Diazgranados and Zarate2009; Browne & Lucki, Reference Browne and Lucki2013; Sanacora & Schatzberg, Reference Sanacora and Schatzberg2015). Several studies have shown that a single injection of a low dose of ketamine induces fast and long-lasting antidepressant effects (Berman et al., Reference Berman, Cappiello, Anand, Oren, Heninger, Charney and Krystal2000; Zarate et al., Reference Zarate, Singh, Carlson, Brutsche, Ameli, Luckenbaugh, Charney and Manji2006; Browne & Lucki, Reference Browne and Lucki2013; Ballard et al., Reference Ballard, Ionescu, Vande Voort, Niciu, Richards, Luckenbaugh, Brutsche, Ameli, Furey and Zarate2014), with remarkable effects in treatment-resistant patients (Zarate et al., Reference Zarate, Singh, Carlson, Brutsche, Ameli, Luckenbaugh, Charney and Manji2006, Reference Zarate, Duman, Liu, Sartori, Quiroz and Murck2013). Recent work with intranasal administration of s-ketamine (Canuso et al., Reference Canuso, Singh, Fedgchin, Alphs, Lane, Lim, Pinter, Hough, Sanacora, Manji and Drevets2018; Daly et al., Reference Daly, Singh, Fedgchin, Cooper, Lim, Shelton, Thase, Winokur, Van Nueten, Manji and Drevets2018) has paved the way for its registration by the Federal Drug Administration (FDA) for use as a therapeutic option in treatment-resistant depression (TRD) (FDA, 2019). Importantly, the clinical data are supported by data from animal studies demonstrating ketamine and other NMDA modulators to show robust antidepressant-like effects (Li et al., Reference Li, Lee, Liu, Banasr, Dwyer, Iwata, Li, Aghajanian and Duman2010, Reference Li, Liu, Dwyer, Banasr, Lee, Son, Li, Aghajanian and Duman2011; Autry et al., Reference Autry, Adachi, Nosyreva, Na, Los, Cheng, Kavalali and Monteggia2011; Zanos et al., Reference Zanos, Moaddel, Morris, Georgiou, Fischell, Elmer, Alkondon, Yuan, Pribut, Singh, Dossou, Fang, Huang, Mayo, Wainer, Albuquerque, Thompson, Thomas, Zarate and Gould2016). The mechanism underlying ketamine’s action has been the focus of intense research and prompted the development of more selective drugs, with fewer pharmacological actions than ketamine. The most prominent of these drugs is rapastinel, formerly GLYX-13, a partial agonist of the glycine site at the NMDA receptor (Burgdorf et al., Reference Burgdorf, Zhang, Weiss, Matthews, Disterhoft, Stanton and Moskal2011, Reference Burgdorf, Zhang, Nicholson, Balster, Leander, Stanton, Gross, Kroes and Moskal2013, Reference Burgdorf, Kroes, Zhang, Gross, Schmidt, Weiss, Disterhoft, Burch, Stanton and Moskal2015a, b). Through this mechanism, rapastinel is proposed to act by reducing the activation of NMDA receptors and it was shown that it induces several beneficial behavioural effects similar to ketamine, but without the psychostimulant effects (Burgdorf et al., Reference Burgdorf, Zhang, Weiss, Matthews, Disterhoft, Stanton and Moskal2011, Reference Burgdorf, Zhang, Nicholson, Balster, Leander, Stanton, Gross, Kroes and Moskal2013, Reference Burgdorf, Zhang, Weiss, Gross, Boikess, Kroes, Khan, Burch, Rex, Disterhoft, Stanton and Moskal2015a, b). Rapastinel is already in advanced clinical trials (NCT03560518, 2019; Ragguett et al., Reference Ragguett, Rong, Kratiuk and McIntyre2019).
One of the main problems in the search for better antidepressant drugs, mainly for treatment-resistant cases, is the lack of appropriate animal models for such a condition (Samuels et al., Reference Samuels, Leonardo, Gadient, Williams, Zhou, David, Gardier, Wong and Hen2011; Willner & Belzung, Reference Willner and Belzung2015). Important in developing such as model is its translational validity for TRD. Willner and Belzung (Willner and Belzung Reference Willner and Belzung2015) emphasise models that incorporate predisposing factors leading to heightened stress responsiveness, and Brand and Harvey (Reference Brand and Harvey2017a, b) considered the construct of high comorbidity of TRD in patients suffering from posttraumatic stress syndrome (PTSD). It is well known that dysregulation of the HPA axis in depression is considered a core feature in depression (McAllister-Williams et al., Reference McAllister-Williams, Ferrier and Young1998; Cowen, Reference Cowen2010), and early studies from the 1950s show higher peripheral concentrations of cortisol in emerging depression, with a return to normal in remitted patients (Quarton et al., Reference Quarton, Clark, Cobb and Bauer1955). The volume of pituitary and adrenal glands have been reported to be increased in patients with depression (Kessing et al., Reference Kessing, Willer and Knorr2011) and evidence of an impaired ACTH response to Corticotrophin-releasing hormone (CRH), and of an elevated cortisol response to ACTH in depression has been observed (Kellner et al., Reference Kellner, Rubinow, Gold and Post1983). Importantly, HPA-axis abnormalities may not only be associated with the pathogenesis of depression but also with poor outcome in patients with depression, since dysregulation of the HPA axis can be linked to with an impaired response to antidepressants (Zobel et al., Reference Zobel, Nickel, Künzel, Ackl, Sonntag, Ising and Holsboer2000; Young et al., Reference Young, Altemus, Lopez, Kocsis, Schatzberg, DeBattista and Zubieta2004), and relapse following successful treatment (Appelhof et al., Reference Appelhof, Huyser, Verweij, Brouwer, Van Dyck, Fliers, Hoogendijk, Tijssen, Wiersinga and Schene2006; Aubry et al., Reference Aubry, Gervasoni, Osiek, Perret, Rossier, Bertschy and Bondolfi2007). These findings highlight the role of ACTH as an important hormone in the hypothalamic–pituitary–adrenal (HPA) stress axis, which plays a crucial role in the neurobiology of depression (Sorrells et al., Reference Sorrells, Caso, Munhoz and Sapolsky2009). In line with these studies, recent evidence suggests that chronic treatment with ACTH may abrogate the antidepressant effect of monoaminergic drugs in different animal behavioural tests, such as the forced swim test (FST) (Kitamura et al., Reference Kitamura, Araki and Gomita2002, Reference Kitamura, Fujitani, Kitagawa, Miyazaki, Sagara, Kawasaki, Shibata, Sendo and Gomita2008; Walker et al., Reference Walker, Burnett, Hasebe, McGillivray, Gray, McGee, Walder, Berk and Tye2013) and the novelty suppressed feeding test (Antunes et al., Reference Antunes, Ruff, de Oliveira Espinosa, Piegas, de Brito, Rocha, de Gomes, Goes, Souza, Donato, Boeira and Jesse2015), thus being proposed as a model to study TRD. However, in order to be considered an appropriate TRD model, in addition to resistance to chronic treatment with conventional antidepressants, the model should positively identify novel antidepressants that are effective in TRD, such as ketamine and rapastinel.
Considering the aforementioned data, the present work aimed to (i) confirm that 14 days of ACTH treatment induces resistance to a conventional monoaminergic antidepressant, (ii) examine the effectiveness of s-ketamine and rapastinel in rats repeatedly treated with ACTH and exposed to the FST, (iii) estimate the plasma levels of corticosterone (CORT) and ACTH in order to validate the causal role of a disordered HPA axis in the model. Through such an approach, this study provides new information on the effects of s-ketamine and rapastinel in antidepressant treatment-resistance in animal models, and provides further predictive confirmation for chronic ACTH exposure as a model of TRD.
Material and methods
Animals
Male Sprague Dawley rats weighing 270–300 g (8 weeks, Taconic A/S, Copenhagen, Denmark) at the beginning of each experiment were housed in pairs in cages (Cage 1291H Eurostandard Type III H, 425 mm × 266 mm × 185 mm, Techniplast, Italy) at 20 ± 2 °C and 60 ± 5% relative humidity on a 12-h light/dark cycle (lights on at 06:00 a.m.) with free access to food and tap water. All animals were kept in the same room with the conditions for at least 1 week before the start of each experiment. Each cage had bedding material made of wooden chips along with access to a tunnel shelter, nesting material, and a wooden stick. The animal colony and all experimental facilities were protected from outside noise. The behavioural procedures were carried out in specially equipped rooms in the animal facility between 08:00 a.m. and 12:00 p.m. All animals were randomly assigned to the test groups and all experimental analysis were performed by an evaluator blinded to the groups. Procedures were conducted in conformity with ARRIVE guidelines (Kilkenny et al., Reference Kilkenny, Browne, Cuthill, Emerson and Altman2010) for the care and use of laboratory animals, which comply with international laws and politics. Additional information about the experimental procedures accordingly to the ARRIVE guidelines is given in Supplementary Material. All animal procedures were carried out under the approval of the Danish National Committee for Ethics in Animal Experimentation (Protocol no. 2012-15-2934-00254). All efforts were made to minimise animal suffering.
Drugs
The following drugs were used: s-ketamine (Pfizer), NMDA receptor antagonist; raspastinel (WuXi AppTec, China), partial agonist of glycine site on the NMDA receptor; imipramine (Sigma-Aldrich); Adrenocorticotropic Hormone 1–24 (ACTH, China Peptides, China). All drugs were diluted in sterile saline immediately before use.
Animal model of treatment-resistance to antidepressant treatment
The protocol to induce an antidepressant treatment-resistance consists of a single daily subcutaneous injection of adrenocorticotropic hormone 1–24 (ACTH) (100 µg/0.1 ml/rat) for 14 days. The ACTH injections were performed between 09:00 and 11:00 am. This protocol has been used to successfully induce resistance to monoaminergic antidepressants in rodents (Kitamura et al., Reference Kitamura, Araki and Gomita2002, Reference Kitamura, Fujitani, Kitagawa, Miyazaki, Sagara, Kawasaki, Shibata, Sendo and Gomita2008; Walker et al., Reference Walker, Burnett, Hasebe, McGillivray, Gray, McGee, Walder, Berk and Tye2013; Antunes et al., Reference Antunes, Ruff, de Oliveira Espinosa, Piegas, de Brito, Rocha, de Gomes, Goes, Souza, Donato, Boeira and Jesse2015).
Forced swim test
The FST was developed accordingly to previous works (Porsolt et al., Reference Porsolt, Le Pichon and Jalfre1977). First, animals were placed individually to swim in acrylic cylinders (24 cm diameter by 60 cm height containing 40 cm of water at 24 ± 1 °C) for 15 min (pre-test) (Fischer et al., Reference Fischer, Eskelund, Budac, Tillmann, Liebenberg, Elfving and Wegener2015; Pereira et al., Reference Pereira, Romano, Wegener and Joca2015). After 24 h, the animals were exposed to a 5-min session in the open-field test (OFT) immediately followed by a 5-min FST session. The water of the cylinders was changed after each trial to avoid the influence of alarm substances (Abel & Bilitzke, Reference Abel and Bilitzke1990). The test session was recorded digitally, and the immobility time was measured afterwards, thus allowing detailed blinded analysis of the behaviour by an independent person.
Open-field test
The OFT was developed in a squared arena (100 cm × 100 cm × 50 cm, 10 lux) as previously described (Liebenberg et al., Reference Liebenberg, Joca and Wegener2015; du Jardin et al., Reference du Jardin, Liebenberg, Muller, Elfving, Sanchez and Wegener2016a). All animals received vehicle or drug injection and after 50 min they were placed in the centre of the arena. All animals were recorded digitally, and the travelled distance was measured during the 5 min of the test through software tracking (Noldus Ethovision XT version 14, Waacheningen, The Netherlands).
Measurement of CORT and ACTH in plasma by Luminex
Right after the test session in the FST, animals were euthanised without anaesthesia, and blood collected from the neck wound into tubes containing Ethylenediaminetetraacetic acid (EDTA). The tubes were manually mixed by slightly shaking them four times. After mixing, the samples were centrifuged at 1500 × g at 4 °C for 10 min. Thus, the plasma samples were collected and frozen at −80 °C until use. CORT and ACTH were assayed with the MILLIPLEX® MAP kit Rat Stress Hormone Magnetic Bead Panel (RSHMAG-69K) on a Luminex 200 instrument (BIORAD). The determination was processed according to the manufacturer’s specifications (http://www.millipore.com). The standard curve was run in duplicate and the samples in single. Two internal quality controls were included on the plate. They were all within the expected ranges for the given analyte.
Statistical analysis
All statistical analyses were performed with GraphPad Prism version 5.01 for Windows (GraphPad software, San Diego, CA, USA). The results of Experiments 1 and 3 were analysed as group means by two-way ANOVA, followed by the Bonferroni post-hoc test, with comparisons to the vehicle group, where appropriate. Experiment 2 was analysed with a one-way ANOVA, followed by Dunnett’s post-hoc test. The results of Experiment 4 regarding CORT measurements were analysed as group means by two-way ANOVA. Then the data were pooled and analysed as group means by Mann–Whitney test. Bartlett’s test for equal variances was applied to verify normality and homogeneity of variances. The data were analysed as nonparametric measurements when significantly different variances were found. Mann–Whitney tests were used for nonparametric analyses. In order to detect outliers, Grubb’s test was carried out. Differences with p < 0.05 were considered significant.
Experimental design
Experiment 1: Effects of s-ketamine and imipramine in animals exposed to chronic ACTH and tested in the open field and forced swim:
Experimentally naïve animals received a single daily injection of ACTH (100 µg/0.1 ml/rat) or vehicle for 14 days. The last ACTH injection was given right after the pre-test session of the FST on the 14th day of treatment. On the next day, the animals received an intraperitoneal (i.p.) injection of vehicle, s-ketamine or imipramine and after 50 min they were exposed to the OFT. Right after the OFT, the animals were exposed to the test session of the FST. The group treated with imipramine received three injections at 0, 5 and 23 h after the pre-test session of FST. The dose chosen for s-ketamine (15 mg/kg) and imipramine (15 mg/kg) were based on previously published papers (Joca & Guimaraes, Reference Joca and Guimaraes2006; Li et al., Reference Li, Lee, Liu, Banasr, Dwyer, Iwata, Li, Aghajanian and Duman2010; Sales et al., Reference Sales, Biojone, Terceti, Guimaraes, Gomes and Joca2011; Liebenberg et al., Reference Liebenberg, Joca and Wegener2015)
Experiment 2: Effects of different doses of rapastinel in rats exposed to the OFT and forced swim:
In order to obtain possible effective doses of rapastinel, intraperitoneal injection of s-ketamine (15 mg/kg) and rapastinel (3, 10 and 30 mg/kg) was carried out. Fifty minutes following the injections the animals were exposed to the OFT. Right after the OFT, the animals were exposed to the test session of the FST.
Experiment 3: Effects of rapastinel in animals exposed to chronic ACTH and tested in the open field and forced swim:
Experimentally naïve animals received a single daily injection of ACTH (100 µg/0.1 ml/rat) or vehicle for 14 days. The last ACTH injection was given right after the pre-test session of the FST on the 14th day of treatment. On the next day, the animals received an injection of vehicle, rapastinel (10 mg/kg), or imipramine (15 mg/kg) and after 50 min they were exposed to the OFT. Right after the OFT, the animals were exposed to the test session of the FST. The group treated with imipramine received three i.p. injections at 0, 5 and 23 h after the pre-test session of FST. Rapastinel’s dose was based on the literature (Burgdorf et al., Reference Burgdorf, Zhang, Nicholson, Balster, Leander, Stanton, Gross, Kroes and Moskal2013).
Experiment 4: Effects of ACTH repeated treatment on the levels of CORT and ACTH
The animals from Experiment 2 were euthanised right after the exposure to the FST and plasma was collected as described above. The plasma samples were used for analysis of the levels of CORT, and ACTH by a Luminex 200 instrument.
Results
Experiment 1: Effects of s-ketamine and imipramine in animals exposed to chronic ACTH and tested on the open field and forced swim:
A two-way ANOVA analysis of the FST data revealed no significant interaction between ACTH/no-ACTH and treatment [F (2,71) = 2.702, p = 0.0739], but a significant effect of ACTH/no-ACTH [F (1,71) = 16.93, p < 0.0001] or treatment [F (2,71) = 12.22, p < 0.0001] alone (Fig. 1A). Post-hoc tests using the Bonferroni correction showed that in the non-ACTH, treatment with imipramine (0, 5 and 23 h after pre-test, p = 0,0013) or s-ketamine (1 h before test, p = 0,0002) reduced the immobility time of the animals compared to vehicle (Fig. 1A). However, in the group of animals repeatedly treated with ACTH, only the animals receiving s-ketamine showed a reduction in the immobility time (p = 0,0143, Fig. 1A).
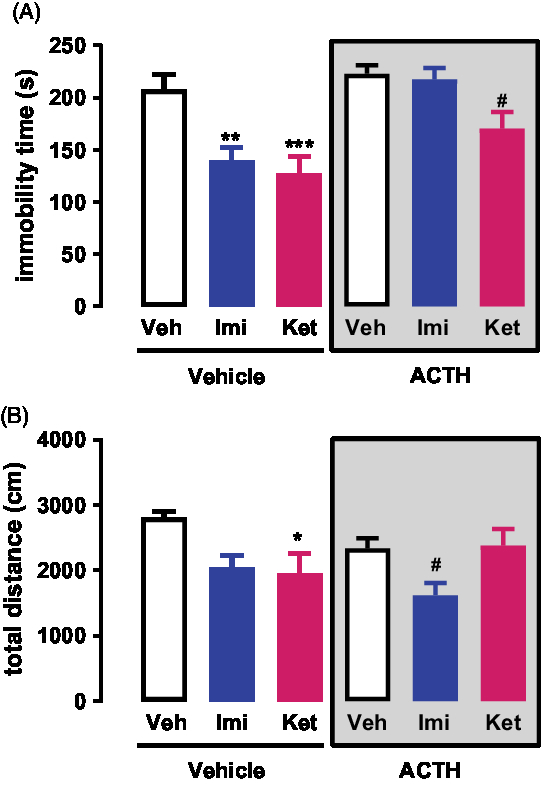
Fig. 1. (A) Effects of s-ketamine or imipramine in rats repeatedly treated with vehicle or ACTH for 14 days and exposed to the FST; n = 12–14. (B) Effects of s-ketamine or imipramine in rats repeatedly treated with vehicle or ACTH for 14 days and exposed to the open-field test; n = 11–14. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to the vehicle + vehicle group; #p < 0.05 compared to the ACTH + vehicle group; Bonferroni posttest. Data represent Mean ± SEM.
Similarly, a two-way ANOVA analysis of the Open-Field data revealed no significant interaction between ACTH/no-ACTH and treatment [F (2,66) = 2.85, p = 0.0650], no significant effect of ACTH/no-ACTH [F (1,66) = 0.7714, p < 0.383] but a significant effect of treatment [F (2,66) = 5.715, p < 0.0051] (Fig. 1B). Post-hoc tests using the Bonferroni correction showed that in the non-ACTH, treatment with imipramine (0, 5 and 23 h after pre-test, p = 0,0428) or s-ketamine (1 h before test, p = 0,0185) reduced the exploration time of the animals compared to vehicle (Fig. 1B). However, in the group of animals repeatedly treated with ACTH, only the animals receiving imipramine showed a reduction in the exploration time (p = 0,0361, Fig. 1B).
Experiment 2: Effects of different doses of rapastinel in rats exposed to the OFT and FST:
Intraperitoneal injection of s-ketamine (15 mg/kg) and rapastinel (3, 10 and 30 mg/kg) was carried out. We found that rapastinel at 10 mg/kg and s-ketamine at 15 mg/kg reduced the immobility time of animals exposed to the test session of FST (Fig. 2A). However, rapastinel at 3 and 30 mg/kg did not induced any changes on the immobility time during the FST (F (4,29) = 7.612; p < 0.05; Dunnett).
The OFT results show that none of the treatments induced hyper locomotion effects when comparted to controls (F (4,29) = 12.67; p < 0.05; Dunnett, Fig. 2B).
Experiment 3: Effects of rapastinel in animals exposed to chronic ACTH and tested on the open field and forced swim:
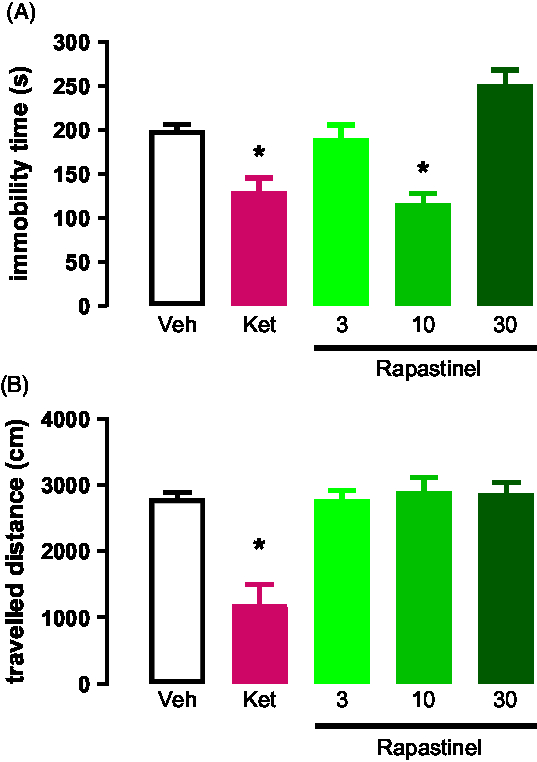
Fig. 2. (A) Effects of s-ketamine or rapastinel in rats exposed to the FST; n = 5–7. (B) Effects of s-ketamine or rapastinel in rats exposed to the OF; n = 5–7. *p < 0.05 compared to the vehicle group. Dunnett’s post-hoc test. Data represent Mean ± SEM.
A two-way ANOVA analysis of the FST data revealed a significant interaction between ACTH/no-ACTH and treatment [F (2, 31) = 4.022, p = 0.0280], a significant effect of treatment [F (2,31) = 9.291, p < 0.0007], but no effect of ACTH/no-ACTH [F (1,31) = 0.7339, p < 0.3982] alone (Fig. 3A). Post-hoc tests using the Bonferroni correction showed that in the non-ACTH, treatment with imipramine (0, 5 and 23 h after pre-test, p = 0.0388) or rapastinel (1 h before test, p = 0.0366) reduced the immobility time of the animals compared to vehicle (Fig. 3A). However, in the group of animals repeatedly treated with ACTH, only the animals receiving rapastinel showed a reduction in the immobility time (p = 0.0026, Fig. 3A).
Similar analysis of the open-field data revealed no significant interaction between ACTH/no-ACTH and treatment [F (2,42) = 0.082, p = 0.9213], no significant effect of ACTH/no-ACTH [F (1,42) = 2.507, p < 0.1209] but a significant effect of treatment [F (2,42) = 9.665, p < 0.0004] (Fig. 3B). Post-hoc tests using the Bonferroni correction did not show any difference in exploration time of the animals compared to vehicle (Fig. 3B).
Experiment 4: Effects of repeated ACTH treatment on plasma CORT and ACTH levels
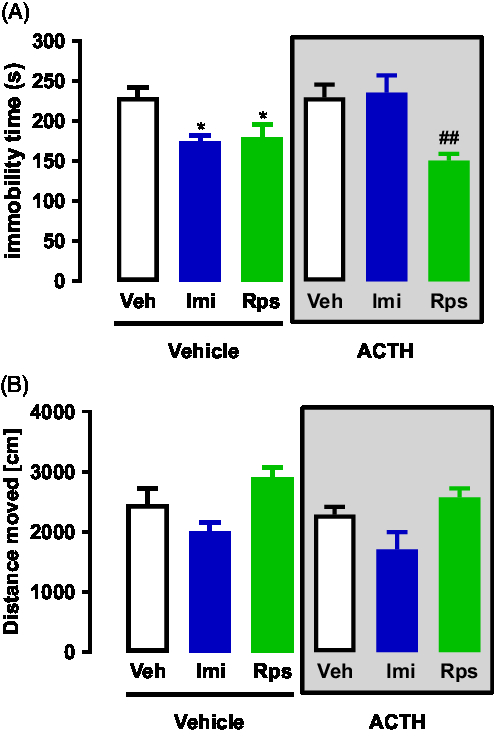
Fig. 3. (A) Effects of imipramine or rapastinel in rats repeatedly treated with vehicle or ACTH for 14 days and exposed to the FST; n = 5–7. (B) Effects of imipramine or rapastinel in rats repeatedly treated with vehicle or ACTH for 14 days and exposed to the open-field test; n = 5–7. *p < 0.05 compared to the vehicle + vehicle group; ##p < 0.01 compared to the ACTH + vehicle group; Bonferroni posttest. Data represent Mean ± SEM.
A two-way ANOVA analyses of plasma levels of CORT revealed is an effect of the first treatment (vehicle or ACTH) while there is no effect of any of the drugs given as second treatment (vehicle, imipramine, rapastinel, Interaction: F(2,42) = 0.3695; First Treatment: F(1,42) = 4.783*; Second Treatment: F (2,42) = 1.106; *p < 0.05 – Fig. 4A). Thus, all animals pre-treated with vehicle or ACTH were pooled appropriately to be analysed. The pooled analyses showed that animals pre-treated with ACTH presented higher levels of CORT when compared to vehicle (U = 181.0*, *p < 0.05 – Mann–Whitney’s test – Fig. 4B).
Regarding the analyses of the plasma levels of ACTH, several samples did not reach detectable levels, which led to undesirable variances between the numbers of samples in each group. Therefore, to run appropriate statistical analyses the pooled data based on the pre-treatment with vehicle or ACTH was analysed. The analyses show that ACTH levels were higher in the animals pre-treated with ACTH as compared to the group pre-treated with vehicle (U = 78.50*, *p < 0.05 – Mann–Whitney’s test – Fig. 4C).
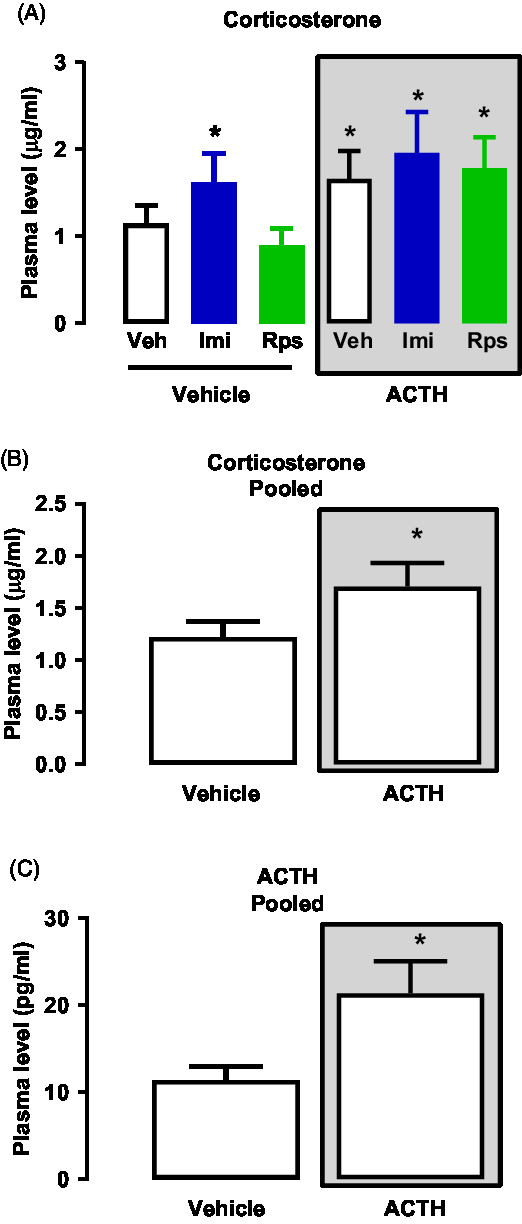
Fig. 4. (A) Effects of vehicle, imipramine or rapastinel on the plasma levels of CORT of rats repeatedly treated with vehicle or ACTH for 14 days and exposed to the FST. Two-way ANOVA, p > 0.05. (B) Effects of repeated treatment with vehicle or ACTH for 14 days on the plasma levels of CORT of rats exposed to the FST – Mann–Whitney, *p < 0.05. (C) Effects of repeated treatment with vehicle or ACTH for 14 days on the plasma levels of ACTH of rats exposed to the FST – Mann–Whitney, *p < 0.05. Data represent Mean ± SEM. n = 5–7.
Discussion
The main finding in the present paper is that repeated ACTH treatment resulted in animals mimicking a treatment-resistant state of depression, without response to imipramine, but with a clear effect of both s-ketamine and rapastinel. Both drugs have been shown to be clinically effective in human TRD, with intranasal s-ketamine recently approved for clinical use in MDD by FDA (FDA, 2019) and rapastinel in fast track FDA clinical phase-III trial (NCT03560518, 2019; Ragguett et al., Reference Ragguett, Rong, Kratiuk and McIntyre2019). Our results are in accordance with previous studies showing that repeated treatment with ACTH for 14 days induced a behavioural phenotype in the animals which is insensitive to treatment with conventional antidepressants and mood-stabilisers, such as desipramine, amitriptyline and lithium, when assessed in the FST or novelty suppressed feeding (Kitamura et al., Reference Kitamura, Araki and Gomita2002, 2008; Walker et al., Reference Walker, Burnett, Hasebe, McGillivray, Gray, McGee, Walder, Berk and Tye2013; Antunes et al., Reference Antunes, Ruff, de Oliveira Espinosa, Piegas, de Brito, Rocha, de Gomes, Goes, Souza, Donato, Boeira and Jesse2015).
Importantly, we demonstrate that the rapid-acting antidepressant drugs, s-ketamine and phase-III drug candidate rapastinel, induced antidepressant-like effects in animals following repeated administration with ACTH. This finding is in accordance with the literature where it was demonstrated that ketamine induces antidepressant effects in individuals with TRD (Zarate et al., Reference Zarate, Singh, Carlson, Brutsche, Ameli, Luckenbaugh, Charney and Manji2006, Reference Zarate, Duman, Liu, Sartori, Quiroz and Murck2013). The antidepressant-like effects of ketamine has been demonstrated in a variety of animal models, including stress based and genetic models of depression, for example the chronic unpredictable stress model (Li et al., Reference Li, Liu, Dwyer, Banasr, Lee, Son, Li, Aghajanian and Duman2011; Jiang et al., Reference Jiang, Wang, Sun, Lian, Sun, Wang, Du, Li and Sun2017; Zhu et al., Reference Zhu, Ye, Wang, Luo and Hao2017), flinders sensitive line (FSL) rats (Ardalan et al., Reference Ardalan, Wegener, Polsinelli, Madsen and Nyengaard2016; Eskelund et al., Reference Eskelund, Budac, Sanchez, Elfving and Wegener2016; du Jardin et al., Reference du Jardin, Liebenberg, Muller, Elfving, Sanchez and Wegener2016a, b, 2017; Ardalan et al., Reference Ardalan, Rafati, Nyengaard and Wegener2017a, b; Silva Pereira et al., Reference Silva Pereira, Elfving, Joca and Wegener2017), and more recently in a novel gene × environment (stress) model of TRD (Brand & Harvey, Reference Brand and Harvey2017b). In a previous study, it was attempted to examine the effect of 10 mg/kg racemic ketamine in a similar treatment-resistance condition (Walker et al., Reference Walker, Foley, Sutor, McGillivray, Frye and Tye2015). Although that study reported positive effects of ketamine, it was only following a median split into responders and non-responders. Apart from the afore noted study by Brand and Harvey using a gene × stress model of TRD (Brand & Harvey, Reference Brand and Harvey2017b), the present study represents the first demonstration of an antidepressant-like effect of s-ketamine in a condition mimicking treatment-resistance, induced by repeated exposure to ACTH. It is nonetheless of interest that Willner and Belzung (Reference Willner and Belzung2015) have emphasised the importance of a heightened stress response when developing a TRD model. In fact, Brand and Harvey (Reference Brand and Harvey2017a, b) combined a PTSD paradigm with a stress-sensitive genetic model of depression, the FSL rat, to successfully model TRD, while the ACTH model presented here is based on a construct of a disordered HPA axis. The latter is a deeply entrenched pathological feature of depression and TRD (Baumeister et al., Reference Baumeister, Lightman and Pariante2016).
There has been great interest in the development of antidepressants targeting the glutamatergic system in the last years. Rapastinel has also shown promising results in animal models (Burgdorf et al., Reference Burgdorf, Zhang, Nicholson, Balster, Leander, Stanton, Gross, Kroes and Moskal2013; Moskal et al., Reference Moskal, Burgdorf, Stanton, Kroes, Disterhoft, Burch and Amin Khan2016). The present results add to the previous studies, demonstrating rapastinel is capable of inducing an antidepressant-like effect in a treatment-resistance condition in animals. This is particularly noteworthy as the route of administration used here (viz. i.p. injection) was different from the majority of previous studies that have used intravenous (i.v.) administration, although other studies have also successfully used intraperitoneal administration (Yang et al., Reference Yang, Zhang, Han, Yao, Yang, Ren, Ma, Chen and Hashimoto2016). As we did not assess the bioavailability and brain content of rapastinel, the behavioural findings must be interpreted with care. Future studies should perform such bioavailability studies in both i.p. and i.v. treated cohorts for comparative reasons. In addition, we also note that, although not statistically confirmed, rapastinel in some, but not all, of our experiments may have a stimulatory effect on locomotion, which may positively bias an antidepressant-like effect in the FST. Given these limitations, it remains objectives of future work to confirm these findings by using more comprehensive bio-behavioural testing, as noted.
Importantly, the analyses of plasma samples confirm that ACTH treatment for 14 days increased the activity of the HPA axis of the animals, as reflected in the increased CORT and ACTH level. Neither imipramine nor rapastinel were able to reduce the plasma CORT and ACTH levels, unfortunately such levels were not performed in s-ketamine-treated animals. Whether this may be relevant for the observed phenotype remains to be clarified, but it is noteworthy that previous studies on alterations in CORT levels after repeated treatment of ACTH are contradictory. For instance, one study showed that CORT levels were unaltered after 14 days of daily ACTH treatment (Walker et al., Reference Walker, Burnett, Hasebe, McGillivray, Gray, McGee, Walder, Berk and Tye2013), whereas another study supports our findings, showing that repeated treatment with ACTH increase CORT levels (Kitamura et al., Reference Kitamura, Araki and Gomita2002). As HPA-axis hyperactivation and maladaptation to stress is a common feature of major depression (Nestler et al., Reference Nestler, Barrot, DiLeone, Eisch, Gold and Monteggia2002; McEwen, Reference McEwen2004; Bale, Reference Bale2006), our findings are valid with regard to the use of repeated treatment with ACTH as a model for TRD. It is speculated that this association successfully translates abnormal features of stress adaptation and clinical depression. Finally, while this study specifically examined treatment response in a TRD model, future work should also consider time to onset of action, as well as bolstering the antidepressant response of a traditional antidepressant (Brand & Harvey, Reference Brand and Harvey2017b). Indeed, ketamine first came to prominence as an adjunctive treatment to hasten antidepressant response (Li et al., Reference Li, Lee, Liu, Banasr, Dwyer, Iwata, Li, Aghajanian and Duman2010, 2011; Autry et al., Reference Autry, Adachi, Nosyreva, Na, Los, Cheng, Kavalali and Monteggia2011; Zanos et al., Reference Zanos, Moaddel, Morris, Georgiou, Fischell, Elmer, Alkondon, Yuan, Pribut, Singh, Dossou, Fang, Huang, Mayo, Wainer, Albuquerque, Thompson, Thomas, Zarate and Gould2016).
The mechanism whereby ACTH induces a treatment-resistant state and how this may be reversed by modulating the glutamatergic system is particularly interesting, and several explanations could be proposed. Within the context of this paper and the drugs used, it seems to hypothesise the involvement of a dysfunctional prefrontal cortical circuitry, combined with GABAergic deficits following chronic stress and the state of TRD (Ghosal et al., Reference Ghosal, Hare and Duman2017). However, further studies are warranted to test this hypothesis.
In conclusion, the present work reinforces the role of the glutamatergic system in the neurobiology of depression as it corroborates that s-ketamine and rapastinel are antidepressant drugs that deserve further investigation. The study also re-affirms and establishes s-ketamine and rapastinel as effective pharmacotherapy for a treatment-resistant state. The experimental protocol used here is suggested to be a useful future tool in the study of TRD. Nonetheless, further studies are necessary to elucidate the mechanism through which the repeated exposition to ACTH leads to resistance to a classical antidepressant, and how this is amenable to treatment with an NMDA receptor modulator.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/neu.2019.25.
Author ORCIDs
Vitor Silva Pereira, 0000-0002-9051-3154; Samia Joca, 0000-0003-0255-5889; Brian Harvey, 0000-0002-9864-7894; Betina Elfving, 0000-0001-6939-5088; Gregers Wegener, 0000-0002-0081-0068
Acknowledgements
We would like to thank CNPq for the grant provided to support Dr. Vitor Silva Pereira (PDE –203647/2014-9) and research fellowship to SJ (306648/2014-8), Aarhus University Research Foundation (AU-IDEAS initiative (eMOOD) (GW), The Lundbeckfoundation (Grant R93A8923) and The Independent Innovation fund Denmark (Grant DFF060202293B).
Author contributions
VSP and GW designed the experimental protocol. VSP carried out the experiments. VSP and GW analysed the data and wrote the first draft of this paper. All authors contributed to and approved the final version.
Conflicts of Interest
Gregers Wegener reported having received research support/lecture/consultancy fees from H. Lundbeck A/S, Servier SA, AstraZeneca AB, Eli Lilly A/S, Sun Pharma Pty Ltd., Pfizer, Inc., Shire A/S, HB Pharma A/S, Arla Foods Amba., Janssen Pharma A/S, and Mundipharma International, Ltd. All other authors declare no conflict of interest. Gregers Wegener is Editor-in-Chief of Acta Neuropsychiatrica but was not involved and actively withdrew during the review and decision process of this manuscript.






