Introduction
Qualifying level of consciousness (LoC) of patients with a prolonged disorder of consciousness (PDoC) is challenging, in clinical practice as well as in research. Although neuroimaging techniques (i.e. fMRI or EEG) help our understanding of neural correlates of consciousness and aid in the diagnosis of PDoC, behavioral assessment scales remain the gold standard for qualifying LoC.
In the acute phase, the Glasgow Coma Scale is the most commonly used instrument (Formisano et al., Reference Formisano, Giustini, Aloisi, Contrada, Schnakers, Zasler and Estraneo2019). When patients are no longer dependent on intensive care treatment, but still do not show clear signs of consciousness, the use of other behavioral observation scales is the standard assessment method to determine the different LoC and possible changes over time (Giacino, Fins, Laureys & Schiff, Reference Giacino, Fins, Laureys and Schiff2014).
Within the context of the evaluation of a treatment program for young (<25 years) PDoC patients in the early 2000s, the ‘Post-acute Level Of Consciousness scale’ (PALOC-s) was developed (Eilander et al., Reference Eilander, van de Wiel, Wijers, van Heugten, Buljevac, Lavrijsen and Prevo2009; Eilander, Wijnen, Scheirs, de Kort & Prevo, Reference Eilander, Wijnen, Scheirs, de Kort and Prevo2005). This scale offers a simple assessment tool using systematically observed behaviors, which can be classified into eight hierarchical levels, from coma to full consciousness. The classification was based on the landmark publications from two specialized task forces: the ‘International Working Party on the Management of the Vegetative state’ (Andrews, Reference Andrews1996) and the ‘Aspen Neurobehavioral Conference’ (Giacino et al., Reference Giacino, Zasler, Katz, Kelly, Rosenberg and Filley1997). The eight levels were coma (level P1), vegetative state with three sublevels (levels P2, P3 and P4), the low awareness state with three sublevels (levels P5, P6 and P7) and consciousness (level P8). Ideally, the PALOC-s was designed to be used in combination with another standardized instrument, like the Western Neuro Sensory Stimulation Protocol (Ansell & Keenan, Reference Ansell and Keenan1989) or the JFK Coma Recovery Scale (CRS) (Giacino, Kezmarsky, DeLuca & Cicerone, Reference Giacino, Kezmarsky, DeLuca and Cicerone1991), but it could also be used as an observational scale at any other moment.
Over the past 20 years, the progress in PDoC research changed our understanding of behaviors related to different levels of consciousness. Therefore, revision of the PALOC-s on this aspect is of importance. Second, the terminology has been changed. In 2010, the ‘Vegetative State (VS)’ has been proposed to be renamed as the ‘Unresponsive Wakefulness Syndrome’ (UWS) (Laureys et al., Reference Laureys, Celesia, Cohadon, Lavrijsen, Leon-Carrion, Sannita and Dolce2010). Also, the term ‘Low Awareness State’ is no longer used and replaced by the term ‘Minimally Conscious State’ (MCS) (Giacino et al., Reference Giacino, Ashwal, Childs, Cranford, Jennett, Katz and Zasler2002). To prevent possible misdiagnosis and to prevent ambiguous descriptions, an update of the PALOC-s is needed.
Levels of consciousness
The validity of LoC measurements in PDoC patients and the underlying cerebral processes has been a subject of academic debate for decades (Nettleton, Kitzinger & Kitzinger, Reference Nettleton, Kitzinger and Kitzinger2014; Wade, Reference Wade2017). Different interrelated concepts can be distinguished, without knowing the exact underlying mechanism: wakefulness (not sleeping), alertness (being able to process information) and consciousness (to be aware of oneself or their surroundings) (Lindsley, Reference Lindsley1988). In the past 20 years, active functional neuroimaging or electrophysiological paradigms have been developed to detect willful brain activity in unresponsive patients (Schnakers et al., Reference Schnakers, Hirsch, Noe, Llorens, Lejeune, Veeramuthu and Estraneo2020.) However, in clinical practice, these methods cannot be used easily. Therefore, behavioral observation in a standardized situation is the preferred way of examining the LoC. The commonly used instrument is the Coma Recovery Scale Revised (CRS-R) (Giacino, Kalmar & Whyte, Reference Giacino, Kalmar and Whyte2004), as was recommended by the American Congress of Rehabilitation Medicine (Seel et al., Reference Seel, Sherer, Whyte, Katz, Giacino, Rosenbaum and Zasler2010).
Until recently, the categories that could be scored in the CRS-R were UWS, MCS and consciousness. In the latest update, the distinction between MCS+ and MCS− has been added, based on a study by Thibaut, Bodien, Laureys & Giacino (Reference Thibaut, Bodien, Laureys and Giacino2020).
For clinical use, research, as well as for communication with relatives, it can be useful to have the possibility to distinguish more (sub)levels, as described more than 20 years ago by the ‘International Working Party on the Management of the Vegetative state’ (Andrews, Reference Andrews1996). Research on the reliability and validity of the PALOC-s, distinguishing eight levels of consciousness, demonstrated a high interrater reliability (Eilander et al., Reference Eilander, van de Wiel, Wijers, van Heugten, Buljevac, Lavrijsen and Prevo2009). This means that it is possible to classify the behavior in a reliable manner. Moreover, the use of the mapping in these eight levels can be of additional value in the clinical description of the observed behavior, due to the overall behavioral repertoire represented by the PALOC-sr.
Classification
The category MCS, as described in 2002, is very heterogeneous. Therefore, clinicians and researchers felt the need to differentiate this level into sublevels (Giacino et al., Reference Giacino, Ashwal, Childs, Cranford, Jennett, Katz and Zasler2002). In 2011, two sublevels were proposed: the minimally conscious state – (MCS−) and the minimally conscious state + (MCS+) (Bruno, Vanhaudenhuyse, Thibaut, Moonen & Laureys, Reference Bruno, Vanhaudenhuyse, Thibaut, Moonen and Laureys2011). Patients in MCS− mainly show involuntary, nonreflexive behaviors, such as visual pursuit of a moving mirror, while patients in MCS+ show language-dependent behaviors, such as executing simple commands like shaking someone’s hand on request and/or intelligible verbalization and/or intentional communication. This subdivision is frequently used in new publications and is shown to be relevant for the prediction of the long-term functional recovery (Thibaut et al., Reference Thibaut, Bodien, Laureys and Giacino2020). Therefore, this distinction is also of importance to the revision of the PALOC-s. Meanwhile, views have also changed about the distinction between UWS and MCS. In 2000, withdrawal of a limb following noxious stimulation was considered compatible with UWS, nowadays this behavior is judged as a sign of MCS− (Giacino et al., Reference Giacino, Katz, Schiff, Whyte, Ashman, Ashwal and Armstrong2018). The difference between UWS and MCS is of importance for both prognosis and treatment policies, for example, managing active treatment, pain relief and medical-ethical decision-making by professionals and proxies (Jox et al., Reference Jox, Kuehlmeyer, Klein, Herzog, Schaupp, Nowak and Bender2015). Previous research showed that patients admitted to an early rehabilitation program, initially scoring P4 on the PALOC-s, had the same 100% chance of recovery to level P7 or P8 as the patients who scored P5 or P6, while patients scoring P2 or P3 at admission only had half of that chance (Eilander et al., Reference Eilander, van Heugten, Wijnen, Croon, de Kort, Bosch and Prevo2013). This is in agreement with recovery patterns reported worldwide (Estraneo et al., Reference Estraneo, De Bellis, Masotta, Loreto, Fiorenza, Lo Sapio and Trojano2019).
In Table 1, we present the PALOC-sr. Level P4 (patients showing reactions in stimulated limbs, visual pursuit or localization) is no longer considered as UWS, but as MCS−. So, levels P2 and P3 are categorized as UWS, levels P4 and P5 as MCS− and the levels P6 and P7 as MCS+. With respect to the description of the conscious state, Giacino et al. (Reference Giacino, Fins, Laureys and Schiff2014, page 101) stated that patients with severe brain injury ‘newly emerged from MCS remain acutely confused and disoriented and may be prone to episodes of agitation, a condition termed acute confusional state’. We included the term ‘confused’ in the PALOC-sr to point out that after recovery to consciousness, cognitive functions may still be severely impaired. The descriptions of the eight hierarchical levels in the PALOC-sr remain unchanged compared with the PALOC-s.
Table 1. PALOC-sr (Post-Acute Level Of Consciousness scale revised), revision 2020
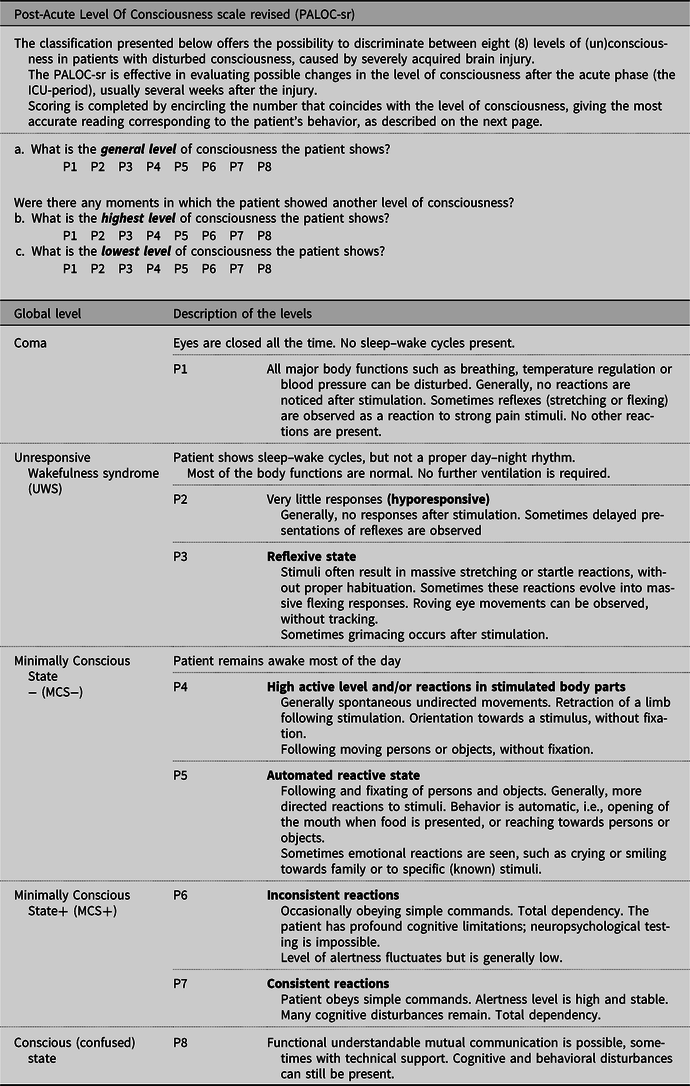
The PALOC-sr is suitable for all unresponsive patients from 2 years of age at all stages after an acute brain injury of any etiology. It is not intended for use in patients suffering from a progressive (neurodegenerative) disease.
Conclusion
The evaluation of the LoC in hyporesponsive patients is currently a matter of systematic behavioral observation. The PALOC-sr, a qualitative, descriptive scale that goes beyond the snapshot evaluations and instead does justice to the overall behavioral clinical picture, allows a detailed description of the LoC of PDoC patients, in research as well as in clinical practice. Especially the distinction in eight levels of consciousness can contribute to a better understanding of recovery processes.
The adaptation of the PALOC-s not only contributes to a more accurate description of the LoC of PDoC patients but may also aid in formulating a reliable prognosis regarding the long-term outcome.
The PALOC-sr should preferably be administered in combination with other instruments in order to prevent possible misdiagnosis. Using multiple instruments is recommended by the American Academy of Neurology (Giacino et al., Reference Giacino, Katz, Schiff, Whyte, Ashman, Ashwal and Armstrong2018) and in the UK by the Royal College of Physicians (2020).
Scoring the PALOC-sr can be done in minutes, so it does not add to the burden of the patient or the practitioner.
Further research testing the validity of the PALOC-sr in a considerable cohort is recommended, especially in the patients aged 25 years and above. In the Netherlands, a nationwide network of institutions treating patients with disorders of consciousness makes such a validation study possible. Hopefully, international validation studies will also be performed, providing an opportunity to compare outcome studies worldwide.
Acknowledgements
The authors thank Manju Virk for her grammatical contribution.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.



