The intestinal immune system is in a state of balance in which pro-inflammatory and anti-inflammatory cells and molecules are carefully regulated to promote host mucosal defence without damage to the intestinal tissue. Once this regulatory balance is disturbed, non-specific stimulation and activation can cause the overproduction and release of potent destructive immunological and inflammatory molecules( Reference Schreiber, Raedler and Stenson 1 ). The incapacity of the intestinal mucosa to control inflammatory events is the basis for the development of inflammatory bowel disease (IBD)( Reference Fiocchi 2 ).
IL-1β is a multifunctional cytokine playing a major role in both the initiation and amplification of many inflammatory conditions( Reference Böcker, Damião and Holt 3 ). IL-1β is released by various cell types including monocytes–macrophages, neutrophils and endothelial cells( Reference Martin and Wallace 4 ), and increased concentrations have been found in the intestinal tissue of IBD patients( Reference Ligumsky, Simon and Karmeli 5 – Reference Reimund, Wittersheim and Dumont 7 ). A number of effects of IL-1β on intestinal epithelial cells have been shown in vitro, including the activation of redox-sensitive intracellular cascades leading to the increased transcriptional activity and secretion of IL-8( Reference Schuerer-Maly, Eckmann and Kagnoff 8 ), IL-6( Reference Parikh, Salzman and Fischer 9 ), PGE2 and/or NO( Reference Van De Walle and Hendrickx 10 ). Moreover, in vitro and in vivo studies have provided evidence that the overproduction of pro-inflammatory cytokines such as TNF-α, interferon-γ and IL-1 can alter the tight junction (TJ) permeability of intestinal epithelia. This allows the permeation of inflammatory cells contributing to perpetuate the inflammatory processes in the gut mucosa( Reference Al-Sadi and Ma 11 ).
Although pharmacological therapy currently plays a major role in the management of IBD, dietary compounds with immunomodulatory properties, including n-3 fatty acids and antioxidants, have also been shown to be effective( Reference Geerling, Stockbrügger and Brummer 12 – Reference Hur, Kang and Jung 14 ).
Indicaxanthin (Ind), the yellow pigment characterising the edible fruit of the cactus Opuntia ficus-indica, is a water-soluble, nitrogen-containing compound belonging to a class of betalains, comprising phytochemicals having in common the structure of betalamic acid. Ind is the adduct of betalamic acid with proline (Fig. 1). Recent and growing interest in these substances has resulted in various studies showing that betalains can be considered as bioactive dietary compounds( Reference Livrea and Tesoriere 15 ). The cyclic amine associated with a polyene system in the Ind molecule was first considered as the reactive group conferring the molecule with reducing activity( Reference Butera, Tesoriere and Di Gaudio 16 ). More interestingly, measurements of the redox potential and radical-scavenging activity( Reference Butera, Tesoriere and Di Gaudio 16 , Reference Tesoriere, Allegra and Butera 17 ) have suggested that Ind might behave as an effective scavenger of radicals formed in biological environments. A large amount of data has recently been published on the lipoperoxyl radical-scavenging activity and antioxidant effects of Ind in various biological contexts, ranging from LDL systems( Reference Tesoriere, Butera and D'Arpa 18 ) to either healthy( Reference Tesoriere, Butera and Allegra 19 ) or pathological( Reference Tesoriere, Allegra and Butera 20 ) erythrocytes, as well as cell cultures( Reference Gentile, Tesoriere and Allegra 21 , Reference Tesoriere, Attanzio and Allegra 22 ). From a nutritional perspective, in vitro studies aimed at elucidating the chemical and biochemical transformations of the pigment during the digestive and intestinal absorptive processes have provided evidence that Ind is quite stable under digestive conditions( Reference Tesoriere, Fazzari and Angileri 23 ), is not metabolised by enterocytes and is absorbed through a paracellular mechanism characterised by a high permeability coefficient( Reference Tesoriere, Gentile and Angileri 24 ). More importantly, human studies have provided evidence that Ind from a diet-consistent amount of cactus pear fruits is highly bioavailable, reaching a plasma concentration of 7 μm, with a half-life of 2·36 h( Reference Tesoriere, Allegra and Butera 25 ).
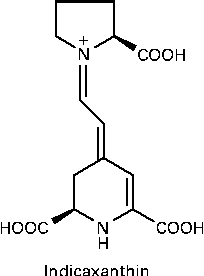
Fig. 1 Molecular structure of indicaxanthin.
Reactive oxygen species (ROS) overproduction and cell redox imbalance play a central role in the pathophysiology of the inflammatory response( Reference Rezaie, Parker and Abdollahi 26 , Reference Zhou and Li 27 ). Although the aetiopathogenesis of IBD must be considered to be multifactorial, modulation of the cell redox environment can somewhat control the intestinal inflammatory response( Reference Romier, Schneider and Larondelle 13 ). Taking into account the redox chemistry and bioactivity of Ind, in the present study, we investigated the effects of this pigment in an in vitro model of enterocytes during an acute phase of IBD. To this aim, Caco-2 cells, an established model of the human intestinal barrier( Reference Hidalgo, Raub and Borchardt 28 ), exposed to IL-1β, known as an IBD causative pathophysiological agent( Reference Ligumsky, Simon and Karmeli 5 – Reference Reimund, Wittersheim and Dumont 7 ), were used and the influence of Ind on a number of inflammatory parameters, including the release of pro-inflammatory mediators and levels of inducible inflammatory enzymes, was evaluated. The effects of Ind on the imbalance of cell redox, activation of membrane NADPH oxidase 1 (NOX-1) and of NF-κB, as well as the influence on the IL-1β-induced increase of the epithelial TJ permeability, were also investigated.
Materials and methods
Reagents
Unless otherwise specified, all reagents and chemicals were obtained from Sigma Chemical Company.
Isolation of indicaxanthin
Ind was isolated from cactus pear (O. ficus-indica) fruits (yellow cultivar). The phytochemical was separated from a methanol extract of the pulp by liquid chromatography on Sephadex G-25( Reference Butera, Tesoriere and Di Gaudio 16 ). Fractions containing the pigment were submitted to cryodessiccation (Freezone 4·5; Labconco). The dried material was resuspended in 1 % acetic acid in water and submitted to semi-preparative HPLC using a Varian Pursuit C18 column (250 × 10 mm inner diameter; 5 μm; Varian), eluted from solvent A (1 % acetic acid in water) to 20 % solvent B (1 % acetic acid in acetonitrile) with a 20 min linear gradient, an injection volume of 200 μl and a flow rate of 3 ml/min( Reference Tesoriere, Allegra and Butera 20 ). Spectrophotometric revelation was at 482 nm. The elution volumes relevant to the Ind peak were collected. After cryodessiccation, the samples were stored at − 80°C. Dried Ind was resuspended in Dulbecco's modified Eagle's medium at a suitable concentration and filtered through a Millex HV 0·2 μm filter (Millipore) before use. The concentration of the samples was evaluated spectrophotometrically with a DU-640 Beckman spectrophotometer using a molar coefficient of 42 800 at 482 nm.
Cell culture
Human colon adenocarcinoma-derived Caco-2 cells were obtained from the American Type Culture Collection and used between passages 27 and 31. The cells were grown in 175 cm2 flasks in Dulbecco's modified Eagle's medium (Gibco Life Technologies) supplemented with 10 % fetal bovine serum (Gibco Life Technologies), 1 % non-essential amino acids, 10 mm-HEPES, 50 units/ml of penicillin, 50 μg/ml of streptomycin and 100 μg/ml of gentamicin and were maintained at 37°C in 5 % CO2. For the experiments, the cells were seeded at a density of 1·25 × 105 in twenty-four-well plates (Corning Costar, Inc.) in a culture medium for 21 d to obtain fully differentiated cells. The culture medium was changed thrice a week. For the measurement of intestinal epithelial TJ permeability, experiments were carried out using Transwell® inserts (polycarbonate membrane, 0·4 μm pore size and 24 mm diameter). The inserts were placed into six-well plates. Caco-2 cells were seeded at 5 × 104 cells/cm2 on the membrane inserts with 1·5 ml of the medium on the apical/luminal side and 2·5 ml of the medium on the basolateral side. The culture medium was changed thrice a week throughout the 21 d of growth, and the transepithelial electrical resistance values across the cell monolayers were measured using a Millicell-ERS volt ohm meter (Millipore Corporation). Caco-2-plated filters with epithelial resistance of 400–500 Ω cm2 were used.
Cell treatments
Before each treatment, Caco-2 cells were made quiescent through overnight incubation in a serum-free medium. The cells were then placed in Dulbecco's modified Eagle's medium with 2 % fetal bovine serum and challenged with IL-1β (25 ng/ml) at 37°C either in the absence (control) or in the presence of Ind for the incubation times shown in the figures. Cell viability was routinely checked using the Trypan Blue Exclusion method. To evaluate the effect of the treatments on TJ permeability, IL-1β was added on the basolateral side and Ind on the apical/luminal side.
Assay for IL-6 and IL-8
The extracellular media were collected and centrifuged at 15 000 g for 10 min. The secretion of IL-6 and IL-8 was evaluated using a sandwich ELISA method (BD Biosciences Pharmingen) in accordance with the manufacturer's protocol.
Measurement of arachidonic acid cascade activity through PGE2 production
The activation of the arachidonic acid cascade was estimated through the production of PGE2 after the addition of arachidonic acid. Briefly, 24 h after the treatment, the cells were washed and incubated with 10 mm-arachidonic acid in PBS for 10 min. The secretion of PGE2 in the extracellular medium was quantified (pg/ml) using a Prostaglandin E2 Enzyme Immunoassay Kit (Cayman Chemical Corporation, Inc.) in accordance with the manufacturer's protocol.
Assay for nitric oxide
The production of NO in Caco-2 cell monolayers stimulated by IL-1β (25 ng/ml) for 24 h was evaluated. NO, present in the medium as nitrite, was assayed according to the Griess reaction. Briefly, 100 μl of the cell culture medium were mixed with 100 μl of Griess reagent (equal volumes of 1 % sulphanilamide (w/v) in 5 % phosphoric acid (v/v) and 0·1 % naphtylethylenediamine–HCl (w/v)) and incubated at room temperature for 10 min and then absorbance was measured at 550 nm using a microplate reader (GloMax®-Multi Microplate Reader; Promega Corporation). Fresh culture medium was used as the blank. The amount of nitrite in the samples was evaluated by referring to a sodium nitrite serial dilution standard curve.
Detection of intracellular reactive oxygen species
The intracellular levels of ROS were assessed by measuring the fluorescence resulting from the intracellular oxidation of 2′,7′-dichlorofluorescin diacetate. 2′,7′-Dichlorofluorescin diacetate in PBS (10 μm) was added to the medium 30 min before ending the treatment of the cells to label intracellular ROS. The medium was then removed, and the cells were washed with PBS, resuspended in the same buffer and immediately subjected to fluorescence-activated cell sorting analysis with an Epics XL™ flow cytometer, using the Expo32 software (Beckman Coulter). At least 1 × 104 cells were analysed for each sample.
Assay for total reduced thiols
After the treatment, the cells were centrifuged for 5 min at 2000 g , washed twice with cold PBS containing 0·1 m-butylated hydroxytoluene and ruptured by sonication. Cell lysates were mixed with 10 % SDS and 30 μm-5,5′-dithiobis (2-nitrobenzoic acid) and incubated at 30°C for 30 min. The levels of total reduced thiols, including protein thiols and glutathione, were measured spectrophotometrically at 412 nm.
Western blot analysis
Caco-2 cells were rinsed twice with ice-cold PBS and harvested by scraping into 200 μl/well ice-cold hypotonic lysis buffer (10 mm-HEPES, 1·5 mm-MgCl2, 10 mm-KCl, 0·5 mm-phenylmethylsulphonyl fluoride, 1·5 μg/ml soyabean trypsin inhibitor, 7 μg/ml pepstatin A, 5 μg/ml leupeptin, 0·1 mm-benzamidine and 0·5 mm-dithiothreitol). After 15 min of incubation on ice, the lysates were centrifuged at 13 000 g for 10 min, and the supernatants (cytosolic fraction) were immediately portioned and stored at − 80°C up to 2 weeks. The nuclear pellet was resuspended in 60 μl of a high-salt extraction buffer (20 mm-HEPES, pH 7·9, 420 mm-NaCl, 1·5 mm-MgCl2, 0·2 mm-EDTA, 25 % (v/v) glycerol, 0·5 mm-phenylmethylsulphonyl fluoride, 1·5 μg/ml soyabean trypsin inhibitor, 7 μg/ml pepstatin A, 5 μg/ml leupeptin, 0·1 mm-benzamidine and 0·5 mm-dithiothreitol) and incubated with shaking at 4°C for 30 min. The nuclear extract was then centrifuged for 15 min at 13 000 g , and the supernatant was portioned and stored at − 80°C up to 2 weeks. The protein concentration of each sample was determined using a Bradford protein assay reagent (Bio-Rad). Protein samples (80 μg/line) were separated on 12 % SDS–PAGE gel and transferred onto nitrocellulose membranes. The immunoblots were incubated overnight at 4°C with a blocking solution (5 % skimmed milk), followed by incubation with human anti-IκB-α monoclonal antibodies (clone 417208, catalogue no. MAB4299; R&D Systems) or human anti-phospho-IκB-α polyclonal antibodies (S32/S36, catalogue no. AF4809; R&D Systems), human anti-NF-κB p65 monoclonal antibodies (clone 532301, catalogue no. MAB 5078; R&D Systems) and human anti-poly(ADP-ribose) polymerase monoclonal antibodies (clone D-1, catalogue no. SC-365315; Santa Cruz Biotechnology) for 1 h at room temperature. The blots were washed twice with Tween 20/Tris-buffered saline and incubated with a 1:2000 dilution of rabbit anti-human horseradish peroxidase-conjugated anti-IgG antibodies (Dako Denmark) for 1 h at room temperature. The blots were again washed five times with Tween 20/Tris-buffered saline and then developed by enhanced chemiluminescence (Amersham Life Science). Immunoreactions were also performed using β-actin antibodies as the loading controls. The immunoreactive bands were densitometrically analysed using an Image Tool program (Image J 1·455; National Institutes of Health).
Evaluation of activated NADPH oxidase complex
The activity of NOX-1 was evaluated through Western blot analysis of the cytosolic subunit NOX activator 1 assembled in the membrane NOX-1 enzyme complex. To this aim, the cytosolic fractions of Caco-2 cells (see above) were centrifuged at 100 000 g for 1 h at 4°C. The pellet, containing the membrane fraction, was suspended in a cold buffer containing 10 mm-Tris–HCl, pH 7·4, 1 mm-EDTA and 0·5 % Triton X-100 (v/v) for 20 min. The membrane proteins (200 μg) were incubated overnight at 4°C with rabbit anti-NOX activator 1 polyclonal antibodies (ab103007; Abcam). Protein A-coupled agarose beads were used for immune complex purification. After 2 h of incubation, the beads were washed thrice in a radioimmunoprecipitation assay buffer, denatured in boiling Laemmli buffer and used for Western blot analysis. The antibodies employed for immunodetection were rabbit anti-NOX-1 polyclonal primary antibodies (ab55831; Abcam) (1:250 dilution) and mouse anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:2500 dilution). The immunoreactive bands were subjected to a densitometric analysis as described previously.
Uptake of indicaxanthin in Caco-2 cell monolayers
Caco-2 cell monolayers were incubated (37°C, 5 % CO2) with 25 μm-Ind. The incubation medium was removed at time intervals, and the cells were washed with sodium taurocholate and extracted with methanol for the HPLC measurement of the pigment. HPLC was performed according to the method of Tesoriere et al. ( Reference Tesoriere, Fazzari and Angileri 23 ) using an RP-18e Performance column (100 × 4·6 mm; Merck), equipped with an RP-18e Chromolith guard cartridge (5 × 4·6 mm; Merck), eluted from solvent A (1 % acetic acid in water) to 20 % solvent B (1 % acetic acid in acetonitrile) with a 20 min linear gradient at a flow rate of 1 ml/min. Spectrophotometric detection of Ind (retention time 9·3 min) was carried out at 482 nm. Quantification was done by referring to standard curves constructed with 5–100 ng of the pigment and by relating its amount to the peak area.
Statistical analysis
Results are given as means and standard deviations. Unless specified, three independent observations were carried out for each experiment replicated thrice. Calculations and graphs were obtained using the INSTAT-3 statistical software (GraphPad Software, Inc.) with a test for normality followed by ANOVA, with Bonferroni's correction for multiple comparisons. In all cases, significance was accepted if the null hypothesis was rejected at the P< 0·05 level.
Results
Effects of indicaxanthin on IL-1β-induced secretion of inflammatory mediators and increase of tight junction permeability
The eventual cytotoxic effect of Ind was preliminarily checked by assaying the extracellular activity of lactate dehydrogenase released from Caco-2 cells. No significant activity of lactate dehydrogenase was detected in the culture media after 24 h of incubation of the cells with amounts of Ind ranging from 25 to 100 μm (data not shown). The incubation of Caco-2 cells with 25 ng/ml of IL-1β, for 24 h, led to an increased secretion of IL-6 and IL-8, to the extent of 10-fold and 24·8-fold, respectively, compared with the cells incubated in the absence of the cytokine (Fig. 2). The co-incubation of the cells with Ind at amounts ranging from 5 to 25 μm resulted in a concentration-dependent inhibition of the release of both pro-inflammatory cytokines. Treatment with 25 μm-Ind reduced the levels of IL-6 and IL-8 in the inflamed cells by 75 and 65 %, respectively (Fig. 2).
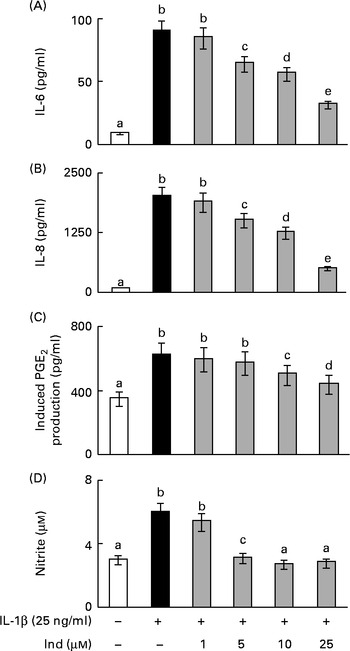
Fig. 2 Effect of indicaxanthin (Ind) on the release of inflammatory mediators by IL-1β-treated Caco-2 cell monolayers. Caco-2 cell monolayers were incubated with IL-1β either in the absence or in the presence of Ind. The release of (A) IL-6, (B) IL-8, (C) PGE2 and (D) nitric oxide into the medium was measured after 24 h of incubation. The levels of IL-6 and IL-8 were evaluated using ELISA. The activation of the arachidonic acid cascade was determined as the amount of PGE2 secreted after incubation for an additional 10 min with arachidonic acid and is referred to as the induced PGE2 production. Nitric oxide present in the medium as nitrite was assayed using the Griess colorimetric method. Values are means of three experiments carried out in triplicate, with standard deviations represented by vertical bars. a,b,c,d,eMean values with unlike letters were significantly different (P< 0·05; Bonferroni's test).
PGE2 and NO are the effectors of an inflammatory response; however, basal levels of PGE2 (0·31 (sd 0·05) ng/mg protein) and NO (3·0 (sd 0·4) μm) were observed in the colon epithelial cells due to the activities of COX-1 and constitutive NOS, respectively, even in the absence of inflammatory stimulation (Fig. 2). Under inflammatory stimulation, a sharp increase in the levels of PGE2 and NO, by the activity of the inducible COX-2 and iNOS, is expected. Accordingly, the activation of Caco-2 cell monolayers with IL-1β for 24 h brought about a 5·2-fold and a 2-fold increase in the levels of PGE2 and NO levels, respectively. The co-treatment of the cells with Ind inhibited the production of PGE2 and NO in a dose-dependent manner. The release of PG was inhibited by 70 % in the presence of 25 μm pigment, whereas the IL-1β-dependent NO formation was totally prevented by 10 μm-Ind (Fig. 2). The incubation of Caco-2 cell monolayers with Ind (25 μm) for 24 h did not significantly modify the basal release of any of the considered inflammatory mediators (not shown). Amounts of Ind as low as 1·0 μm were not effective in decreasing the levels of any of the measured inflammatory mediators (Fig. 2(A)–(D)).
The effect of IL-1β on the TJ permeability of filter-grown Caco-2 monolayers was evaluated by measuring the transepithelial electrical resistance with respect to the untreated cells. A drop in the transepithelial electrical resistance of Caco-2 cells by about 30 % was evident after 24 h of treatment with IL-1β, which was prevented by co-incubating the cells with 25 μm-Ind (Fig. 3).
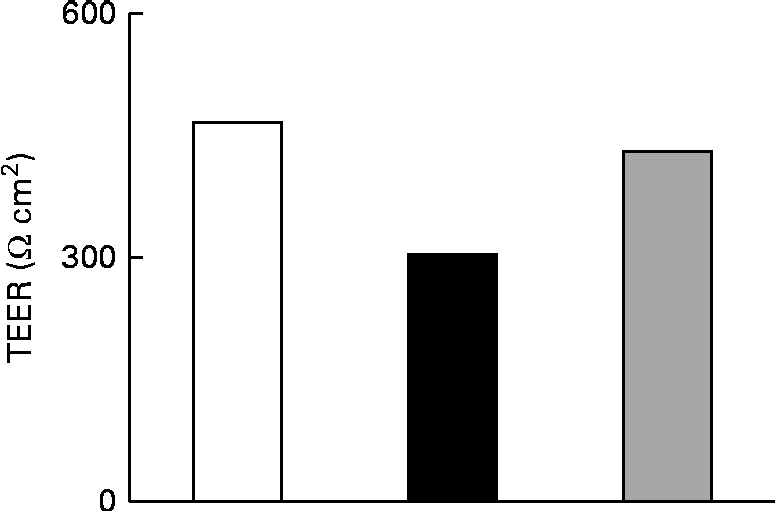
Fig. 3 Effect of indicaxanthin (Ind) on IL-1β-induced decrease of tight junction permeability in Caco-2 cells. Caco-2 cells grown in Transwells were incubated for 24 h with IL-1β (25 ng/ml) either in the absence or in the presence of Ind (25 μm). Control, cells treated with the vehicle. Transepithelial electrical resistance (TEER) values across the cell monolayers were measured using a volt ohm meter as reported in the Materials and methods section. Data are the mean of two experiments carried out in duplicate. □, Control; ■, IL-1β; ![]() , IL-1β+Ind.
, IL-1β+Ind.
Effect of indicaxanthin on the IL-1β-induced cell redox imbalance and NADPH oxidase activation
ROS are considered as intracellular second messengers of the signalling pathway associated with the Toll/IL-1β receptor( Reference O'Neill 29 ). The levels of intracellular ROS were monitored from 30 min to 3 h in Caco-2 cell monolayers incubated with IL-1β, either in the absence or in the presence of Ind. Cytofluorimetric analysis with 2′,7′-dichlorofluorescin diacetate showed a rapid production of ROS, with a significant peak after 1 h of treatment and a plateau in the following 2 h (Fig. 4(A)). As a consequence of the production of ROS, the levels of cell total thiols progressively decreased (Fig. 4(B)). The co-incubation of the cells with IL-1β and Ind resulted in the inhibition of both the production of ROS and the loss of thiols, an effect dependent on the concentration of Ind (Fig. 4(A) and (B)).
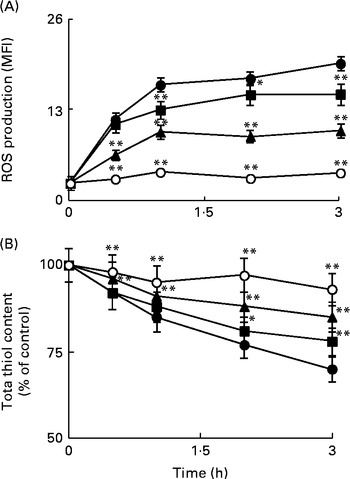
Fig. 4 Effect of indicaxanthin (Ind) on oxidative stress induced by IL-1β in Caco-2 cells. (A) Reactive oxygen species (ROS) production. (B) Thiol depletion. Caco-2 cells were treated with IL-1β (25 ng/ml) either alone or in combination with Ind at different time intervals. Control, cells treated with the vehicle. The levels of cellular ROS and total thiols were assayed using flow cytometry (2′,7′-dichlorofluorescin diacetate staining) and by the 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) reaction, respectively, as reported in the Materials and methods section. Values are means of three separate experiments carried out in triplicate, with standard deviations represented by vertical bars. Mean value was significantly different from that of the relevant control: * P< 0·05, ** P< 0·01 (Student's t test). MFI, mean fluorescence intensity. ●, Control; ■, 5 μm-Ind; ◆, 10 μm-Ind; ○, 25 μm-Ind.
The membrane NOX-1 has been identified as a source of ROS in the gastrointestinal tract. Under inflammatory stimulation, the activation of NOX-1 requires the assembly of cytosolic subunits (NADPH oxidase organiser 1 (NOXO1), NOX activator 1 and Ras-related C3 botulinium toxin substrate 1 (Rac1)) with the membrane-bound functional heterodimer (NOX-1 and p22phox)( Reference Bedard and Krause 30 ). The activation of the membrane NOX-1 complex in Caco-2 cells, after 30 min of incubation with either IL-1β or IL-1β and Ind, was evaluated by Western blot analysis of the cytosolic subunit NOXA-1 assembled in the membrane enzyme complex (Fig. 5). With respect to the control cells, the densitometric analysis of IL-1β-activated cells showed the appearance of NOX-1-activated complex. When Caco-2 cells were co-incubated with IL-1β and 25 μm-Ind, the activation of the NOX-1 complex was prevented (Fig. 5).
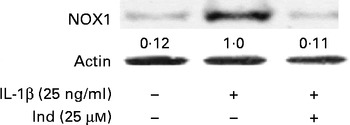
Fig. 5 Effect of indicaxanthin (Ind) on the activation of NADPH oxidase 1 (NOX-1). Caco-2 cells were treated with IL-1β (25 ng/ml) either alone or in combination with Ind for 15 min. The activation of NOX-1 was detected by Western blot analysis. After immunoprecipitation with NOXA1 antibodies, cell membrane proteins were separated by SDS–PAGE and immunoblotted with membrane enzyme component NOX-1 as reported in the Materials and methods section. The numbers below each line represent the relative expression level normalised to actin, as determined on the basis of the intensity of the band. Representative image of three experiments with similar results.
Effect of indicaxanthin on IL-1β-induced cyclo-oxygenase-2 and inducible nitric oxide synthase expression and NF-κB activation
The expression of COX-2 and iNOS was assessed by Western blot analysis in IL-1β-stimulated Caco-2 cells and in cells treated simultaneously with IL-1β and Ind. While COX-2 and iNOS were undetectable in the control cells, both enzymes were noticeably induced in cells treated with IL-1β for 24 h. Ind significantly reduced the expression of both COX-2 and iNOS; the higher the concentration, the lower the enzyme level (Fig. 6).
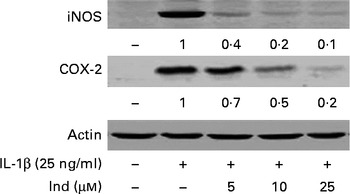
Fig. 6 Effect of indicaxanthin (Ind) on the IL-1β-induced increase of inducible nitric oxide synthase (iNOS) and cyclo-oxygenase-2 (COX-2) expression in Caco-2 cells. Caco-2 cells were incubated for 24 h with IL-1β either in the absence or in the presence of Ind. Cell lysates were subjected to Western blot analysis with the indicated antibodies as reported in the Materials and methods section. The numbers below each line represent the relative expression level normalised to actin, as determined on the basis of the intensity of the band. Representative image of three experiments with similar results.
The transcriptional activation of pro-inflammatory genes is regulated by NF-κB. IL-1β induces the activation of the NF-κB transcription factor by phosphorylation of its inhibitor IκB-α, which is then degraded by the proteasome, allowing the translocation of NF-κB into the nucleus( Reference Surh, Chun and Cha 31 ). A Western blot analysis of Caco-2 cells treated with IL-1β for 12 h showed a net decrease in the levels of cytosolic IκB-α with an increase in the levels of its phosphorylated form (phospho-IκB-α), accompanied by the nuclear translocation of the NF-κB p65 subunit. The co-incubation of the cells with Ind and IL-1β inhibited the activation of NF-κB in a dose-dependent manner (Fig. 7).
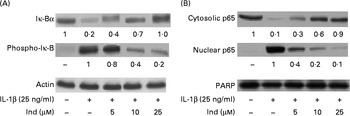
Fig. 7 Effect of indicaxanthin (Ind) on IL-1β-induced (A) Iκ-B phosphorylation and (B) p65 nuclear translocation in Caco-2 cells. Caco-2 cells were incubated for 12 h with IL-1β either in the absence or in the presence of Ind. Control, cells treated with the vehicle. Cellular and nuclear lysates were subjected to Western blot analysis with the indicated antibodies as reported in the Materials and methods section. The numbers below each line represent the relative expression level normalised to actin (cytosolic protein) or poly(ADP-ribose) polymerase (nuclear proteins) as determined on the basis of the intensity of the band. Representative image of three experiments with similar results.
Uptake of indicaxanthin in Caco-2 cell monolayers
HPLC measurements of cell extracts were carried out to explore the uptake of Ind (25 μm) in IL-1β-treated cells when compared with control Caco-2 cells. Under both conditions, a quite comparable (P>0·05, Student's t test) concentration-dependent uptake was observed, reaching equilibrium within 20 min of incubation (Fig. 8).
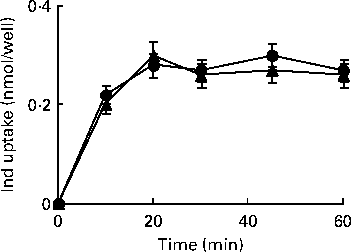
Fig. 8 Uptake of indicaxanthin (Ind) in Caco-2 cells. Caco-2 cell monolayers were incubated with 25 μm-Ind either in the absence or in the presence of IL-1β (25 ng/ml) for the indicated times. The gathered cells were extracted for the HPLC analysis of Ind as reported in the Materials and methods section. Values are means of three separate experiments carried out in triplicate, with standard deviations represented by vertical bars. ![]() , Ind;
, Ind; ![]() , Ind+IL-1β.
, Ind+IL-1β.
Discussion
Ind, a dietary pigment that characterises the fruit of cactus pear( Reference Livrea and Tesoriere 15 ), has recently attracted attention for a number of biological activities based on in vitro data, ranging from cytoprotective effects( Reference Tesoriere, Butera and Allegra 19 , Reference Tesoriere, Allegra and Butera 20 ) to the modulation of intracellular redox-dependent signalling( Reference Gentile, Tesoriere and Allegra 21 , Reference Tesoriere, Attanzio and Allegra 22 ), and for its high bioavailability in humans( Reference Tesoriere, Allegra and Butera 25 ). In the present study, we provide evidence of a remarkable anti-inflammatory effect of the molecule in an in vitro model of intestinal inflammation consisting of IL-1β-activated Caco-2 cell monolayers. The effect was found to be associated with the inhibition of the production of NOX-1 and ROS and the activation of NF-κB and downstream events, such as the release of inflammatory mediators and the over-expression of COX-2 and iNOS. Finally, the epithelial permeability appeared to be preserved.
Inflammatory mediators such as IL-8, IL-6, PG and NO are constitutively produced by intestinal cells as a part of the physiological immune response of the mucosa. The IL-8 chemokine directs the migration of polymorphonuclear leucocytes, monocytes and macrophages towards the site of inflammation, and the inflammatory IL-6 cytokine stimulates the chemotaxis of neutrophils and is associated with necrosis in the colon, which in turn leads to tissue destruction( Reference Böcker, Damião and Holt 3 , Reference Reinecker, Steffen and Witthoeft 6 ). NO regulates blood flow, pain transmission and immune system activation( Reference Kolios, Valatas and Ward 32 ), whereas PGE2 stimulates mucus and bicarbonate secretion, elevates mucosal blood flow, regulates leucocyte recruitment, and mediates fever and pain( Reference Martin and Wallace 4 , Reference Tanabe and Tohnai 33 ). IL-1β, a key mediator of cell communication within the inflammatory intestinal area, stimulates the synthesis and secretion of all these factors( Reference Ligumsky, Simon and Karmeli 5 – Reference Reimund, Wittersheim and Dumont 7 ) and then allows recruitment to the intestinal mucosa and activation of other immune cells such as neutrophils, which promote a self-sustaining inflammatory loop. In our model, the activation of Caco-2 cells by IL-1β resulted in a dramatic increase in the secretion of IL-6, IL-8, PGE2 and NO, an effect remarkably inhibited by co-treatment with Ind, with a concurrent inhibition of the expression of COX-2 and iNOS. As an apparent contrast with our findings, other authors have reported that the release of NO from Caco-2 cells is not modified by IL-1β treatment( Reference Romier-Crouzet and Van De Walle 34 ). Differences including cell phenotype, passage number and seeding can account for this discrepancy( Reference Chen and Kitts 35 ).
The NF-κB transcription factor is crucial for a number of cell processes including inflammation, proliferation and apoptosis and plays a pivotal role in chronic inflammatory diseases( Reference Barnes and Karin 36 ). In resting cells, the most abundant form of NF-κB is the cytosolic inactive p65/p50 heterotrimer, with the p65 subunit, containing the transcriptional activation domain, bound to the IκB inhibitory protein. A number of extracellular stimuli, including inflammatory cytokines, activate signalling pathways that converge on the phosphorylation and inactivation of IκB, followed by nuclear translocation of the p65/p50 dimer and its binding to specific response elements in the DNA( Reference Karin 37 ). In the present study, the remarkable increase in the levels of the nuclear p65 subunit consequent to the stimulation of IL-1β was inhibited by Ind pre-treatment of Caco-2 cells. Considering that NF-κB, in synergy with other transcriptional activators, coordinates the gene expression of pro-inflammatory enzymes and cytokines, including IL-6( Reference Hershko, Robb and Wray 38 ), IL-8( Reference Hoffmann, Dittrich-Breiholz and Holtmann 39 ), COX-2( Reference Tanabe and Tohnai 33 ) and iNOS( Reference Surh, Chun and Cha 31 ), these findings suggest that the anti-inflammatory activity of Ind is, at least in part, mediated by the inhibition of the NF-κB activation pathway in our cell model. Consistent with these conclusions, in an in vivo model of IBD( Reference Comalada, Camuesco and Sierra 40 , Reference Ukil, Maity and Karmakar 41 ), the anti-inflammatory effects of polyphenolic redox-active/antioxidant phytochemicals such as quercitrin and curcumin have been shown to be mediated through the suppression of iNOS and COX-2 and the down-regulation of NF-κB signalling.
Signal transduction and inflammatory response induced by IL-1β have been reported to be related to the activity of NOX-1 in a number of cells. NOX-1, a member of the NOX-1 family, is highly expressed in colon tissue, as well as in Caco-2 cells( Reference Geiszt, Lekstrom and Brenner 42 , Reference Geiszt, Lekstrom and Witta 43 ), and ROS produced by this enzyme have been suggested to be second messengers in the immune response of these cells( Reference Geiszt, Lekstrom and Brenner 42 , Reference Rokutan, Kawahara and Kuwano 44 ), therefore being potentially involved in the pathogenesis of IBD( Reference Szanto, Rubbia-Brandt and Kiss 45 ). In accordance with the expected inflammatory sequence of events, we report that the stimulation of Caco-2 cells with IL-1β results in a very rapid ROS production with thiol loss, concomitant with the transport of the NOX activator 1 subunit and activation of NOX-1 at the plasma membrane. Ind inhibited the IL-1β-induced ROS generation and maintained the cell redox balance. Moreover, the activated NOX-1 complex was not detected in the presence of the pigment. Then, although Ind is an effective radical scavenger( Reference Butera, Tesoriere and Di Gaudio 16 , Reference Tesoriere, Allegra and Butera 17 ), the observed anti-inflammatory effect may not be limited to a mere scavenging of NOX-1-derived oxidant species. Physicochemical characteristics( Reference Tesoriere, Gentile and Angileri 24 ) allow Ind to interact with and locate in the phospholipid bilayers of biomimetic membrane aqueous dispersions( Reference Turco-Liveri, Sciascia and Lombardo 46 ) and enter into erythrocytes both in vivo and in vitro and, as observed in the present study, in Caco-2 cells under either normal conditions or an inflammatory stimulus. In this context, the activity of Ind could be hypothesised to be at the cell membrane level, e.g. interference with the binding of IL-1β with its cell surface receptor and/or the ensuing recruiting of effectors leading to the activation of NF-κB.
A leaky epithelial barrier is a hallmark of many diseased states and, in particular, of those affecting the intestine such as IBD, resulting in aberrant exposure of the bloodstream to luminal substances and pathogens, toxins and allergens. This perturbation is attributed to the effect of cytokines on the structure and function of TJ( Reference Capaldo and Nusrat 47 , Reference Groschwitz and Hogan 48 ). It has been reported that IL-1β induces an increase of intestinal epithelial TJ permeability both in vivo ( Reference Al-Sadi, Guo and Dokladny 49 ) and in Caco-2 cell monolayers( Reference Al-Sadi and Ma 11 ), as a result of the activation of NF-κB-dependent pathways. Under the conditions of the present study, Ind inhibited the activation of NF-κB and significantly corrected the increase of permeability caused by the inflammatory stimulus. Although there is active research on the anti-inflammatory activity of putative nutraceuticals at the intestinal level( Reference Romier, Van De Walle and During 50 , Reference Trishna, Da and Beong 51 ), such a protective effect on TJ permeability has not been reported. Polyphenols such as epigallocatechin-3-gallate and genistein, reported to inhibit the production of inflammatory mediators, have been shown to not attenuate the loss of epithelium integrity in inflamed Caco-2 monolayers( Reference Sergent, Piront and Meurice 52 ). Although pathways downstream the activation of NF-κB may have been inhibited or modulated by Ind, it may be interesting to mention that, differently from the great majority of phytochemicals, Ind does not undergo metabolism in the mucosal cells and can cross the intestinal epithelial barrier through the paracellular route, thus interacting with the TJ( Reference Tesoriere, Gentile and Angileri 24 ). This concept has recently put forward that epithelial permeability and TJ may be modified by dietary means( Reference Mullin, Skrovanek and Valenzano 53 ). The specific activity of Ind at the level of the complex structure of intestinal TJ, in both normal and diseases states, deserves to be investigated.
The modulation of redox-sensitive signalling pathways that support inflammatory processes by dietary redox-active phytochemicals in the intestinal mucosa may be of particular interest, the region in which these compounds can attain relatively high concentrations. In this context, Ind has been shown to be quite stable under digestive conditions( Reference Tesoriere, Fazzari and Angileri 23 , Reference Tesoriere, Gentile and Angileri 24 ), with only a 30 % loss at the gastric level. This would allow this phytochemical to reach even a 100 μm concentration in the small intestine, when ingested with dietary amounts of cactus pear fruit pulp (200 g, 24 mg Ind)( Reference Tesoriere, Fazzari and Angileri 23 , Reference Tesoriere, Allegra and Butera 25 ) and considering an intestinal volume of 600 ml( Reference Mahe, Huneau and Marteau 54 ). On the basis of this, Ind concentrations effective in modulating the immune response of the intestinal cells in our model are physiologically relevant. Given the interest in the identification of natural compounds for the prevention and/or progression of inflammatory states and inflammation-associated diseases( Reference Gosslau, Li and Ho 55 ), our data concerning a dietary highly bioavailable compound may offer an interesting perspective for a multimodal approach to IBD therapy.
Acknowledgements
The present study was supported by Italian Government's funds from MIUR (University of Palermo, ex 60 %). MIUR had no role in the design and analysis of the study or in the writing of this article.
L. T. and M. A. designed the research; A. A. and C. G. carried out the experimental work; M. A. L. coordinated the work and discussed the experimental data. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest.










