The current trend in fish production is to increase the lipid content in diets to spare proteins, improve feed conversion and decrease the amount of waste produced by fish. These diets, however, alter body composition and slaughter quality, particularly through an increase in lipid deposition (Cowey, Reference Cowey, Kaushik and Luquet1993; Hillestad & Johnsen, Reference Hillestad and Johnsen1994; Vergara et al. Reference Vergara, López-Calero, Robaina, Caballero, Montero, Izquierdo and Aksnes1999). The farming industry is concerned with finding methods that control body lipid deposition and/or increase the market value of cultured fish when such diets are used.
Conjugated linoleic acids (CLA) is a collective term for isomers of C18:2 fatty acids that differ in the geometry and position of two conjugated double bonds. Research with CLA indicates that these fatty acids are responsible for many biological properties that relate to health. CLAs have been shown to inhibit atherosclerosis (Lee et al. Reference Lee, Kritchevsky and Pariza1994), chemically induced carcinogenesis (Ha et al. Reference Ha, Storkson and Pariza1990; Ip et al. Reference Ip, Banni, Angioni, Carta, Mcginley, Thompson, Barbano and Bauman1999) and obesity (Park et al. Reference Park, Albright, Liu, Storkson, Cook and Pariza1997; West et al. Reference West, DeLany, Camet, Blohm, Truett and Scimeca1998) and to enhance feed efficiency (Li & Watkins, Reference Li and Watkins1998) in several rodent models. In addition, dietary CLA has been reported to decrease body fat in mice (Park et al. Reference Park, Albright, Liu, Storkson, Cook and Pariza1997), rats (Sisk et al. Reference Sisk, Hausman, Martin and Azain2001), pigs (Ostrowska et al. Reference Ostrowska, Muralitharan, Cross, Bauman and Dunshea1999) and chickens (Szymczyk et al. Reference Szymczyk, Pisulewski, Szczurek and Hanczakowski2001), although in man the results are limited and contradictory (Risérus et al. Reference Risérus, Arner, Brismar and Vessby2002, Reference Risérus, Smedman, Basu and Vessby2003; Gaullier et al. Reference Gaullier, Halse, Hoye, Kristiansen, Fagertun, Vik and Gudmundsen2005).
In fish, studies with CLA are very scarce, but in certain species, CLAs have been shown to alter growth responses, feed efficiency and lipid deposition (Choi et al. Reference Choi, Kang, Ha, Ackman, Xiong, Ho and Shahidi1999; Twibell et al. Reference Twibell, Watkins, Rogers and Brown2000, Reference Twibell, Watkins and Brown2001; Twibell & Wilson, Reference Twibell and Wilson2003). Dietary CLA did not, however, affect the growth performance or proximate composition of rainbow trout (Figueiredo-Silva et al. Reference Figueiredo-Silva, Rema, Bandarra, Nunes and Valente2005) or Atlantic salmon juveniles (Berge et al. Reference Berge, Ruyter and Asgard2004; Kennedy et al. Reference Kennedy, Campbell, Porter and Tocher2005), although the fatty acid profile of those fish was strongly modified (Berge et al. Reference Berge, Ruyter and Asgard2004; Bandarra et al. Reference Bandarra, Nunes, Andrade, Prates, Pereira, Monteiro, Rema and Valente2006). Fish are not a naturally rich source of CLA but are capable of incorporating high levels of CLA into tissue lipids after dietary supplementation (Twibell et al. Reference Twibell, Watkins, Rogers and Brown2000, 2001; Kennedy et al. Reference Kennedy, Campbell, Porter and Tocher2005; Bandarra et al. Reference Bandarra, Nunes, Andrade, Prates, Pereira, Monteiro, Rema and Valente2006), this being a suitable route to produce ‘functional foods’.
High-lipid diets result in a marked increase in the flesh lipid content of fish, which is a component of organoleptic quality (Corraze, Reference Corraze, Guillaume, Kaushik, Bergot and Métailler2001). Fattening makes the raw flesh more tender and slightly modifies the consumer's overall taste perception. The nature of the dietary lipids also affects the taste of the products (Boggio et al. Reference Boggio, Hardy, Babbitt and Brannon1985; Turchini et al. Reference Turchini, Mentasti, Frøyland, Orban, Caprino, Moretti and Valfré2003; Izquierdo et al. Reference Izquierdo, Montero, Robaina, Caballero, Rosenlund and Gines2005), but very little is known about the general effects of these alterations, particularly dietary CLA supplementation, on the sensory characteristics of rainbow trout.
The overall objective of the present study was to evaluate the effect of graded levels of CLA in high-fat diets fed to large-size rainbow trout. The growth performance, body composition, tissue fatty acid deposition, lipogenic enzyme activities (glucose-6-phosphate dehydrogenase, malic enzyme, fatty acid synthetase) and sensory characteristics of the flesh were then assessed using those fish.
Material and methods
Experimental diets
A commercial extruded diet for rainbow trout was supplied by Sorgal S.A. (Ovar, Portugal). The CLA mixture (containing 75 % CLA) was offered by Bioriginal Food and Science Corp. (Saskatoon, SK, Canada). Before oil-coating, the pellets (3 mm diameter) were analysed for proximate composition and then coated with 21·9 % oil containing the different CLA levels (0 %, 0·5 %, 0·75 %, 1 %). The CLA supplement was added to the diets at the expense of fish oil to maintain a constant energy level (25–26 kJ/g DM) between dietary treatments. The ingredients and proximate composition of the experimental diets are presented in Table 1 and the fatty acid profiles in Table 2.
Table 1 Ingredients and proximate composition of diets with different levels (0 %, 0·5 %, 0·75 %, 1 %) of conjugated linoleic acid (CLA)
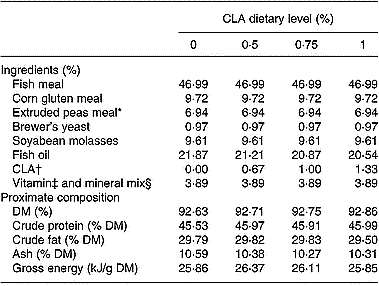
* Aquatex (20·5 crude protein); Sotexpro, Bermericourt, France.
† CLA mixture: total CLA, 75 %; 18 : 2 (cis-9, trans-11), 33·6 %; 18 : 2 (trans-10, cis-12), 32·5 %.
‡ Vitamins (mg/kg diet or IU/kg diet): vitamin A, 8000 IU; vitamin D3, 2000 IU; vitamin E, 100 mg; vitamin K, 10 mg; vitamin B12, 0·02 mg; vitamin B1, 15 mg; vitamin B2, 25 mg; vitamin B6, 15 mg; folic acid, 10 mg; biotin, 1 mg; vitamin C, 100 mg; betaine, 500 mg; inositol, 300 mg; nicotinic acid, 100 mg; pantothenic acid, 50 mg; choline chloride, 1000 mg.
§ Minerals (g/kg diet or mg/kg diet): MnSO4, 20 mg; KI, 0·6 mg; CuSO4, 5 mg; CoSO4, 0·4 mg; MgSO4, 500 mg; Zn (Bioplex; Alltech), 30 mg; Se (Sel-Plex 2000; Alltech), 0·3 mg; FeSO4, 40 mg; CaCO3, 2·15 g; dibasic calcium phosphate, 5 g; KCl, 1 g; NaCl, 0·4 g.
Table 2 Fatty acid profile (% total fatty acids) and total conjugated linoleic acid (CLA) content and CLA isomers (% total lipids) of diets with different levels of CLA (0 %, 0·5 %, 0·75 % and 1 %) (Mean values and standard deviations for three determinations)

a,b,c,d Mean values with unlike superscript letters were significantly different (P < 0·05).
* Saturated: 12 : 0, 14 : 0, 14 : 0 isobr, 15 : 0, 16 : 0, 16 : 0 isobr, 17 : 0, 18 : 0, 19 : 0, 20 : 0, 22 : 0 and 24 : 0.
† Monounsaturated: 16 : 1n-7, 17 : 1n-8, 18 : 1n-9, 18 : 1n-7, 20 : 1n-9, 20 : 1n-7, 22 : 1n-11 and 22 : 1n-9.
‡ Polyunsaturated: 16 : 2n-4, 16 : 3n-3, 16 : 4n-3, 18 : 2n-6, 18 : 2CLA, 18 : 3n-6, 18 : 3n-3, 18 : 4n-3, 20 : 2n-6, 20 : 3n-3, 20 : 4n-6, 20 : 4n-3, 20 : 5n-3, 22 : 2n-6, 22 : 4n-6, 22 : 5n-6, 22 : 5n-3 and 22 : 6n-3.
§ Sum of all CLA isomers detected by HPLC.
∥ Isomers detected by HPLC.
Growth trial
The trial was conducted with rainbow trout (Oncorhynchus mykiss) juveniles produced at the University of Trás-os-Montes and Alto Douro (UTAD, Vila Real, Portugal) rearing facilities. Fish were acclimated to the experimental conditions and fed the control diet ( < 0·003 % CLA) for a period of 2 weeks before the beginning of the experiment. Homogenous groups of twenty-five fish with an average initial body weight of 97·8 (sd 0·02) g were then randomly distributed between twelve square fibre-glass tanks (250 litres) in an open flow-through system. Triplicate groups of fish for each treatment were hand-fed to apparent satiety twice a day (09.30 and 18.00 h) for 12 weeks. The pH, ammonia, nitrite, nitrate and phosphate levels were monitored during the entire trial and maintained at levels compatible with the species. The daily water temperature was 16 ± 1°C and fish were exposed to natural photoperiod. At the end of the growth trial, data on weight gain and feed intake were collected from all the fish. Prior to sampling, fish were fasted for 24 h and then anaesthetised by immersion in an ethylene glycol monophenyl ether (1:2500) bath. A pooled sample of six fish from the initial stock at the beginning of the experiment and three fish per tank at the end of the experiment were taken and stored at − 20°C for subsequent whole-body composition analyses.
At the end of the experiment, liver and viscera from nine fish per dietary treatment were removed and weighed for estimation of the hepatosomatic and viscerosomatic indices. The livers and muscle samples from nine fish per treatment were removed and frozen in liquid N and stored at − 80°C prior to fatty acid determination and analysis of lipogenic enzymes activity. Experiments were conducted according to the European Council Directive 86/609/EEC regarding the protection of animals used for experimental and other scientific purposes.
Analytical methods
Whole fish were ground, and their moisture content was determined (105°C for 24 h). Fish were subsequently freeze-dried before further analysis. Feed, whole-body samples and faeces were then analysed for DM (105°C for 24 h), ash by combustion in a muffle furnace (550°C for 12 h), crude protein (Micro-Kjeldahl; N × 6·25) after acid digestion, lipid content by petroleum ether extraction (at Soxhlet 40–60°C) and gross energy in an adiabatic bomb calorimeter (IKA, Staufen, Germany).
Determinations of the total lipid of the fish tissues were carried out following the Bligh & Dyer (Reference Bligh and Dyer1959) method with small modifications. Muscle samples were analysed individually, whereas in liver, owing to the scarcity of individual samples, the three livers per tank were sliced and divided into two samples that were analysed independently.
Fatty acid methyl esters (FAME) of diets were prepared after lipid extraction carried out according to the methodology of Bligh & Dyer (Reference Bligh and Dyer1959), followed by acid modified transesterification for 2 h at 80°C. To analyse the fatty acid methyl esters and CLA of trout muscle and liver, extracted total lipids were used for methyl ester preparation by base-catalysed transesterification, with 0·5 m-sodium methoxide solution in anhydrous methanol (2 h at 30 °C), as proposed by Park et al. (Reference Park, Albright, Cai and Pariza2001) and Kramer et al. (Reference Kramer, Blackadar and Zhou2002), in order to avoid the isomerisation of CLA. Fatty acid methyl esters prepared in triplicate were analysed in a Varian CP 3800 (Walnut Creek, CA, USA) GC, equipped with an autosampler and fitted with a flame ionisation detector at 250°C. The separation was achieved using a capillary column HP-Innowax (30 m length, 0·25 mm internal diameter, 0·25 (m film thickness) from Agilent (Albertville, MN, USA). After being kept constant at 180°C for 5 min, the temperature was raised by 4°C/min to 220°C and maintained at 220°C for 25 min with the injector at 250°C. The split ratio was 100:1, and quantification was carried out using an area of the internal standard of 21:0. All analytical determinations were done in triplicate.
In order to avoid some possible co-elution of individual CLA isomers using a short GC column (30 m), the methyl esters of the CLA isomers were individually separated by triple Ag ion columns in series (ChromSpher 5 Lipids; 250 mm′ 4·6 mm internal diameter, 5 μm particle size; Chrompack, Bridgewater, NJ, USA), using an HPLC system (Agilent 1100 Series; Agilent Technologies Inc., Palo Alto, CA, USA) equipped with an autosampler and diode array detector adjusted to 233 nm. The mobile phase was 0·1 % acetonitrile in n-hexane maintained at a flow rate of 1 ml/min, using injection volumes of 20 μl. The identification of the individual CLA isomers was achieved by comparing their retention times with commercial and prepared standards, as well as with values published in the literature In addition, the identification of each isomer was controlled by the typical UV spectra of CLA isomers from the diode array detector in the range 190 to 360 nm, using the spectral analysis of Agilent Chemstation for LC 3D Systems rev. A.09.01 (Agilent Technologies). The determination of total and individual CLA isomer content was based on the external standard technique (using 18 : 2 cis-9, trans-11, 18 : 2 trans-10, cis-12, 18 : 2 cis-9, cis-11 and 18 : 2 trans-9, trans-11 as representatives of each of the geometric groups of CLA isomers) and on the method of area normalisation (Association of Official Analytical Chemists, 2000). The CLA isomers were expressed in gravimetric contents as percentages of total lipids (g/100 g lipids).
Enzyme assays
Liver samples were homogenised in three volumes of ice-cold buffer 0·02 m-Tris–HCl, 0·25 m-sucrose, 2 mm-EDTA, 0·1 m-NaF, 0·5 m m-phenyl methyl sulphonyl fluoride and 0·01 m-β-mercaptoethanol at pH 7·4 and centrifuged (30 000 g at 4°C) for 20 min. The soluble protein content of the liver was determined in the supernatant by the method of Bradford (Reference Bradford1976) using bovine serum albumin as a standard. Selected lipogenic enzyme activities were assayed on the supernatant using spectrophotometric procedures: glucose-6-phosphate dehydrogenase (EC 1.1.1.49) according to Bautista et al. (Reference Bautista, Garrido-Pertierra and Soler1988), malic enzyme (ME, EC 1.1.1.40) according to Ochoa (Reference Ochoa, Colowick and Kaplan1955) and fatty acid synthetase (EC 2.3.1.38) according to the methodology of Chang et al. (Reference Chang, Seidman, Teebor and Lane1967) as modified by Chakrabarty & Leveille (Reference Chakrabarty and Leveille1969). Enzyme activity units (IU), defined as millimoles of substrate converted to product per minute at the assay temperature, were expressed per milligrams of hepatic soluble protein-specific activity. In order to take into account possible variations in the hepatosomatic index, the activities were also calculated per 100 g of fish.
Sensory analysis
At the end of the experiment, four fish per tank were killed by a sharp blow on the head and immediately put on ice. They were then stored for 24 h at 4°C before sensory analysis. The same protocol of slaughter and filleting was strictly applied to all the fish. The fillets were skinned, washed with tap water and hand-cut into 20 g portions. These were then put into small plastic cups previously coded with three-digit numbers and microwaved until homogeneous cooking (20 s at a maximum power of 850 W). Samples were randomised and served hot in a consumer-type test to thirty-one panelists who regularly ate fish, who were asked to compare unknown samples with an identified control (0 % CLA), in a room designed for sensory analysis. A hidden control was included among the samples to be classified. An explanation sheet with detailed instructions and questions to be answered was given to each panelist. The samples were evaluated using a scale from 0 (similar to control) to 6 (extremely different from control). If sensory differences were detected between the samples, panelists were asked to describe them to obtain a general idea of attributes of the different fish.
Statistical analysis
Statistical analyses followed methods outlined by Zar (Reference Zar1996). All data were tested for normality and homogeneity of variances by Kolmogorov-Smirnov and Bartlett tests, and were then submitted to a one-way ANOVA with the Statistica 6.0 package for Windows (StatSoft Inc., Tulsa, OK, USA). When data did not meet the assumptions of ANOVA, the non-parametric ANOVA equivalent (Kruskal–Wallis test) was performed. When these tests showed significance (P < 0·05), individual means were compared using the Tukey or Dunn test. The results of sensory analysis were subjected to two-way ANOVA to test the effects of experimental diets and assessors, this being followed by a Dunnett test. Significant differences were considered when P < 0·05.
Results
Rainbow trout fed the experimental diets during 12 weeks did not display significant differences in growth performance (2–2·3 %), feed conversion ratio (1·1–1·2), protein efficiency ratio (1·8–2·0) or feed intake (12·6–12·9 g/kg per day) between treatments (Table 3). Furthermore, neither the hepatosomatic (0·8–0·9) nor the viscerosomatic (9·8–11) index was affected by the dietary inclusion of CLA (P>0·1).
Table 3 Effect of different dietary levels (0 %, 0·5 %, 0·75 %, 1 %) of conjugated linoleic acid (CLA) on growth, efficiency and feed intake in rainbow trout over 12 weeks (Mean values and standard deviations*)

Absence of superscripts letters indicates no significant differences between treatments (P>0·05).
* n 3 except for hepatosomatic index and viscerosomatic index, for which n 9.
† Hepatosomatic index = 100 × (liver weight/body weight), %.
‡ Viscerosomatic index = 100 × (viscera weight/body weight), %.
§ Feed conversion ratio = dry feed intake/weight gain.
∥ Daily growth index = 100 × ((final body weight)1/3 – (initial body weight)1/3)/days.
¶ Protein efficiency ratio = weight gain/crude protein intake.
** Average body weight = (initial mean body weight+final mean body weight)/2.
Data on the whole-body composition and retention of rainbow trout fed the different diets are presented in Table 4. By the end of the feeding trial, whole-body protein and lipid content were similar between treatments, ranging from 15·8 % to 16·5 % and 14·9 % to 16·5 %, respectively. Moreover, no significant differences were observed for protein, lipid and energy retention values between dietary treatments (P>0·1).
Table 4 Whole-body composition and retention of rainbow trout fed different levels of conjugated linoleic acid (CLA; 0 %, 0·5 %, 0·75 %, 1 %) for 12 weeks (Mean values and standard deviations for three determinations)

Absence of superscript letters indicates no significant differences between treatments (P>0·05).
The fatty acid profile of the rainbow trout muscle is presented in Table 5. Total saturated fatty acid, MUFA and PUFA were not significantly affected by the incorporation of CLA. Moreover, muscle total lipids were similar between treatments. Within the saturated fraction, stearic acid (18 : 0) varied significantly with dietary CLA inclusion, although the expected decrease in 18 : 1 fatty acid was not seen. Among the PUFA, dietary CLA supplementation mainly resulted in a significant increase in the biologically active trans-10, cis-12 and cis-9, trans-11 CLA isomers. The total CLA content of the muscle reflected the dietary inclusion levels, the highest level being attained when fish were fed 1 % CLA. The n-3 PUFA, in particular 20 : 5n-3 (EPA), 22 : 5n-3 (docosapentaenoic acid) and 22 : 6n-3 (DHA), were not affected by dietary CLA inclusion.
Table 5 Fatty acid profile (% total fatty acids), total conjugated linoleic acid (CLA; % total lipids) content, CLA isomers (% total lipids) and total lipids (% wet weight) in muscle of rainbow trout fed different dietary levels of CLA (0 %, 0·5 %, 0·75 %, 1 %) for 12 weeks (Mean values and standard deviations for nine determinations)

a,b,c,d Mean values with unlike superscript letters were significantly different (P < 0·05).
* Saturated: 12 : 0, 14 : 0, 14 : 0 isobr, 15 : 0, 16 : 0, 16 : 0 isobr, 17 : 0, 18 : 0, 19 : 0, 20 : 0, 22 : 0 and 24 : 0.
† Monounsaturated: 16 : 1n-7, 17 : 1n-8, 18 : 1n-9, 18 : 1n-7, 20 : 1n-9, 20 : 1n-7, 22 : 1n-11 and 22 : 1n-9.
‡ Polyunsaturated: 16 : 2n-4, 16 : 3n-3, 16 : 4n-3, 18 : 2n-6, 18 : 2CLA, 18 : 3n-6, 18 : 3n-3, 18 : 4n-3, 20 : 2n-6, 20 : 4n-6, 20 : 3n-3, 20 : 4n-3, 22 : 2n-6, 20 : 5n-3, 22 : 4n-6, 22 : 5n-6, 22 : 5n-3 and 22 : 6n-3.
§ Sum of all CLA isomers detected by HPLC.
∥ Isomers detected by HPLC.
Contrary to what was observed in muscle, the liver fat content was significantly reduced by the inclusion of 0·75 % or 1 % CLA (Table 6). The ingestion of increasing amounts of CLA induced significant changes in the fatty acid profile of the liver that were reflected in all fractions. Both saturated and polyunsaturated fractions increased significantly with CLA incorporation, whereas there was a significant reduction in MUFA. The most evident changes in saturated fatty acids and MUFA were related to the increase in 18 : 0 and decrease in 18 : 1 fatty acids. With regard to PUFA, the levels of 18 : 2 CLA were significantly raised as a result of both biologically active isomers, although the incorporation of the cis-9, trans-11 isomer was always greater. Moreover, the liver deposition of 20 : 5n-3 (EPA) and of 22 : 5n-3 (docosapentaenoic acid) showed the opposite trend: the level of the former increased significantly, whereas that of docosapentaenoic acid declined with dietary CLA incorporation. DHA deposition was similar in all treatments.
Table 6 Fatty acid profile (% total fatty acids), total conjugated linoleic acid (CLA; % total lipids) content, CLA isomers (% total lipids) and total lipids (% wet weight) in the liver of rainbow trout fed different dietary levels of CLA (0 %, 0·5 %, 0·75 %, 1 %) for 12 weeks (Mean values and standard deviations for six determinations)

a,b,c Mean values with unlike superscripts letters were significantly different (P < 0·05).
* Saturated: 12 : 0, 14 : 0, 14 : 0 isobr, 15 : 0, 16 : 0, 16 : 0 isobr, 17 : 0, 18 : 0, 19 : 0, 20 : 0, 22 : 0 and 24 : 0.
† Monounsaturated: 16 : 1n-7, 17 : 1n-8, 18 : 1n-9, 18 : 1n-7, 20 : 1n-9, 20 : 1n-7, 22 : 1n-11 and 22 : 1n-9
‡ Polyunsaturated: 16 : 2n-4, 16 : 3n-3, 16 : 4n-3, 18 : 2n-6, 18 : 2CLA, 18 : 3n-6, 18 : 3n-3, 18 : 4n-3, 20 : 2n-6, 20 : 4n-6, 20 : 3n-3, 20 : 4n-3, 22 : 2n-6, 20 : 5n-3, 22 : 4n-6, 22 : 5n-6, 22 : 5n-3 and 22 : 6n-3.
§ Sum of all CLA isomers detected by HPLC.
∥ Isomers detected by HPLC.
Activities of lipogenic enzymes
Data on the activities of the three lipogenic enzymes assayed are reported as both IU/mg protein and IU/100 g whole fish (Table 7). Glucose-6-phosphate dehydrogenase and fatty acid synthetase displayed the highest (7·8–8·9 IU/100 g) and lowest (60–90 mIU/100 g) activity values, respectively, but dietary CLA level had no significant effect on either of these two lipogenic enzymes. Malic enzyme activity in fish fed 0·75 % CLA was lower than that in fish fed the control diet (0 % CLA), although this difference was less evident when the results were expressed per 100 g fish.
Table 7 Effects of different dietary levels (0%, 0·5%, 0·75% and 1 %) of conjugated linoleic acid (CLA) on the hepatic lipogenic enzyme activities of rainbow trout (Mean values and standard deviations for nine determinations)

a,bMean values with unlike superscript letters were significantly different (P < 0·05).
Sensory analysis
All panelists correctly identified the hidden control, and non-significant differences were detected between the panelists' results (P < 0·01). Sensory data indicated significant differences in the organoleptic characteristics between trout fed the control diet and trout fed the different CLA levels (0·5 %, 0·75 %, 1 % CLA). These differences were classified as slight-to-moderate for 0·5 % and 0·75 % CLA, and between slight and slight-to-moderate for 1 % CLA, suggesting that CLA levels above 0·75 % were less perceptible in sensory terms than were lower values. Descriptors from the panelists included attributes of flavour and texture in the mouth, although panelists were divided concerning their preferences: approximately half of the panelists had the overall impression that the samples with CLA were better than the control, whereas the other half expressed the opposite view.
Discussion
The growth rates and nutrient utilisation of rainbow trout were good, and the incorporation of increasing dietary levels of CLA (0–1 %) led to similar final weights of the fish at the end of the 12-week trial. Moreover, the independency of growth performance upon dietary CLA level confirm earlier observations in juvenile rainbow trout growing from 5 to 70 g and fed low-fat diets with the same CLA level (Figueiredo-Silva et al. Reference Figueiredo-Silva, Rema, Bandarra, Nunes and Valente2005), and are in agreement with results from other studies in various fish species fed 0·5–5 % CLA (Twibell et al. Reference Twibell, Watkins and Brown2001; Twibell & Wilson, Reference Twibell and Wilson2003; Berge et al. Reference Berge, Ruyter and Asgard2004; Kennedy et al. Reference Kennedy, Campbell, Porter and Tocher2005). However, common carp fed 1 % CLA increased their weight gain (Choi et al. Reference Choi, Kang, Ha, Ackman, Xiong, Ho and Shahidi1999), whereas hybrid striped bass reduced their feed intake and improved their feed efficiency (Twibell et al. Reference Twibell, Watkins, Rogers and Brown2000). Nevertheless, higher CLA inclusion levels reduced weight gain and feed efficiency in Nile tilapia, rockfish and common carp (Choi et al. Reference Choi, Kang, Ha, Ackman, Xiong, Ho and Shahidi1999). It can be concluded that the effect of CLA on growth performance and feed efficiency is clearly dependent on the species considered.
In the present study, dietary CLA up to 1 % had no effect on the whole-body composition of rainbow trout, which is in agreement with previous findings in smaller-sized trout (Figueiredo-Silva et al. Reference Figueiredo-Silva, Rema, Bandarra, Nunes and Valente2005), hybrid striped bass (Twibell et al. Reference Twibell, Watkins, Rogers and Brown2000), channel catfish (Twibell & Wilson, Reference Twibell and Wilson2003) and Atlantic salmon (Kennedy et al. Reference Kennedy, Campbell, Porter and Tocher2005). Several studies have, however, reported a decrease in lipid deposition in certain fish species (Choi et al. Reference Choi, Kang, Ha, Ackman, Xiong, Ho and Shahidi1999; Twibell et al. Reference Twibell, Watkins, Rogers and Brown2000, Reference Twibell, Watkins and Brown2001; Twibell & Wilson, Reference Twibell and Wilson2003). The reduction in lipid deposition is particularly desirable when high-fat diets are used. In the present study, however, CLA administration did not affect the body composition of the fish.
With respect to the influence of CLA on tissue composition, a decrease in liver lipid content was obtained when high levels of CLA (>0·5 %) were fed. This is in accordance with earlier results from striped bass (Twibell et al. Reference Twibell, Watkins, Rogers and Brown2000) and yellow perch (Twibell et al. Reference Twibell, Watkins and Brown2001). In contrast, dietary CLA had no significant effect on the liver lipid content of catfish (Twibell & Wilson, Reference Twibell and Wilson2003), Atlantic salmon (Kennedy et al. Reference Kennedy, Campbell, Porter and Tocher2005) or rainbow trout juveniles fed low-fat diets (Bandarra et al. Reference Bandarra, Nunes, Andrade, Prates, Pereira, Monteiro, Rema and Valente2006), whereas increased muscle lipid concentrations were observed in Atlantic salmon (Kennedy et al. Reference Kennedy, Campbell, Porter and Tocher2005). In the present work, the hepatosomatic index was not affected by different CLA treatments, despite the reduction in liver lipid content by dietary CLA supplementation.
The reduction in liver lipid content was accompanied by a slight reduction in malic enzyme specific activity, whereas glucose-6-phosphate dehydrogenase and fatty acid synthetase were not significantly affected by the dietary CLA level. The positive correlation between malic enzyme specific activity and liver lipid content suggests an inhibition of malic enzyme activity and consequent liver lipid content by increasing dietary CLA levels. An inhibition of de novo lipogenesis by CLA isomers has been demonstrated in the mammary glands of sow (Bee, Reference Bee2000) and mice (Lin et al. Reference Lin, Loor and Herbein2004). Moreover, other studies have demonstrated that CLA upregulates not only the mRNA expression, but also the activity of various enzymes involved in lipogenesis and fatty acid oxidation in mouse liver (Takahashi et al. Reference Takahashi, Kushiro, Shinohara and Ide2003; Ide, Reference Ide2005). In rats, the ingestion of a CLA mixture increased malic enzyme activity in the liver, whereas the activities of both glucose-6-phosphate dehydrogenase and fatty acid synthetase were unaffected (Faulconnier et al. Reference Faulconnier, Arnalb, Mirand, Chardignyc and Chilliarda2004). Figueiredo-Silva et al. (Reference Figueiredo-Silva, Rema, Bandarra, Nunes and Valente2005) did not observe any significant changes in the activities of the lipogenic enzymes in rainbow trout juveniles fed low-fat diets (16 %) and increasing CLA levels, suggesting that the effect of CLA on hepatic lipogenic enzymes was dependent on the species considered and might be related to the various nutritional and physiological conditions involved in the regulation of hepatic fatty acid oxidation and synthesis.
Several studies have shown that the dietary CLA affects the fatty acid profile of fish independently of any effect on body fat level (Twibell et al. Reference Twibell, Watkins, Rogers and Brown2000; Berge et al. Reference Berge, Ruyter and Asgard2004; Bandarra et al. Reference Bandarra, Nunes, Andrade, Prates, Pereira, Monteiro, Rema and Valente2006). In the present work, the total percentage of the main fatty acids groups in muscle were not as strongly affected by the dietary inclusion of CLA as they were in rainbow trout juveniles fed low-fat diets (Bandarra et al. Reference Bandarra, Nunes, Andrade, Prates, Pereira, Monteiro, Rema and Valente2006). This suggests that the developmental stage of the fish and the dietary lipid content might affect the pattern of lipid metabolism. The saturated lipid fraction in muscle did not vary between dietary treatments despite the increasing concentration of stearic acid (18 : 0). In liver, however, the saturated fraction of the control group was significantly lower than that found in fish fed CLA, stearic acid concentration being mainly responsible for these differences. This was a clear effect of dietary CLA as the control diet contained higher amounts of saturated fatty acids. Moreover, the level of MUFA decreased significantly in both muscle and liver. The significant increase in 18 : 0 and the concomitant decrease in 18 : 1n-9 suggests an inhibition of Δ-9 desaturase activity by CLA. This was more evident in liver than muscle. Similar results have been reported in rainbow trout juveniles (Bandarra et al. Reference Bandarra, Nunes, Andrade, Prates, Pereira, Monteiro, Rema and Valente2006), hybrid striped bass (Twibell et al. Reference Twibell, Watkins, Rogers and Brown2000), yellow perch (Twibell et al. Reference Twibell, Watkins and Brown2001) and Atlantic salmon (Berge et al. Reference Berge, Ruyter and Asgard2004; Kennedy et al. Reference Kennedy, Campbell, Porter and Tocher2005). The dietary incorporation of CLA led to a significant increase in the polyunsaturated fraction in the liver. In muscle, besides CLA deposition, a reduction in 22 : 5n-6 was observed, whereas the n-3 fatty acid concentrations remained unchanged. Different results were previously reported in rainbow trout juveniles (Bandarra et al. Reference Bandarra, Nunes, Andrade, Prates, Pereira, Monteiro, Rema and Valente2006) and striped bass muscle (Twibell et al. Reference Twibell, Watkins, Rogers and Brown2000), for which CLA incorporation resulted in decreased levels of DHA (22 : 6n-3). In larger rainbow trout (>250 g), this negative effect was not observed in muscle and liver, where a prominent biosynthesis and deposition of DHA was reported.
CLA was successfully incorporated into tissue lipids, levels in flesh being 2-fold higher (2·1–4·2 %) than those in liver (0·8–1·9 %). Moreover, muscle CLA incorporation was not related to a decrease in DHA, as previously reported in juvenile rainbow trout fed low-fat diets (Bandarra et al. Reference Bandarra, Nunes, Andrade, Prates, Pereira, Monteiro, Rema and Valente2006), as well as other species (Twibell et al. Reference Twibell, Watkins, Rogers and Brown2000; Kennedy et al. Reference Kennedy, Campbell, Porter and Tocher2005). In muscle and liver lipids, cis-9, trans-11 and trans-10, cis-12 isomers were retained in proportions similar to those found in the diets. Results reported by Burdge et al. (Reference Burdge, Lupoli and Russell2004) show that there was no differential incorporation of cis-9, trans-11 and trans-10, cis-12 into the plasma phospholipids of healthy men. Nevertheless, the extent of incorporation of total CLA isomers seems to be tissue dependent, showing a higher deposition in muscle lipid than liver, as previously reported for rainbow trout juveniles (Bandarra et al. Reference Bandarra, Nunes, Andrade, Prates, Pereira, Monteiro, Rema and Valente2006), Atlantic salmon (Kennedy et al. Reference Kennedy, Campbell, Porter and Tocher2005) and mammalian species (Ostrowska et al. Reference Ostrowska, Cross, Muralitharan, Bauman and Dunshea2003; Lauridsen et al. Reference Lauridsen, Mu and Henckel2005).
It is well known that different dietary fat sources will affect the fatty acid composition of fish, and that this can be reflected in their sensory qualities (Boggio et al. Reference Boggio, Hardy, Babbitt and Brannon1985; Turchini et al. Reference Turchini, Mentasti, Frøyland, Orban, Caprino, Moretti and Valfré2003; Izquierdo et al. Reference Izquierdo, Montero, Robaina, Caballero, Rosenlund and Gines2005). In the present study, the sensory characteristics of trout fillet were slightly affected by the dietary supplementation with CLA. Schabbel et al. (Reference Schabbel, Maaβ, Steinhart, Reiter and Schwarz2004) did not find any difference in trout previously fed with diets containing 0 %, 1 % and 3 % CLA, although the flavour of deep-frozen fillets with 3 % CLA and 600 mg vitamin E supplementation was considered to be positively distinct.
The results obtained in the present experiment clearly show that CLA can be incorporated in a proportion of up to 1 % in rainbow trout diets without altering fish performance, feed efficiency and whole-body composition. The effects of CLA in liver metabolism were more pronounced than reported in previous studies (Figueiredo-Silva et al. Reference Figueiredo-Silva, Rema, Bandarra, Nunes and Valente2005; Bandarra et al. Reference Bandarra, Nunes, Andrade, Prates, Pereira, Monteiro, Rema and Valente2006), suggesting that the dietary oil content and developmental stage of the fish are important factors in determining the effects of CLA. The present results clearly suggest that CLA can be incorporated at a level of up to 1 % in rainbow trout diets, contributing to the production of a functional food. It is now important to determine the minimal administration period of dietary CLA to obtain the desirable deposition levels of CLA in the muscle of commercially sized rainbow trout.
Acknowledgements
Special thanks go to Sorgal, S.A., Ovar, Portugal, for supplying the extruded diet before coating, and to Bioriginal Food and Science Corp., Saskatoon SK, Canada, for offering the CLA mixture. We also thank António Júlio Pinto for his technical assistance during the growth trial and samplings, and Engs. Maria João Monteiro and Susana Teixeira, from Escola Superior de Biotecnologia, Universidade Católica Portuguesa, for their work and facilities for sensory testing. This study was supported by project POCTI/CVT/39 237/2001 (FCT, Portugal, with the support of the European fund FEDER).









