Central neurocytomas (CN) account for 0.1–0.5% of tumours of the central nervous system, and are most commonly found in young adults.Reference Patel, Schmidt and Liu1 They typically present in the lateral ventricles.Reference Qian, Lin, Zhang and Cao2 Though relatively benign in histology, these tumours can invade the foramen of Monro causing obstructive hydrocephalus. Additionally, these lesions can cause an intraventricular haemorrhage.Reference Patel, Schmidt and Liu1 Therefore, when symptomatic, intraventricular CNs are treated with surgical resection.Reference Patel, Schmidt and Liu1 Surgical access to the lateral ventricles is technically challenging and associated with increased postoperative complications compared to other intracranial procedures.Reference John, Robin and Pabaney3 Reported complications postintraventricular surgery include hydrocephalus, subdural hygroma, subdural haematoma and intraventricular haemorrhage.Reference Qian, Lin, Zhang and Cao2, Reference Chen, Yang, Ma, Yu, Xu and Zhou4, Reference Tanaka, Sugita, Kobayashi, Takemae and Hegde5
We present a case of a CN resected through a transcortical approach. The postoperative course was complicated by progressively enlarging bilateral subdural hygromas – a manifestation of external hydrocephalus – resulting in bilateral uncal herniation.
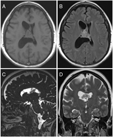
Our patient was a 60-year-old female who initially presented with intractable headaches, anosmia, and forgetfulness. Neurological examination revealed no focal neurological deficits, and the patient was otherwise healthy. Computed tomography (CT) and magnetic resonance imaging (MRI) of the head revealed a 2.8 × 3.0 × 2.7 cm mixed cystic and solid mass attached to the septum pellucidum extending into both lateral ventricles, with obstruction of the foramen of Monro (Figure 1).
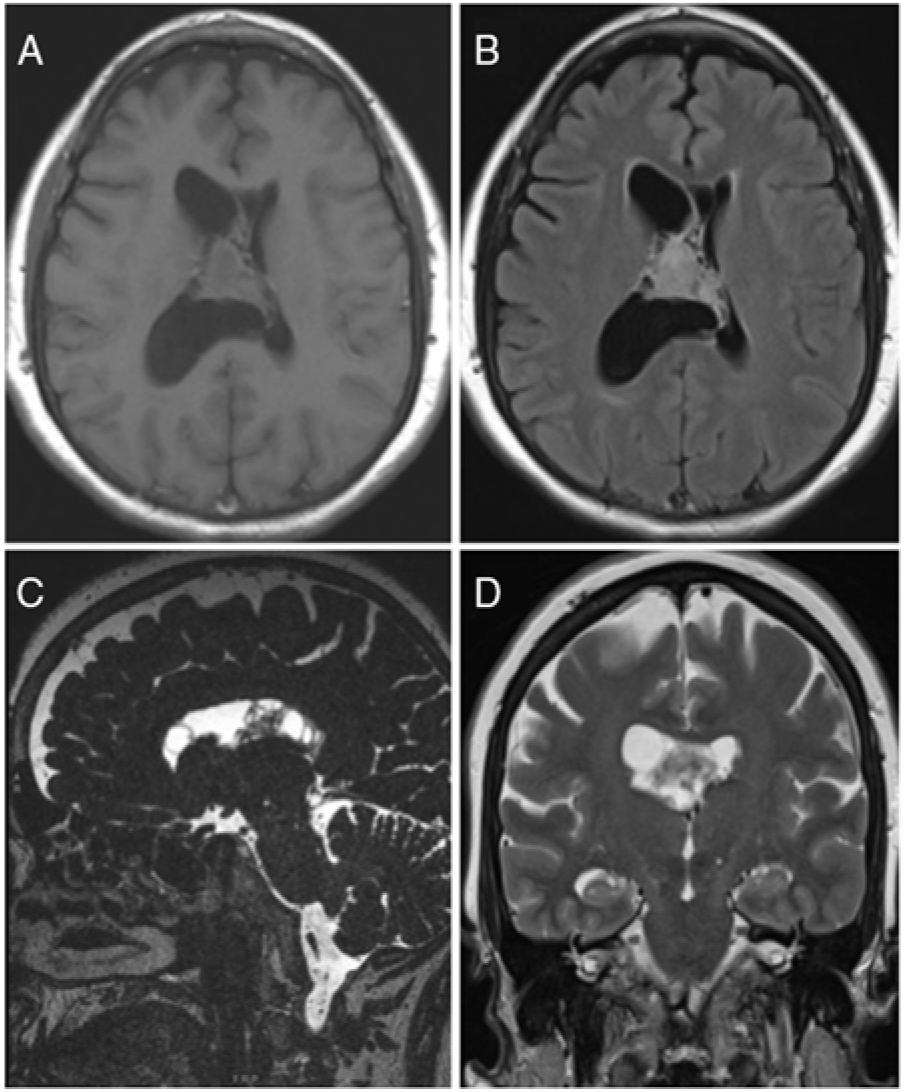
Figure 1: Preoperative MRI showed an intraventricular mass. (A) Axial T1-weighted image, (B) Axial T2-weighted FLAIR image, (C) Sagittal CISS image and (D) Coronal T2-weighted image demonstrating a mixed cystic and solid mass centred on the septum pellucidum with extension into bilateral lateral ventricles (right greater than left). FLAIR – Fluid Attenuation Inversion Recovery, CISS – Constructive Interference Steady State.
An elective right frontal mini craniotomy was performed under image guidance, and the right lateral ventricle was accessed transcortically (transgyral) using a 16 mm-wide METRx tubular retractor system (Medtronic) after sequential dilation.Reference Almenawer, Crevier, Murty, Kassam and Reddy6, Reference Eliyas, Glynn and Kulwin7 A wide septostomy was performed, and the tumour was resected using a Nico Myriad (NICO Corporation). Maximal safe resection was achieved with residual tumour in the posterosuperior aspect of the right lateral ventricle. At the end of the procedure, the surgical tract was packed with Gelfoam (Pfizer). Microscopic examination of the resected mass confirmed the diagnosis of CN, WHO grade II, with a Ki67 proliferation index <2%.
Postoperatively, the patient complained of nausea, vomiting and headaches. A CT performed on postoperative day (POD) 1 showed subtotal resection of the tumour (Figure 2A–C). She was discharged on POD 2 with a minimal headache.
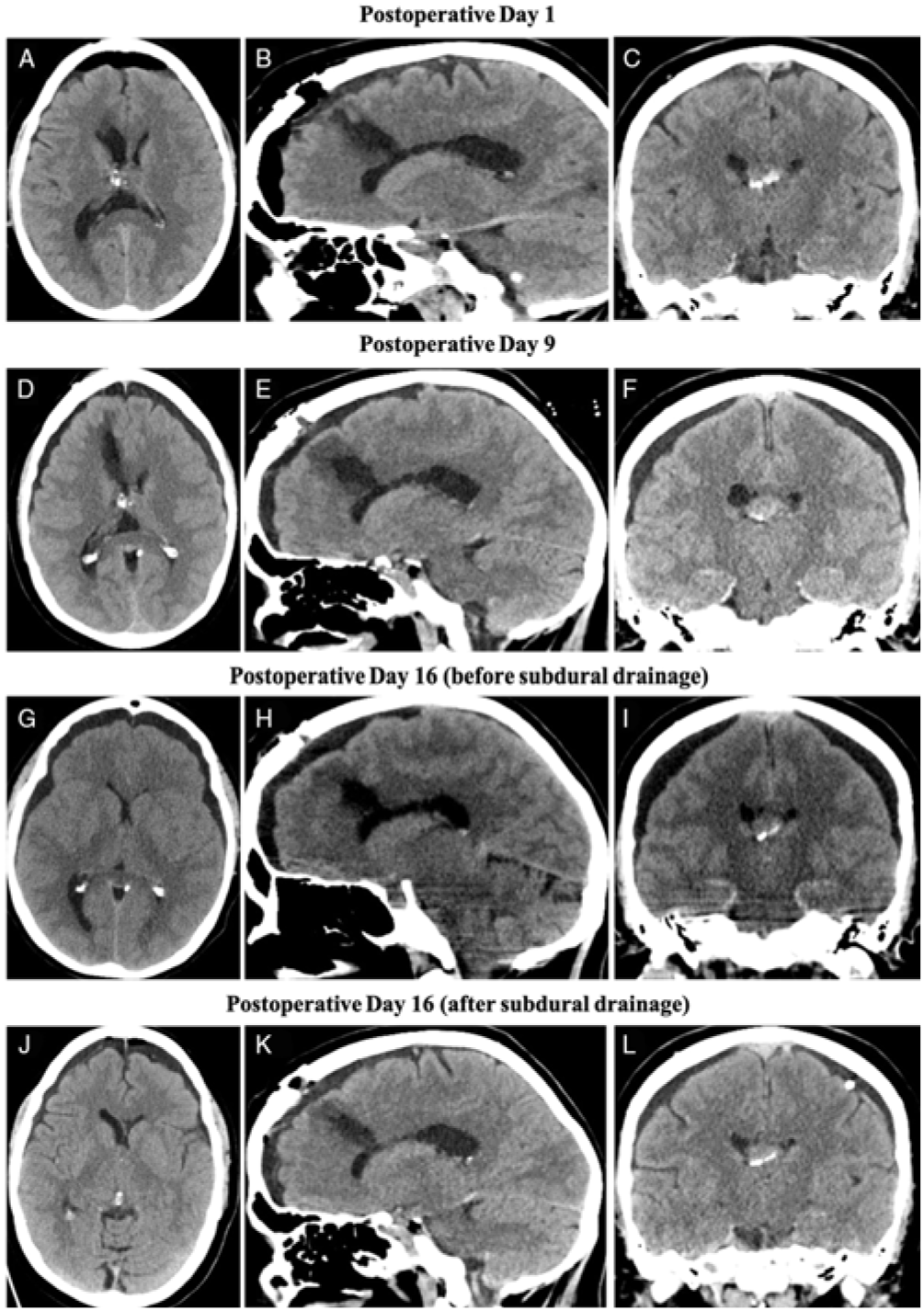
Figure 2: CT images of bilateral subdural hygromas monitored through the patient’s postoperative course. (A–C) Axial, sagittal and coronal images on POD 1 illustrating residual tumour in the right lateral ventricle, as well as expected postoperative changes. Of note, there was pneumocephalus, but no ventriculomegaly, and a surgical tract was appreciated. (D–F) Axial, sagittal and coronal images on POD 9 revealed bilateral subdural hygromas, without significant mass effect or herniation. (G–I) CT scan performed on POD 16 prior to twist-drill craniostomy. Compared to POD 9, there was an interval increase in hygroma size with evidence of diffuse sulcal effacement, descending transtentorial herniation and bilateral uncal herniation. There was no ventriculomegaly or midline shift noted. (J–L) CT images on POD 16 posttwist-drill craniostomy. Of note, the bilateral hygromas decreased in size and there was partial resolution of mass effect as noted by less sulcal effacement.
The patient was seen routinely by the multidisciplinary neuro-oncology team on POD 8 and was asymptomatic at that time. However, on POD 9, the patient presented to the emergency department (ED) with intractable nausea and vomiting, but no focal neurological deficits. A CT showed small bilateral subdural hygromas with mild mass effect, without ventriculomegaly or intracranial haemorrhage (Figure 2D–F). The patient was discharged after symptomatic treatment with instructions to return if her symptoms persist or worsen. The patient presented to the ED again on POD 13 with the same presentation and was again treated symptomatically without repeated imaging.
The patient then presented on POD 16 to the ED with decreased level of consciousness, bilateral fixed and dilated pupils (left greater than right), left-sided ptosis and left sixth nerve palsy. Repeat CT showed enlargement of the hygromas, with significantly worsened global mass effect including diffuse sulcal effacement, bilateral uncal herniation, and compression of the basal cisterns (Figure 2G–I). Urgent left-sided twist drill craniostomy was performed and a subdural drain was left in place. The CSF egress was under high pressure with large amount of light-pink-coloured fluid drainage. The patient had immediate improvement of left oculomotor nerve palsy. A post-procedure CT demonstrated reduction in the hygroma size (Figure 2J–L). MRI with CSF flow study at that time only showed slightly diminished CSF flow at the cervicomedullary junction with a bilaterally patent foramen of Monro. Our patient continued to improve with subdural drainage and returned to her neurological and functional baseline over the next two days.
This constellation of imaging and clinical findings points to a diagnosis of external hydrocephalus due to redistribution of CSF along the recently established surgical tract from the tubular retractors into the subdural space. Further, CSF analysis showed elevated protein (1.2 g/L) which may have contributed to reduced CSF absorption. Thus, a right-sided ventriculoperitoneal shunt was inserted. Postoperatively, the patient has been doing well and followed by the multi-disciplinary neuro-oncology team without further concerns.
Subdural hygromas following intraventricular surgery are common and often are self-resolving.Reference Tanaka, Sugita, Kobayashi, Takemae and Hegde5 In a series of 92 intraventricular CNs, subdural hygromas were found in 13 (14.1%) patients.Reference Qian, Lin, Zhang and Cao2 In the same series, 13 (14.1%) patients also developed shunt-dependant hydrocephalus; however, there was no correlation reported between developing subdural hygromas and postoperative hydrocephalus. Another study of 38 patients with transcortical surgery reported 40% of their patients developing subdural hygromas.Reference Tanaka, Sugita, Kobayashi, Takemae and Hegde5 However, after serial imaging, 24% were persistent, and of those, 11% were symptomatic requiring further treatment. Predisposing factors for persistent and symptomatic subdural hygromas were preoperative ventriculomegaly and a frontal operative approach.Reference Tanaka, Sugita, Kobayashi, Takemae and Hegde5 Regarding postoperative hydrocephalus, reported etiologies have exclusively been due to obstruction of foramen of Monro due to either residual tumour, postoperative swelling or intraventricular heamorrhage, with no reported association to postoperative subdural hygromas.Reference Qian, Lin, Zhang and Cao2 However, our patient did not have obstruction of CSF at the foramen of Monro. Rather, they had decreased CSF reabsorption as well as a surgical tract which allowed an alternative path for CSF flow into the subdural space.
With regard to the surgical technique, the use of tubular retractor for intraventricular surgery has been previously proposed due to the potential advantages over typical brain retractors for concerns that the blades cause transection of white matter tracts and place unequal pressure on the parenchyma which can result in significant injury to surrounding structures.Reference Vigneswaran, Pradilla, Chaichana and Quiñones-Hinojosa8 The safety and efficacy of using tubular retractor systems for intraventricular surgery have previously been studied with no reports of subdural hygromas or external hydrocephalus; however, the evidence has thus far been reserved to single-arm case series.Reference Almenawer, Crevier, Murty, Kassam and Reddy6–Reference Vigneswaran, Pradilla, Chaichana and Quiñones-Hinojosa8 To the best of our knowledge, this is the first reported case of acute neurological deterioration with bilateral uncal herniation syndrome associated with external hydrocephalus following intraventricular surgery.
This case highlights the need to carefully observe patients postintraventricular surgery to halt the insidious progression of external hydrocephalus, with diligent repeat imaging especially in symptomatic patients within the first few postoperative weeks. Further, these authors endorse having external hydrocephalus on the differential diagnosis for patients with subdural hygromas following intraventricular surgery.
Disclosures
The authors have nothing to disclose.