Introduction
Rationale
‘Blanket’ or systematic dry-cow therapy (antimicrobial treatment of all quarters in all cows) has been recommended for decades as part of an overall effort to reduce intramammary infection (IMI) in the dry period (Neave et al., Reference Neave, Dodd, Kingwill and Westgarth1969). IMI at dry-off is an important risk factor for the development of clinical mastitis in early lactation (Green et al., Reference Green, Green, Medley, Schukken and Bradley2002; Piepers et al., Reference Piepers, De Vliegher, de Kruif, Opsomer and Barkema2009). Mastitis treatment and prevention is a large driver of antimicrobial use for dairy cattle (United States Department of Agriculture, 2008). Increased concern for antimicrobial use and its relationship with the development of antimicrobial resistance (World Health Organization, 2015) has resulted in nation-specific regulations (Santman-Berends et al., Reference Santman-Berends, Swinkels, Lam, Keurentjes and van Schaik2016) and general pressure to reduce group-level (as opposed to individual animal) prophylactic use of antimicrobials (ECDC/EMEA, 2015).
In Scandinavia, selective dry-cow therapy has been common for several decades (Vilar et al., Reference Vilar, Hovinen, Simojoki and Rajala-Schultz2018), and has been established more recently as the only legal use of dry-cow therapy in Holland (Vanhoudt et al., Reference Vanhoudt, van Hees-Heijps, van Knegsel, Sampimon, Vernooij, Nielen and van Werven2018). However, IMI risk during the dry period in these countries may be different than in North America (Ruegg, Reference Ruegg2017), and it is possible that the effect of these therapies varies depending on herd-level factors, or details surrounding the intervention, such as how cows are selected for antimicrobial therapy.
Systematic reviews of controlled trials provide the highest level of evidence for the efficacy of an intervention under field conditions (Sargeant et al., Reference Sargeant, Kelton and O'Connor2014a), and if sufficient primary studies on a given comparison are available, allow for exploration of heterogeneity of the effect size among studies. Establishing the relative efficacy of selective dry-cow therapy on udder health and production outcomes would serve to improve decision-makers' ability to engage in effective stewardship of antimicrobials with knowledge of implications on animal health and welfare.
This systematic review was conducted based on methods proposed by the Cochrane Collaboration (Higgins and Green, Reference Higgins, Thomas, Chandler, Cumpston, Li, Page and Welch2011) and recommendations for conducting systematic reviews in animal agriculture and veterinary medicine (O'Connor et al., Reference O'Connor, Anderson, Goodell and Sargeant2014a, Reference O'Connor, Sargeant and Wang2014b; Sargeant and O'Connor, Reference Sargeant and O'Connor2014a, Reference Sargeant and O'Connor2014b; Sargeant et al., Reference Sargeant, Kelton and O'Connor2014a, Reference Sargeant, Kelton and O'Connor2014b). It was reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (Liberati et al., Reference Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche, Ioannidis, Clarke, Devereaux, Kleijnen and Moher2009).
Objective
The objective of this review was to determine the relative efficacy of selective antimicrobial treatment at dry-off compared to blanket dry-cow treatment (all quarters of all cows) to decrease frequency of IMI at calving, frequency of IMI over the first 30 days in milk, and risk of clinical mastitis during the first 30 days in milk of the subsequent lactation.
Methods
Protocol
A review protocol, established in advance and reported in accordance with PRISMA-P guidelines (Moher et al., Reference Moher, Shamseer, Clarke, Ghersi, Liberati, Petticrew, Shekelle and Stewart2015), was published to the University of Guelph's institutional repository (https://atrium.lib.uoguelph.ca/xmlui/handle/10214/10046) on 25 June 2018. The protocol is also available through Systematic Reviews for Animals and Food (SYREAF) (http://www.syreaf.org/contact/).
Eligibility criteria
Primary research studies, both refereed and non-refereed (grey literature), available in English were eligible for inclusion. Controlled trials with natural disease exposure were the only eligible study design, although challenge trials and analytical observational studies were documented during the full-text screening stage. Studies must have enrolled dairy cows after their first (or greater) lactation, and have compared blanket dry-cow antimicrobial therapy (all quarters of all cows treated at dry-off) to selective dry-cow therapy, where cows or quarters are treated at dry-off based on IMI status, as determined by culture, SCC, or SCC proxy. To be eligible, studies must have included at least one of the following outcomes: (i) frequency of IMI (using the trial's authors' definition of IMI) at calving following the intervention, (ii) frequency of IMI during the first 30 days of the subsequent lactation and (iii) incidence of clinical mastitis during the first 30 days of the subsequent lactation. Clinical mastitis was considered an incidence outcome, as it was assumed that enrolled cows did not have clinical mastitis at the time of dry-off. The outcome measures of IMI frequency reflects both incident and prevalent cases, as all cows enrolled (regardless of initial infection status) would contribute to the denominator. Assuming an equal proportion of prevalent cases in both groups, the difference between groups will reflect both the difference in incident cases as well as the ability to accurately classify cows in the selective treatment group. Assuming accurate classification, cure risk should be equal in both groups. Throughout this review, we, therefore, refer to the IMI outcome measures as ‘frequency of IMI’.
Information sources
Databases searched were Agricola (via ProQuest, 1970 to current), CAB Abstracts and Global Health, Epub ahead of print, In-process & other non-indexed citations (via Web of Science, 1910 to current), Ovid MEDLINE®(R) Daily, and Ovid MEDLINE® (R) (via Ovid, 1946 to current), Conference Proceedings Citation Index – Science (via Web of Science, 1990 to current), and Science Citation Index (via Web of Science, 1900 to current). A single reviewer hand-searched the table of contents of the following conferences from 1997 to 2018: Proceedings of the American Association of Bovine Practitioners, World Association for Buiatrics, and the National Mastitis Council Proceedings. The Food and Drug Administration (FDA) website containing the Freedom of Information New Animal Drug Approvals (NADA) summaries were also searched.
Search
The search strategy initially was developed for the Science Citation Index (Web of Science) interface and employed a multi-stranded approach to maximize sensitivity (Table 1). The conceptual structure combined the concepts of ‘dairy cows’ AND ‘dry off’ AND ‘antibiotics’; or ‘dry cow’ AND ‘antibiotics’; or ‘dairy cows’ AND ‘prophylaxis’ AND ‘intra-mammary infections’. An additional precise search line to identify phrases such as ‘dry cow therapy’ and ‘dry cow management’ was also included in order to retrieve any records not identified by the previous two combinations. Database searches were conducted on 28 June 2018 and accessed through the University of York in the UK. Search results were uploaded to EndNoteX7 (Clarivate Analytics, Philadelphia, PA) and duplicate results were documented and removed. Records were then uploaded to DistillerSR (Evidence Partners Inc., Ottawa, ON) and additionally de-duplicated. If the same study and data were available as a conference abstract and as a full publication, the conference abstract was removed. Data only available as a conference abstract were eligible if the full text was >500 words, to allow sufficient detail for data extraction and risk of bias assessment.
Table 1. Full electronic search strategy used to identify studies of antimicrobial treatments during the dry-off period in dairy cattle in Science Citation Index (Web of Science) conducted on 28 June 2018
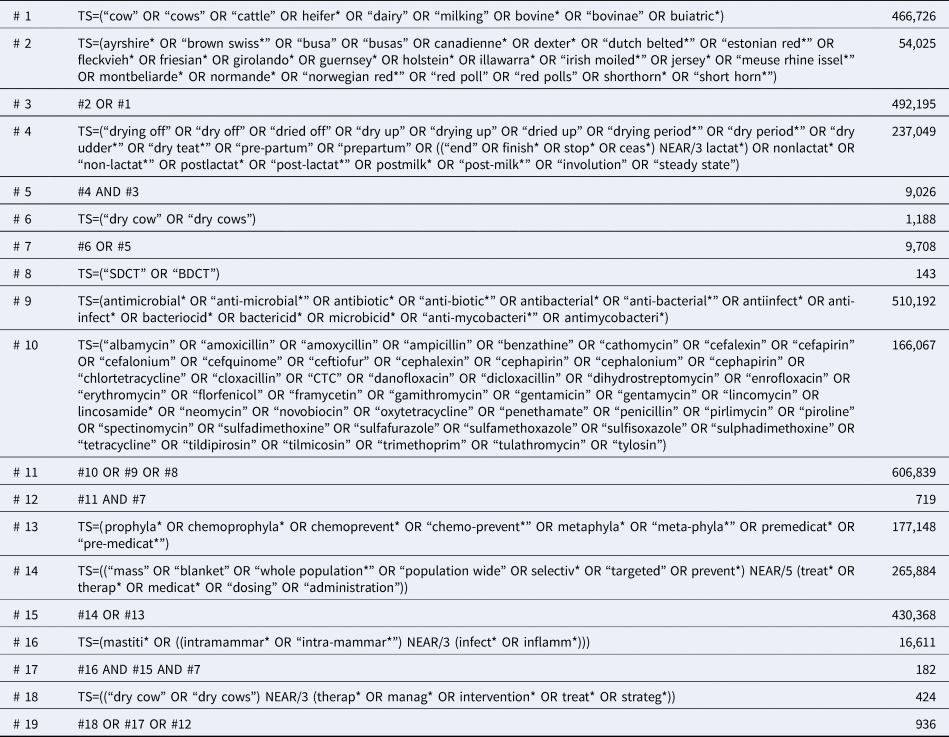
TS = topic field search (includes the title, abstract, author keywords, and keywords plus fields), * = unlimited right-hand truncation symbol, NEAR/N = retrieves records that contain terms (in any order) within a specified number (N) of words of each other.
Validation of the search was done by identifying all articles included in the qualitative syntheses of reviews in the area of dry-cow management as identified from the following papers: Robert et al. (Reference Robert, Seegers and Bareille2006); Halasa et al. (Reference Halasa, Osteras, Hogeveen, van Werven and Nielen2009); Pereira et al. (Reference Pereira, Oliveira, Mesquita, Costa and Pereira2011); van Knegsel et al. (Reference van Knegsel, van der Drift, Cermakova and Kemp2013); Enger et al. (Reference Enger, White, Nickerson and Fox2016). All relevant articles identified in these reviews were found in the search.
This search, and the initial eligibility screening questions (see below) were used to inform two separate reviews: this review, and one examining comparative efficacy of dry-off antimicrobials at an individual cow level.
Study selection
DistillerSR was used for all levels of screening and data extraction. Title and abstracts were initially screened for eligibility. Two reviewers independently evaluated each citation, and all reviewers were trained by CBW and JMS on a pre-test of the title and abstracts of the first 250 citations to ensure clarity of understanding and consistency of question application. The following questions were used to assess relevance:
(1) Does the study involve antimicrobial-containing dry-cow treatments in dairy cattle at the individual level or evaluation of group-level strategies for administering antimicrobial-containing dry-cow treatments (such as selective treatment versus blanket treatment)? YES (neutral), NO (exclude), UNCLEAR (neutral)
(2) Is there a concurrent comparison group? (i.e. controlled trial with natural or deliberate disease exposure, or analytical observational study)? YES (neutral), NO (exclude), UNCLEAR (neutral)
(3) Is the full text available in English? YES (include for full-text screening), NO (exclude), UNCLEAR (include for full-text screening)
Citations were excluded if both reviewers responded ‘NO’ to any of the questions; agreement was at the level of the form. Disagreements were resolved by consensus with mediation by JMS or CBW if agreement could not be reached. The secondary screening was conducted independently by two reviewers on the full text of remaining studies, using the first 10 citations as a pre-test by all reviewers. This level of screening used the initial three questions with only YES (neutral) or NO (exclude) options, and additionally:
(1) Does the study evaluate any of the following outcomes: incidence of clinical mastitis during the first 30 DIM, frequency of IMI at 30 DIM, or frequency of IMI at calving? YES (include) NO (exclude)
(2) What is the study design? Experimental with natural disease exposure (neutral), experimental with deliberate disease exposure (exclude), analytical observational study (exclude)
(3) Does the study evaluate a group level strategy for administering dry-cow treatments (selective treatment versus blanket treatment)? YES (include), NO (exclude)
The agreement was at the question level, with conflicts resolved by consensus or with mediation by JMS or CBW if agreement could not be reached.
Data collection
Data from citations meeting the full-text screening inclusion criteria were independently extracted by two reviewers using a standardized form, which was piloted on the first five citations by all reviewers to ensure consistency. Discrepancies in data extraction were resolved by consensus, with mediation by JMS and CBW if agreement could not be reached. Hierarchical forms were used in DistillerSR for data extraction, with forms nested as (Study Characteristics (Outcome (Arm, Contrast, Risk of bias))). A PDF version of the full data extraction tool is available as Supplemental File S1.
Data items
Study characteristics
Study-level data included study design, country of conduct, year and months of study conduct, setting (research or commercial herd), breed of cattle, number of herds enrolled, inclusion criteria at the cow and herd level, and parity of enrolled animals.
Interventions and comparators
For selective therapy, details on how IMI was determined (criteria for selecting treatment) was extracted, and if selective therapy was given at the level of the cow or the quarter. For all groups, details on the antimicrobial(s) used, route of administration, frequency of administration, dose, dry period length, level of treatment allocation, and level of analysis were recorded. Baseline characteristics and loss to follow up were captured.
Eligible outcomes
Outcomes eligible for inclusion in meta-analysis were:
• Incidence risk of clinical mastitis in the first 30 days of lactation,
• Frequency of IMI at calving, and
• Frequency of IMI in the first 30 days of lactation
Prioritization of these outcomes for meta-analysis was determined during protocol development in consultation with content experts based on the anticipated frequency of use in the primary literature and as proxies to reflect the effect of infection during the dry period. IMI was defined by the primary study authors and may have included multiple samples interpreted in series or parallel.
For outcomes for which data were extracted, the prioritized outcome measure was an adjusted summary effect size (adjusted odds ratio (OR) or relative risk or risk ratio (RR)). Variables included in adjustment and the corresponding precision estimate were recorded. If an adjusted measure was not reported, unadjusted summary effect size (second priority) or treatment arm-level (raw) data (third priority) were recorded, with an applicable variance measure.
For multi-farm trials where clustering at the farm level was not adjusted for (i.e. those reporting raw data for multiple farms), if raw data were available by the farm, data from each farm was extracted as a unique trial.
Risk of bias in individual studies
Risk of bias was assessed by outcome for all outcomes extracted, using the Cochrane Risk of Bias 2.0 tool (Higgins et al., Reference Higgins, Sterne, Savovic, Page, Hróbjartsson and Boutron2016), with signaling questions modified to be specific to the topic of the review. This tool assesses the potential for bias arising from five areas or domains of potential bias: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported results. In some commodity groups, individual animal value is likely to be unknown or equal at the time of treatment allocation; for these livestock groups, the question on allocation sequence concealment may not be important in the bias assessment for the domain related to the randomization process (Moura et al., Reference Moura, Totton, Sargeant, O'Sullivan, Linhares and O'Connor2019). In the case of dairy cattle, a decision was made to include the question on allocation concealment in the risk-of-bias assessment, as individual animal value is likely unequal and known at the time of treatment allocation in most (or all) trials. As well, an additional answer option was provided for the question on random allocation sequence, to identify studies using the word ‘random’ to describe the allocation sequence but not providing details on the method used to generate the random sequence.
Risk of bias was assessed independently in duplicate, with disagreement resolved by consensus and mediation by JMS or CBW if needed. The risk-of-bias tool is available as Supplemental File S2.
Synthesis of results
Pairwise meta-analysis was performed when multiple studies evaluated the same intervention and comparison. Meta-analysis was conducted in R 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria) using RStudio version 1.0.136 (RStudio Inc., Boston, MA) using the ‘metafor’ package (Viechtbauer, Reference Viechtbauer2010). A random effects approach was used, with weighting of studies using the inverse variance method. A random effects approach was chosen as it was assumed that the ‘true’ effect is a distribution among studies and not a single value. Heterogeneity was assessed by the I2 statistic (Viechtbauer, Reference Viechtbauer2010). If substantial heterogeneity was present (>50%), effects of study level moderators were tested using subgroup meta-analysis if at least three studies were included in each group. Tests for subgroup differences were done using the Knapp-Hartung adjustment (Knapp and Hartung, Reference Knapp and Hartung2003).
Risk of bias at the review level
If 10 or more studies were found for a single outcome, a funnel plot (effect estimate versus the inverse of its standard error) was used to visually assess the potential for publication bias (Viechtbauer, Reference Viechtbauer2010).
Results
Study selection
Results of the search and flow of studies through the screening process are presented in Fig. 1, including reasons for full-text exclusions. Full details on all searches are available as Supplemental File S3.
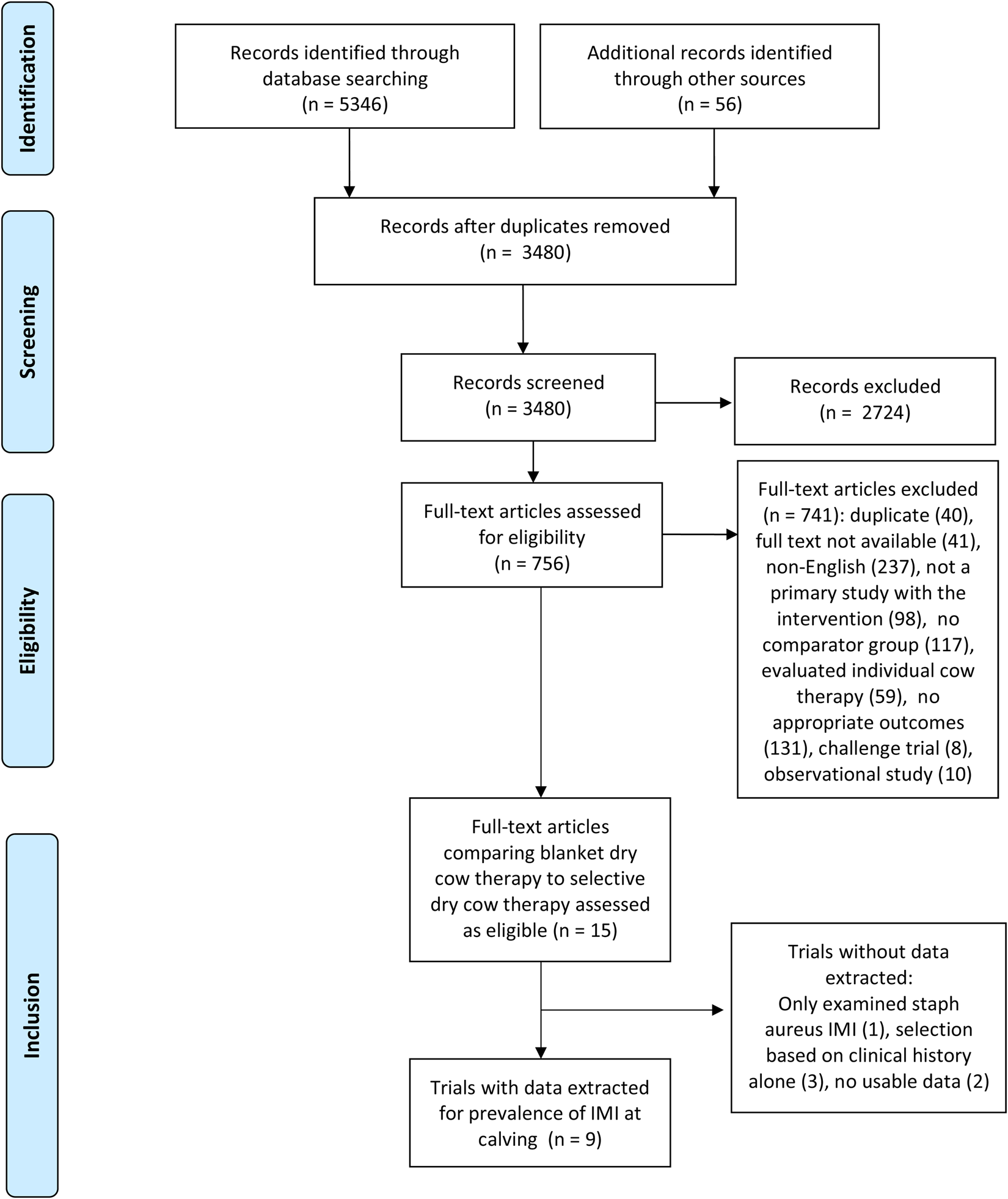
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) study flow diagram (Moher et al., Reference Moher, Shamseer, Clarke, Ghersi, Liberati, Petticrew, Shekelle and Stewart2015) for the systematic review of trials examining the efficacy of selective dry-cow therapy compared to ‘blanket’ therapy (treating all quarters of all cows).
From an initial 3480 articles screened by title and abstract, 756 full texts were reviewed, with 741 articles not meeting full-text eligibility criteria. Fifteen studies (comprising 15 trials) evaluated blanket versus selective dry-cow therapy and were thereby assessed as eligible. Of these, two trials had data that were not usable (e.g. data not presented, data presented in graphs or figures only, etc.), one trial evaluated IMI due to Staphylococcus aureus only (which was determined post hoc as not combinable with all-case IMI), and three trials selected cows solely on the basis of clinical history (which was determined post hoc as not a combinable proxy for IMI status based on culture or SCC history). Therefore, data were extracted for one or more outcomes from nine trials. All of these trials reported the frequency of IMI at calving, one reported the incidence of clinical mastitis during the first 30 DIM, and none reported the frequency of IMI during the first 30 DIM.
Study characteristics
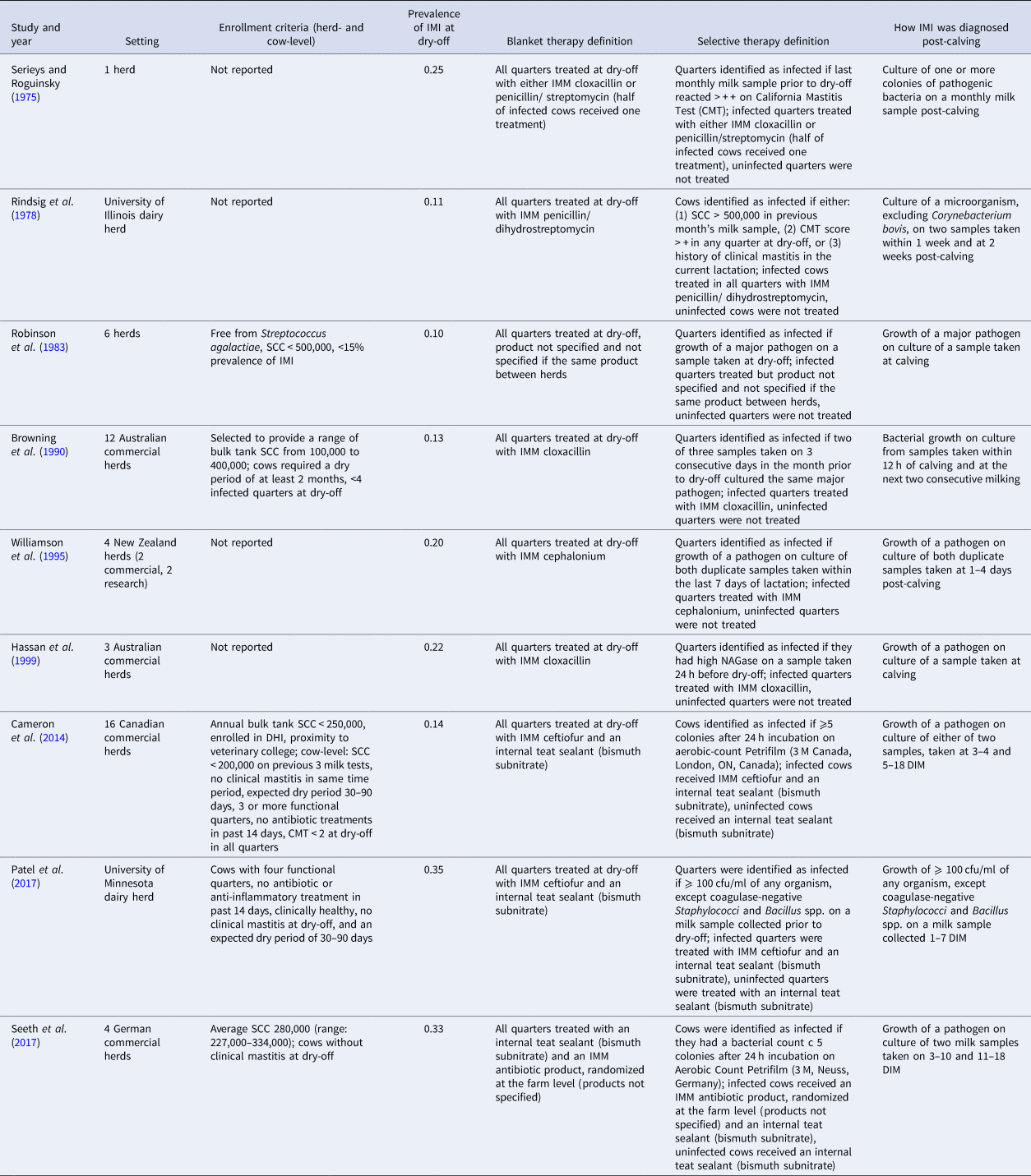
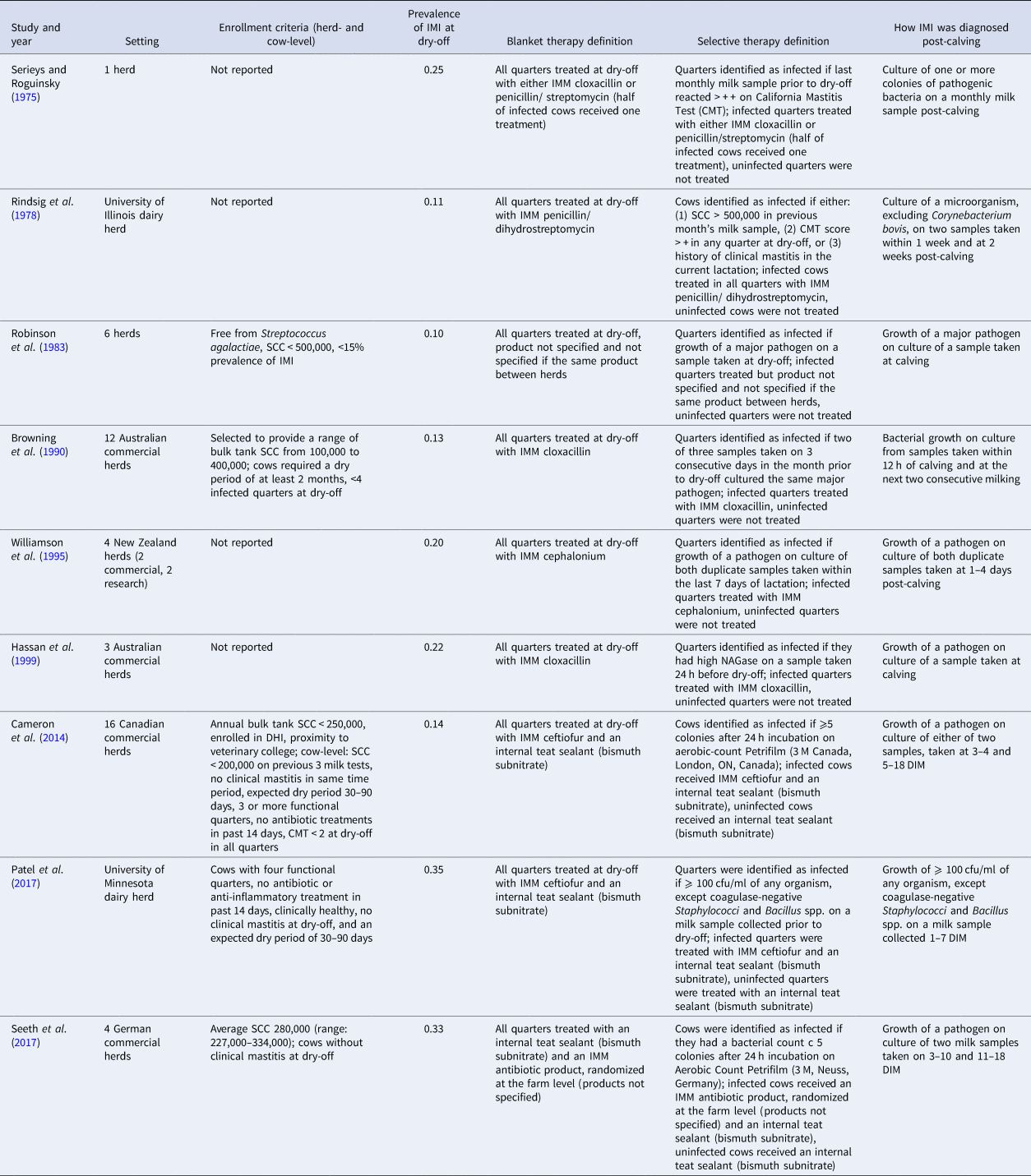
Full details on the study characteristics of the nine trials with data extracted for IMI at calving is shown in Table 2. Studies were conducted in five countries: Australia (n = 2), Canada (n = 1), Germany (n = 1), New Zealand (n = 1), and the USA (n = 2). The country of conduct was not reported in two trials. The study setting was most commonly a commercial dairy (4/9; 44%), with some trials conducted in a research dairy (2/9) or a combination of research and commercial facilities (1). In two trials, the setting was not reported. Two trials were conducted since 2000, four between 1990 and 2000, and two prior to 1990. The number of herds enrolled ranged from 1 to 16. Herd- and/or cow- level enrollment criteria were reported in less than half of the trials (4/9). For cows enrolled in the studies, the frequency of IMI at dry-off ranged from 11 to 35% (combined across intervention groups).
Table 2. Characteristics of the nine trials included in the meta-analysis examining the effect of selective dry-cow therapy compared to ‘blanket’ therapy (treatment of all quarters of all cows)
Outcomes
The frequency of IMI at calving was estimated in nine trials, and incidence of clinical mastitis during the first 30 DIM determined in one trial, while none reported the frequency of IMI during the first 30 DIM. All outcomes were based on the bacterial culture of milk samples taken post-calving, with three trials reporting samples taken ‘at calving’ or ‘post-calving’, and six trials providing further definitions. Days in milk at sampling for these trials ranged from 0 to 18 DIM, with one to three total samples taken per quarter. Definitions of infection varied among trials but were generally more standardized and well-described in more recent publications (Table 2).
Risk of bias – IMI at calving
The results of the risk of bias assessment for the nine trials included in the meta-analysis are presented as Fig. 2. The overall risk of bias was assessed as the highest level for any domain; as a result, all trials received an overall rating of either ‘some concerns’ or ‘high’. Risk of bias is presented by outcome by the domain of bias for each outcome, in order to identify which areas of bias have specific challenges across the body of evidence included in this review.
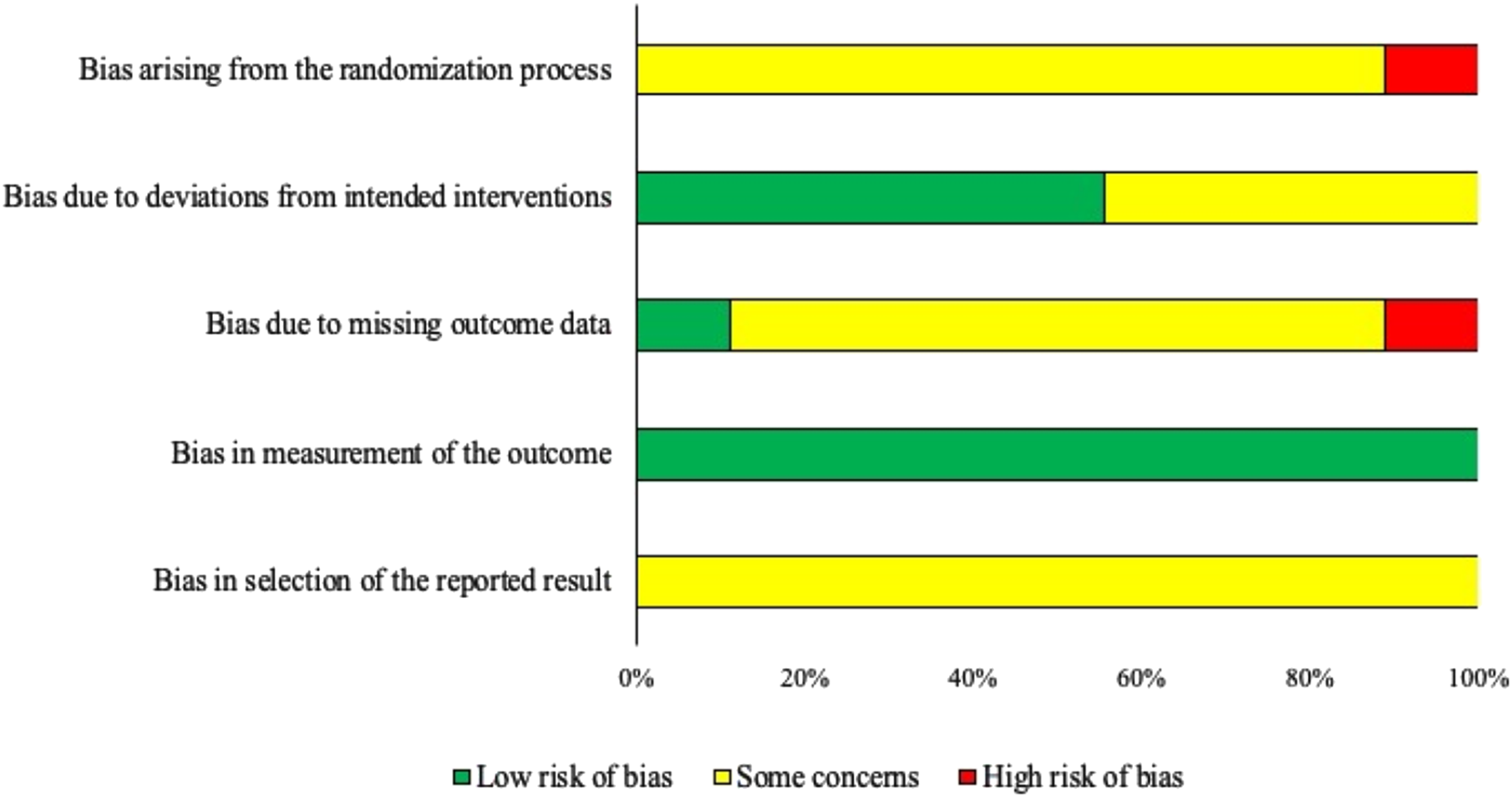
Fig. 2. Risk of bias by domain for trials included in the pairwise meta-analysis assessing the efficacy of selective dry-cow therapy compared to ‘blanket’ therapy (treating all quarters of all cows) on the frequency of intramammary infections (IMI) at calving (n = 9). Risk of bias was assessed according to the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) (Higgins et al., Reference Higgins, Sterne, Savovic, Page, Hróbjartsson and Boutron2016).
For bias arising from the randomization process, eight trials were assessed as ‘some concerns’ and one as ‘high risk’. This was primarily driven by a lack of reporting; information regarding allocation concealment was not provided in any trials, although one trial clearly described a lack of allocation concealment and thus received a ‘high risk’ designation. Two trials reported random assignment of cows to treatment and included evidence of randomization (i.e. described the method used to generate the random sequence), while the word ‘random’ was present in three trials without providing detail to ascertain if an appropriate method was used. Two trials did not use a random method of allocation, and two did not provide sufficient information for assessment.
Bias due to deviations from intended interventions was assessed as ‘low risk’ in five trials and ‘some concerns’ in four trials. Due to the nature of the intervention, blinding of caregivers was not possible, but ‘low risk’ could still be achieved if the trial did not have deviations from intended interventions and if cows or quarters were likely analyzed in the group to which they were assigned. As the intervention was considered short-term (as the application happened at a single instance) it was considered unlikely in any trials that quarters were analyzed in the incorrect group in any trials. Potential for deviation from intended interventions was considered unlikely for trials providing details about the care and management of the study animals such as common housing and feeding (i.e. equivalent management). The four trials assessed as ‘some concerns’ did not provide details about group management, and therefore equivalent care could not be assessed.
Bias due to missing outcome data was assessed as ‘low risk’ in 7/9 trials, ‘some concerns’ in one trial, and ‘high risk’ in one trial. ‘Some concerns’ resulted from a lack of reported information on loss to follow-up, and ‘high risk’ was assessed when there was >5% loss to follow-up that was non-random or unequal between groups.
Bias due to measurement of the outcome was considered ‘low risk’ in all trials as, although only three trials reported that outcome assessors were not aware of treatment group allocation, laboratory diagnosis was considered an objective measurement and thus this resulted in a ‘low risk’ of bias in this domain.
For bias arising from the selection of the reported results, information regarding a priori intentions of outcome measurements and analyses were not available for any studies; this domain generally requires the examination of a trial protocol or statistical analysis plan documented ahead of the trial when there are multiple ways an outcome could be measured or analyzed. As a result, all trials were assessed as ‘some concerns’ for this domain.
Results of individual studies
All results were presented at the quarter level. Six of the nine trials were conducted in multiple herds. Data adjusted for cow as a random effect were presented in two trials, to account for clustering of quarters within cow. Of the six trials with multiple herds, one trial used a random effect of herd to account for non-independence of cows within herd. While adjusted measures were prioritized, as the majority of included trials only had raw data available, these were combined with adjusted summary measures in the meta-analysis by converting the measure of association (OR, RR) to raw values using the study's baseline prevalence in the control (blanket therapy) group.
Pairwise meta-analysis
The comparison of interest was between selective dry-cow therapy compared to blanket therapy. Two trials contained a third control arm where cows did not receive any therapy at dry-off (antimicrobial or otherwise). Data from these control arms were not extracted. In one trial (Seeth et al., Reference Seeth, Wente, Paduch, Klocke, Mansion-de Vries, Hoedemaker and Kromker2017), two selective therapy arms were used, one based on culture and one based on SCC cut points. We extracted data from the selective therapy arm from culture only, in order to avoid a lack of independence in the comparator arms if both intervention arms were extracted. Selective therapy based on bacterial culture was the predominant method used to identify cow with IMI at dry-off in the remaining eight trials (5/8). Only one other trial used an SCC cut point (Serieys and Roguinsky, Reference Serieys and Roguinsky1975), but this cut point was not comparable to that used in the trial by Seeth et al. (Reference Seeth, Wente, Paduch, Klocke, Mansion-de Vries, Hoedemaker and Kromker2017).
Figure 3 shows the meta-analysis for the nine trials comparing selective dry-cow therapy to blanket treatment. Based on the included trials, the frequency of IMI at calving in cows assigned to the selective dry-cow therapy protocol was higher than that of cows in the blanket therapy groups (RR = 1.34, 95%CI = 1.13, 1.59). However, substantial heterogeneity was seen in the analysis (I 2 = 58%), indicating that there was important between-study variation beyond that expected by chance.

Fig. 3. Forest plot showing the effect of selective dry-cow treatment (experimental) compared to ‘blanket’ therapy (treatment of all quarters of all cows) (control) on the risk of intramammary infection at calving. Each study is listed by the first author's last name and year of publication. The squares indicate the individual study's effect size as a risk ratio. The horizontal line shows the corresponding confidence interval. The center of the diamond shows the overall effect size estimate, with the width of the diamond showing the confidence interval of this estimate.
Sub-group meta-analysis
Post-hoc subgroup meta-analysis was performed to explore the effect of two sources of potential between trial variance: the method used to determine infected cows in the selective group (culture, Y/N) and if concurrent therapy was given to all cows in all groups (internal teat sealant (bismuth subnitrate), Y/N). Method of determining infection was considered as a source of heterogeneity, as less accurate methods could result in greater differences between treatment groups. However, the point estimates of the intervention effect size among studies where culture-based methods were used to determine infection (Robinson et al., Reference Robinson, Jackson and Marr1983; Browning et al., Reference Browning, Mein, Barton, Nicholls and Brightling1990; Williamson et al., Reference Williamson, Woolford and Day1995; Cameron et al., Reference Cameron, McKenna, MacDonald, Dohoo, Roy and Keefe2014; Patel et al., Reference Patel, Godden, Royster, Timmerman, Crooker and McDonald2017; Seeth et al., Reference Seeth, Wente, Paduch, Klocke, Mansion-de Vries, Hoedemaker and Kromker2017; RR = 1.37, 95% CI = 1.10, 1.70) did not differ from those using non-culture-based methods (Serieys and Roguinsky, Reference Serieys and Roguinsky1975; Rindsig et al., Reference Rindsig, Rodewald, Smith and Spahr1978; Hassan et al., Reference Hassan, Daniel, O'Boyle and Frost1999; RR = 1.37, 95% CI = 0.97–1.171).
Subgroup analysis based on the use of an internal teat sealant for all cows in all groups resulted in significantly different overall effects (Fig. 4). For studies not including teat sealants, the risk of IMI at calving was significantly higher for selectively treated cows than blanket-treated cows (RR = 1.48, 95% CI = 1.19, 1.82), but substantial heterogeneity was still present in the analysis (I 2 = 55). For studies including the concurrent therapy of internal teat sealants (for all cows in all groups), the overall risk of IMI at calving was not different between selectively treated and blanket-treated cows (RR = 1.09, 95%CI = 0.92, 1.28) and no heterogeneity was seen in the analysis (I 2 = 0%). The point estimates were significantly different between these subgroups (P = 0.03).
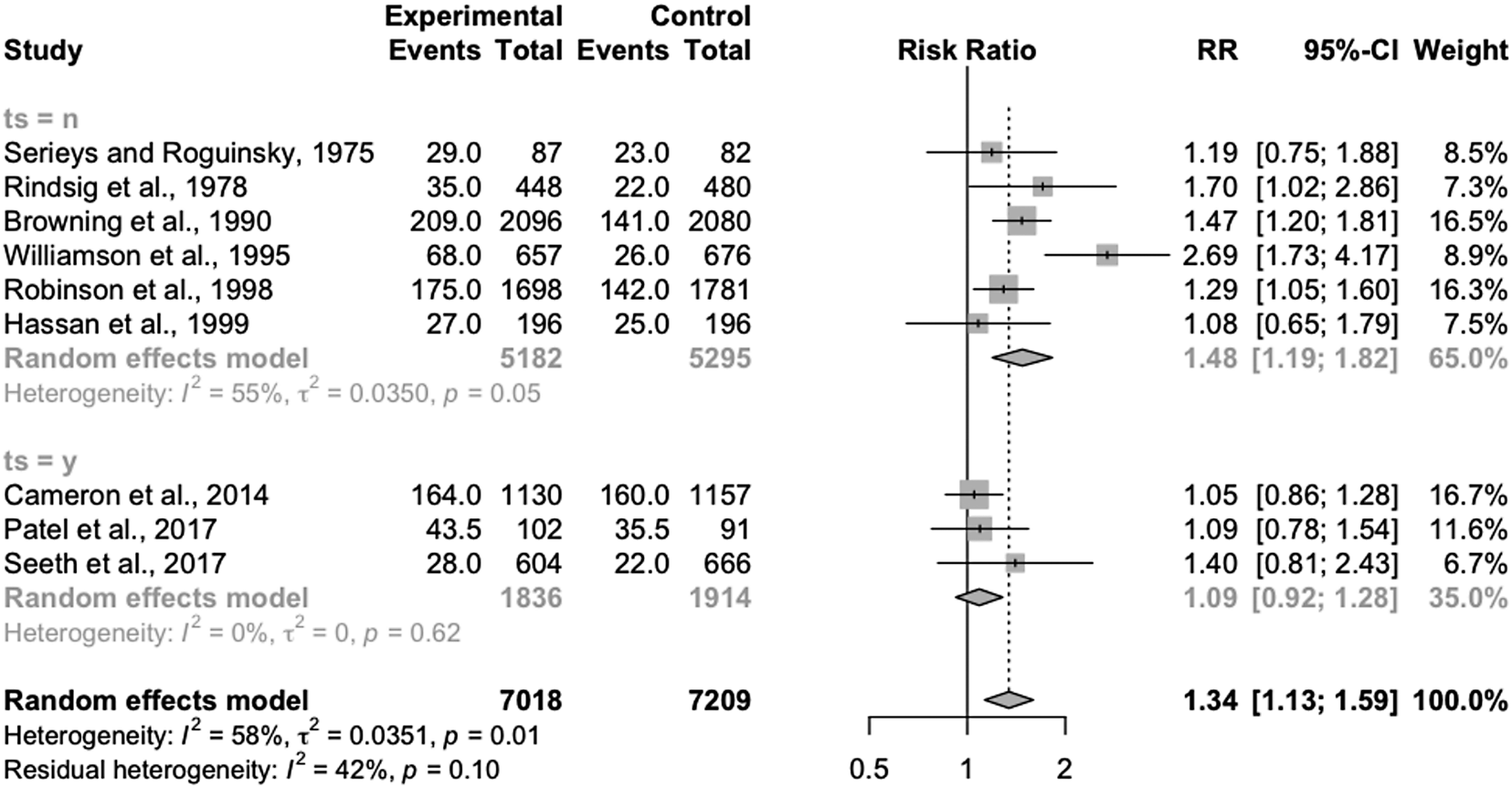
Fig. 4. Forest plots showing the effect of selective dry-cow treatment (experimental) compared to ‘blanket’ therapy (treatment of all quarters of all cows) (control) on risk of intramammary infection at calving, grouped by studies including no concurrent therapy with teat sealant (ts = n) and those where all cows in all groups received an internal teat sealant (bismuth subnitrate) (ts = y). Each study is listed by the first author's last name and year of publication. The squares indicate the individual study's effect size as a risk ratio. The horizontal line shows the corresponding confidence interval. The center of the diamonds shows the overall effect size estimate for each group and for the summary estimate, with the width of the diamond showing the confidence interval of these estimates.
Discussion
Treatment and prevention of mastitis represent a large portion of antimicrobial use in the dairy industry (Lam et al., Reference Lam, van Engelen, Scherpenzeel and Hage2012; USDA-APHIS, 2016), due to the frequency and economic impact of mastitis (Lam et al., Reference Lam, Van Den Borne, Jansen, Huijps, Van Veersen, van Schaik and Hogeveen2013). As the greater concern is directed towards antimicrobial use in the dairy industry, there is increasing pressure to develop alternatives to blanket dry-cow therapy (ECDC/EMEA, 2015). Selective dry-cow therapy is very common in Nordic countries (Vilar et al., Reference Vilar, Hovinen, Simojoki and Rajala-Schultz2018) and blanket therapy without regard to infection status has been banned in Holland since 2012 (Santman-Berends et al., Reference Santman-Berends, Swinkels, Lam, Keurentjes and van Schaik2016). A retrospective study in the Netherlands showed no significant changes in SCC dynamics during the dry period at the herd level comparing annually from 2011 through 2015 (Vanhoudt et al., Reference Vanhoudt, van Hees-Heijps, van Knegsel, Sampimon, Vernooij, Nielen and van Werven2018). However, it is unclear if the same would apply to other countries and management systems should blanket dry-cow therapy be eliminated elsewhere, especially in countries such as the USA, Canada, or the UK, where blanket dry-cow therapy has remained widely adopted (Ruegg, Reference Ruegg2017).
With increasing requirements globally with respect to antimicrobial use in food-producing animals, it is important that decisions surrounding these practices are evidence-based. Systematic reviews of randomized controlled trials yield the highest level of evidence of intervention efficacy (Sargeant et al., Reference Sargeant, Kelton and O'Connor2014a), and sources of heterogeneity can be explored to understand differences in effect sizes between studies. This is notable if there are characteristics of the study population or the implementation of the intervention which may result in a different effect, i.e. if selective dry-cow therapy is more efficacious in herds with specific characteristics, or if cows are selected or treated in specific ways.
Interpretation
The results suggest that the use of selective dry-cow therapy may not increase the risk of IMI at calving if internal teat sealants are used for all cows. However, the small number of included trials, and heterogeneity in the subgroup without internal teat sealants, suggests that the true relative risk between treatments may differ from the determined point estimates based on some of these unmeasured factors. Interestingly, to note that there was no heterogeneity seen among these trials, although strict herd- and cow-level inclusion criteria were applied by Cameron et al. (Reference Cameron, McKenna, MacDonald, Dohoo, Roy and Keefe2014) while Seeth et al. (Reference Seeth, Wente, Paduch, Klocke, Mansion-de Vries, Hoedemaker and Kromker2017) had a wider range of bulk tank SCC and less strict cow-level criteria. This suggests that, although there are likely other factors which drive differences between studies, the use of internal teat sealants still may explain a substantial portion of the heterogeneity seen in the original analysis.
A lack of trials examining the other outcomes identified as important for this review may reflect differences in time at risk measured among trials. However, it was beyond the scope of this review to examine variation in time at risk for the determination of IMI or clinical mastitis during the subsequent lactation.
Strengths and limitations of evidence
Only a small number of trials were included and they were published over a range in time (1975–2017) and likely reflected a range of management practices. Herd-level inclusion criteria were often not stated, but of trials which did, inclusion criteria also varied, which could be an additional source of heterogeneity in the analyses. For example, Cameron et al. (Reference Cameron, McKenna, MacDonald, Dohoo, Roy and Keefe2014) selected herds based on having low BTSCC (<250,000) whereas other studies selected herds with a wide range in BTSCC, either purposively or as a result of their sampling strategy (Robinson et al., Reference Robinson, Jackson and Marr1983; Browning et al., Reference Browning, Mein, Barton, Nicholls and Brightling1990; Seeth et al., Reference Seeth, Wente, Paduch, Klocke, Mansion-de Vries, Hoedemaker and Kromker2017). These study populations may behave differently in response to selective or blanket therapy. While the prevalence of IMI at dry-off also varied between studies, ranging from 11 to 35% of cows infected at dry-off, there was variation in the definition of IMI, and the time at risk varied between studies depending on when the post-calving sample was taken (Table 2). This variation in IMI definition is also likely to result in heterogeneity of effect between studies.
Although the use of teat sealants explained part of the heterogeneity in the original analysis, suggesting that the use of this product concurrently in a selective dry-cow program reduces the risk of IMI at calving, it is important to emphasize the limitations of the small number of studies contributing to the analysis. The three studies using teat sealant concurrently (Cameron et al., Reference Cameron, McKenna, MacDonald, Dohoo, Roy and Keefe2014; Patel et al., Reference Patel, Godden, Royster, Timmerman, Crooker and McDonald2017; Seeth et al., Reference Seeth, Wente, Paduch, Klocke, Mansion-de Vries, Hoedemaker and Kromker2017) were conducted in commercial and research herds, and do reflect some range of BTSCC. However, it is unlikely that they reflect the breadth and depth of the range of herd-level management factors which may influence response to group-level therapy. As well, these three trials were also the three most recent publications. Housing and management of these herds may also have been different than the previous studies not using a teat sealant.
The risk of bias was often categorized as ‘some concerns’ in several domains as a result of failure to report key study items. Adherence to reporting guidelines such as the Reporting guidElines For randomized controlled trials in livEstoCk and food safeTy (REFLECT) (O'Connor et al., Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010; Sargeant et al., Reference Sargeant, O'Connor, Gardner, Dickson and Torrence2010) would allow for better assessment of the potential for bias in future work. A large number of trials were excluded at full-text screening as they were not available in English, and as a result, our conclusions may not reflect the entirety of the literature assessing the efficacy of dry-cow antimicrobial therapy on the prevention of IMI and CM. However, it is possible that some of the articles excluded on language may not have been relevant, as many had only the title available in English, and abstract screening would have been marked as ‘unclear’.
Conclusions
From the evidence available, selective dry-cow therapy appears to be associated with a higher frequency of IMI at calving, although subgroup analysis revealed that for trials where all cows received an internal teat sealant (bismuth subnitrate), the frequency was not significantly different between selective therapy and blanket therapy. More research comparing the efficacy of selective therapy to blanket therapy is warranted in order to determine if there are population- or intervention- level factors which drive heterogeneity in the point estimate. As there were only a small number of trials included in the subgroup, it is possible the homogeneity seen in the teat sealant sub-group could be due to chance. Accordingly, heterogeneity in this sub-group may be seen should additional trials be conducted in different populations or with different inclusion/exclusion criteria for the selective therapy group.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1466252319000306.
Author contributions
CBW assisted with the development of the review protocol, co-coordinated the research team, assisted with data screening, data extraction, and risk of bias assessment, conducted analyses, interpreted results, and wrote the manuscript drafts. JMS developed the review protocol, co-coordinated the research team, commented on manuscripts drafts, and approved the final manuscript. JG and HW developed the search strings, conducted all searches, commented on the manuscript drafts and approved the final manuscript. KJC, MdB, KD, JD, and SM conducted relevance screening, extracted data, conducted risk of bias assessments, commented on manuscript drafts, and approved the final manuscript version. DFK, SJL, TFD, and AMOC co-developed the review protocol, commented on manuscript drafts, and approved the final manuscript.
Financial support
Support for this project was provided by The Pew Charitable Trusts.
Conflict of interest
None of the authors has conflicts to declare.