INTRODUCTION
Reducing the incidence of foodborne zoonoses through the farm-to-fork approach remains one of the biggest challenges in veterinary public health. Reducing the prevalence of contaminated poultry is likely to reduce the risk of human salmonellosis from broiler chicken consumption [1].
During the 1990s and 2000s, a number of field studies attempted to delineate which factors within the modern conventional production cycle impact the burden of Salmonella in broiler flocks [Reference Bailey2–Reference Rose12]. Such aspects as the construction of the broiler house, litter management, farm biosecurity, seasonal effects, management of broiler harvest, transportation to the processing plant, and processing have been extensively surveyed and rigorously screened for associations with Salmonella. However, to the best of the authors' knowledge, the effects of vaccinations routinely administered to grow-out broilers to prevent or lessen infection with other pathogens on the burden of Salmonella in flocks have not been considered.
Broiler production is hierarchically structured. In the USA, a broiler company normally consists of multiple production complexes. A complex usually manages the entire grow-out production cycle and therefore incorporates dedicated broiler parent breeder flocks and one or more hatcheries, feedmills, and processing plants. Grow-out flocks are usually reared at privately owned farms under a contract with the company through the complex. An immunization programme for the grow-out broilers is developed by each company, with consideration of disease threat and cost-effectiveness. The protocols may be modified by the managers of the complexes to target localized risks of infection. Therefore the vaccination protocols differ between the broiler complexes operating in a given geographical area, and within a given complex over time. The vaccines are administered to the broiler embryos (in ovo) or broilers (via spray or injection of 1-day-old birds) at the hatchery, and to the broilers during rearing in the grow-out house on the farm (via spray or drinking water).
MATERIALS AND METHODS
Sampling
In a prospective field observational study, we sampled grow-out broiler flocks throughout their entire production cycles. The flocks were reared on conventional grow-out farms in the US states of Alabama, Mississippi and Texas. The sample collection continued from 2003 to 2006, and is described in detail in Volkova et al. [Reference Volkova13]. Briefly, only one flock was sampled per broiler house involved. Each sampled flock was reared in a single house. Each flock was sampled at the time of placement into the grow-out house (upon arrival from the hatchery) by collecting paper liners from 30 transport trays and gastrointestinal tracts from each of 30 broilers (each bird was selected at random from the 100 birds in the transport tray from which the liner was obtained). When the birds were aged 41–57 days, about 1 week before the end of rearing, 30 broilers were selected from the flock. The whole feathered carcass rinse, crop and one (either the left or the right) caecum were obtained from each bird. On the day the flock was harvested, samples of the house litter and drag swabs of litter were obtained from the grow-out house after the flock's harvest. When the flock arrived at the processing plant, 30 broilers were removed from the hauling cages and the whole feathered carcass rinse, crop and one caecum were collected from each bird carcass. At processing, the broilers were aged 48–61 days (average 56 days), and a sampled flock numbered 15 200–27 200 birds. All the birds sampled were humanely euthanized by cervical dislocation. The 30 hatchery-farm transport tray liners, the 30 birds in grow-out and the 30 birds at arrival to the plant were convenience samples. The flock was followed through processing and sampled by collecting rinses of 30 carcasses (eviscerated carcasses with feathers, head, and feet removed) taken from the processing line immediately before the final carcass rinse prior to the immersion chilling tank, and 30 other carcasses immediately after the chilling tank (the end point of processing). The collection of carcass rinses at these two processing points was timed so that at each point the samples were collected evenly over the course of the flock's passing through that point.
A total of 76 grow-out broiler flocks reared in 76 individual houses on 38 farms were sampled at the time of placement for rearing. From these, 70 flocks were sampled at the end of the grow-out and 66 were sampled upon arrival at the processing plants. All of these 66 flocks were sampled prior to chilling, from which post-chilling samples were available for 64 flocks (samples from the other two flocks were lost in a laboratory accident). The post-harvest litter samples and drag swabs were obtained from 68 of the houses. From the flocks sampled at placement for rearing, four were lost from the study due to damage on the farms from Hurricane Katrina in autumn 2005, and the others due to scheduling conflicts at the flock processing stage. The participating farms reared broilers ‘all-in-all-out’ under contract with ten production complexes owned by two broiler companies. The farms were selected by the companies' personnel prior to the placements so that the flocks sampled, when grown, would be processed at the start of a working week, to facilitate laboratory processing of the samples. Compliance of the growers was absolute. Despite the convenience sampling, we consider that broiler flocks sampled in this study were generally representative of conventional grow-out broilers reared in southeastern USA during the years of study.
Salmonella isolation
All the samples collected were tested for the presence of Salmonella by conventional microbiological techniques as detailed in Volkova et al. [Reference Volkova13]. It should be noted that the sensitivity of microbiological methods used to isolate Salmonella was limited [Reference Rybolt, Wills and Bailey14]. The sensitivity may also have varied between the various types of samples collected in this study. Therefore, some of the samples classified as negatives may have had levels of Salmonella undetectable with the methods used.
Survey of vaccination programmes
One questionnaire was developed for the hatchery managers and another for the broiler production managers. Two pilot tests were conducted for each questionnaire before the final instruments were adopted [Reference Volkova15]. A chart on which to record vaccinations and any other treatments administered to the embryos and birds of the sampled flock at the hatchery was attached to the hatchery manager questionnaire. A chart on which to record vaccinations during grow-out was attached to the broiler manager questionnaire, in which details of coccidiosis control in the flock were also enquired of. Sixty-five completed hatchery manager questionnaires were returned – 30 for sampled flocks from company A and 35 for sampled flocks from company B. The hatchery questionnaires for the remaining 11 flocks sampled at placement for rearing were not returned by company B. The broiler manager survey was less successful. The incomplete responses to the surveys were probably due to competing time demands following Hurricane Katrina in autumn 2005. Grow-out production records routinely archived by company A were available for analysis. Whenever possible the information reported in the two surveys was cross-checked against and complemented by information in the production records of sampled flocks raised for company A.
Analysis
We refer to the diseases targeted by a vaccination programme as its ‘content’, and to the total numbers of immunizations, their timing, and the modes and dosages of deliveries as the ‘design’ of the programme. Individual infections controlled and details of the design were analysed for associations with the burden of Salmonella in the broiler flock. The information on individual items of the vaccination programmes was available for a variable number of sampled flocks. In certain cases the information was only relevant for a part of the flocks (for example, a dosage of Marek's disease vaccine administered in ovo was only relevant for the flocks vaccinated in ovo). Each item was therefore tested individually for associations with the probability of detecting Salmonella at each sampling point throughout the production cycle in the flocks for which this item was characterized. This was done while accounting for potential confounding effects on the response due to variability among the grow-out farms, their production complexes and companies. However, due to the sample size limitations, more complex models assessing the relative significance of the items of the vaccination programme for each outcome (e.g. models with multiple fixed-effects factors) were not considered. Specifically, each item was tested for associations with the probability of detecting Salmonella (an increase in the proportion of Salmonella-positive samples out of total samples of this type collected from the flock at this point) in a multi-level mixed logistic regression model that incorporated the hierarchically structured random effects of the grow-out farms, their production complexes and companies, and the item tested as a single fixed-effects factor. The item was considered to be associated with the outcome if P⩽0·150 in this model. The models were fit using the glimmix procedure in SAS® 9·1 software for Windows (SAS Institute Inc., USA).
Table 1. Practices of broiler vaccination against infections other than Salmonella associated with probabilities of detecting Salmonella in the flock or grow-out house litter
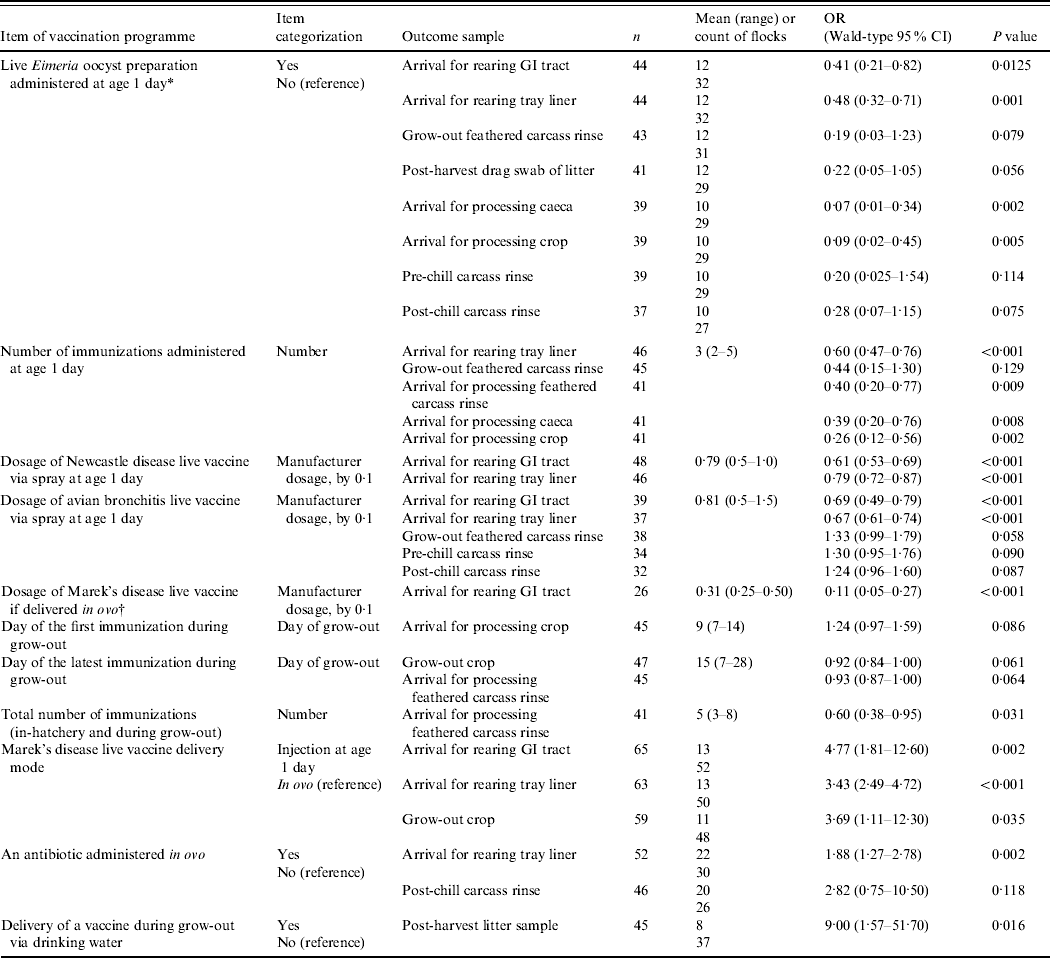
OR, Odds ratio; CI, confidence interval; GI, gastrointestinal.
For a given outcome (an increase in the proportion of Salmonella-positive samples from the flock), each item of vaccination programme was evaluated as a single fixed-effects factor in a multi-level model that accounted for the variation among the farms, their production complexes and companies. n, sample size (number of sampled flocks) for the outcome/item of vaccination programme model.
* One-day-old birds are newly hatched broilers processed (vaccinated, counted, etc.) at the hatchery.
† In-ovo administrations were performed using the Inovoject® system.
RESULTS
None of the flocks studied, for which the vaccination programmes were surveyed, were either immunized against Salmonella or received a competitive exclusion product. Vaccination protocols for the parent breeder flocks of studied flocks were not available. All studied flocks received Marek's disease live vaccine either in ovo or via injection of 1-day-old birds at the hatchery on the day of hatch. Administration of an antibiotic in ovo was reported for some of the flocks; this practice was also analysed. All in-ovo administrations were performed using the Inovoject® system (Embrex Inovoject® Egg Injection System, Pfizer Poultry Health, Pfizer, USA). At the hatchery on the day of hatch, all studied flocks were vaccinated with Newcastle disease and infectious bronchitis (IB) live vaccines via spray; some of the flocks also received live Eimeria oocyst preparations via spray.
The results are detailed in Table 1; the sample size (number of sampled flocks) available to assess the significance of each item of the vaccination programmes is indicated.
DISCUSSION
In terms of the content of the vaccination programme, administration of a live Eimeria oocyst preparation to 1-day-old broilers (delivered via spray at the hatchery in all cases) was associated with reduced probabilities of detecting Salmonella in the flock throughout its entire production cycle – from the time of the flock's placement for rearing to the end of its processing (post-chilling point). No significant associations were observed between the pharmacological groupings of coccidiostatics administered and occurrence of Salmonella Typhimurium in Danish broiler flocks [Reference Chriel, Stryhn and Dauphin6]. The Danish flocks sampled were on average 3 weeks old, while in the current study the flocks were on average 7 weeks old when sampled during rearing, and 8 weeks old upon arrival for processing. Our results suggest that the choice of coccidiosis control method per se – administration of live Eimeria oocysts vs. coccidiostatics – may be important for control of Salmonella in grow-out broilers. The oocyst administration appears beneficial, and the effects are apparent both during rearing and when the flocks reach processing. The underlying biology probably relates to the mucosal responses induced by Eimeria and the overall improved control of coccidiosis, i.e. healthier chicken gut physiology during rearing and, relatedly, to the establishment and maintenance of the normal intestinal microflora in broilers, rather than to any systematic immunizing effects of the oocyst administration. However, the latter could not be ruled out considering the other results of this study. The mucosal responses, inflammatory and immunostimulatory, to eimerial infection are multi-functional [Reference Laurent16] and are beyond the scope of this discussion. That broilers with normal intestinal flora are less likely to be colonized with Salmonella was shown by experimental studies in the 1970s [Reference Nurmi and Rantala17]. Since then field investigations have demonstrated that these relationships are important in production settings, with some of the studies analysing hundreds of broiler flocks [Reference Wierup, Wahlstrom and Engstrom18–Reference Hirn, Nurmi, Johansson and Nuotio21]. Clinical coccidiosis disturbs the balance of intestinal microbiota of chickens [Reference Kimura22].
The interactions between Eimeria and Salmonella in the course of concurrent infection in chickens received extensive attention in laboratory experiments in the 1980s and 1990s (data not given). It is intuitively suggestive that the damage of intestinal mucosal epithelium in a course of clinical coccidiosis may enhance susceptibility to Salmonella colonization. However, experimental evidence also suggests that subclinical eimerial infection in broilers following oocyst administration on the first day of life may lead to organ resistance to Salmonella colonization several days later [Reference Tellez, Kogut and Hargis23]. In particular for colonization of caeca the resistance was attributed to increased thickness of lamina propria following infiltration with inflammatory cells [Reference Tellez, Kogut and Hargis23]. Moreover, in another experimental series, no enhancement of caecal colonization with Salmonella in the presence of clinical coccidiosis was observed in broilers receiving anaerobic adult caecal flora on the first day of life and simultaneously challenged with Eimeria and Salmonella 2 days later [Reference Kogut24]. However, such enhancement was observed in the control groups not receiving the adult caecal flora. Therefore, both the degree of eimerial infection and the sequence/timing of exposures to this and concurrent microbiota may be important.
Returning to the choice of coccidiosis control method, if the levels of eimerial infection caused by the vaccine strain delivered to broilers on the first day of life are carefully controlled, the stimulatory effects on the intestinal mucosal responses may take place throughout the birds' production lifespan. There is also an opportunity for intestinal microflora to develop normally. In contrast, if coccidiostatics are chosen, the 1-day-old broilers are exposed to Eimeria persistent in the grow-out house litter (potentially more virulent than the vaccine strains), and the levels of infection are only controlled following the drug administration. The Eimeria strains in the litter are not routinely monitored, and a mismatch in terms of the strains' resistance to the coccidiostatics used is possible.
Concerning the design of the vaccination programme, a higher total number of individual immunizations (each targeting a particular infection other than Salmonella) administered to the grow-out broilers on the first day of life while still at the hatchery was associated with reduced probabilities of Salmonella detection in the flock – upon arrival at the farm, during rearing, and at arrival for processing. In some cases, the dosage of a vaccine administered in ovo or to the broilers is either higher or lower than the manufacturer's recommendation. Reduced doses may be given because of economic considerations, to decrease the severity of reaction to the vaccine, or if the disease is perceived to be rare. Higher doses may be used if the goal is to increase the potency of immunization. Increased dosages of a Newcastle disease live vaccine and an IB live vaccine, both delivered via spray to the 1-day-old broilers, and of Marek's disease live vaccine delivered in ovo were associated with reduced probabilities of detecting Salmonella in the flock at the time of delivery to the farm.
Newly hatched broilers are highly susceptible to colonization with Salmonella, generally being more susceptible than older birds [Reference Cox25]. These differences have been attributed to the lack of adult intestinal microflora [Reference Nurmi and Rantala17]; although, a recent study suggests that a diverse bacterial community may be present in the chicken gut since day 16 of egg incubation [Reference Pedrosa26]. Whichever of these two is true, our results suggest that the administration of live viral vaccines to the broilers in the late stage of embryonic development and on the first day of life may alter their susceptibility to early colonization with Salmonella. A higher total number of immunizations on the first day of life was associated with reduced Salmonella burden throughout the flock's production lifespan, with the effects manifesting as early as by the time the broilers were delivered to the grow-out farm. The exact interval from the time of the vaccination to the time of the delivery to the farm was impossible to derive. For each flock, this interval was composed of the time spent on the bird-processing line post-vaccination, waiting to be loaded for transportation, and being transported to the farm. The waiting time was surveyed in the hatchery manager questionnaire, and the duration of transportation was recorded at the time of sampling. The waiting time averaged 4·7 h, but ranged from 1 h to 12 h. The duration of transportation was on average 65 min, but ranged from 5 min to 190 min. In practice, effort is made to minimize the duration of bird processing and delivery.
The mechanisms underlying the associations between the extensive immunostimulation of late broiler embryos and newly hatched birds and the reduced burden of Salmonella in the flock may be multi-fold. First, the effects may be direct, i.e. the immunostimulation lessens broilers' susceptibility to colonization with non-host-specific Salmonella at the time when susceptibility is at its highest. Chicken embryos and young birds are able to mount rapid immune responses [Reference Swaggerty27]. Recent evidence suggests that these responses may be more robust than previously considered [Reference Jenkins28, Reference MacKinnon29]. This explanation does not imply that systematic immune responses necessarily play a role in determining the probability of Salmonella colonization. The vaccine preparations proposed to lessen the burden of non-host-specific Salmonella in poultry have had variable success [Reference Barrow30], and the mechanisms allowing the disease-free persistence of such Salmonella in poultry are not fully understood. The broiler flocks studied received live viral vaccines, which in addition to activating systematic responses also stimulate cell-mediated and mucosal immunity [Reference Rauw31, Reference Russell and Koch32]. Second, broilers experience immunosuppression during early-life viral infections [Reference Lam33]. Improved prevention of the clinical diseases helps reduce these effects. Concurrent immunosuppressive viral infections are known to worsen the course of infection with host-specific poultry Salmonella [Reference Gast and Saif34]. It may be that a well-designed routine vaccination protocol tailored to local risks decreases susceptibility of broilers to colonization with non-host-specific Salmonella by effectively protecting them from immunosuppression due to early-life viral infections.
In contrast to the other viral vaccinations, increased dosage of IB viral vaccine delivered via spray to the 1-day-old birds was linked to a higher probability of detecting Salmonella in the flock during rearing, and on the broiler carcasses at the pre-chilling and post-chilling points in processing. A possible explanation is a relatively high frequency of a mild form of the disease following the IB vaccination. Further, a live attenuated IB vaccine containing one or two strains of the virus does not prevent clinical disease caused by other strains [Reference Ignjatovic and Sapats35]. In the field, an IB vaccine is selected according to the virus strains known to circulate in the area. But circulating strains can be replaced over time by new field strains or vaccine strains undergoing virulence reversion. In the case of a mismatch between the vaccine and circulating strains, an increased dose of IB vaccine may result in the flock undergoing both a stronger form of the disease caused by the vaccine strains and the disease caused by circulating strains. The severity of the latter would depend upon the circulating strains. The clinical disease may lead to immunosuppression in affected birds, making them more susceptible to Salmonella colonization, or it may enhance Salmonella shedding in faeces, facilitating bird-to-bird transmission during rearing. A strain mismatch was more likely for IB vaccines than for the other live viral vaccines delivered to the studied flocks.
A later delivery of the first vaccination during grow-out (i.e. after the flock's placement into the house) was associated with a higher probability of Salmonella in the broilers arriving for processing. This observation supports the hypothesized beneficial effects of early-life immunostimulation. However, administering the final vaccination at a later time of rearing was also associated with reduced probabilities of detecting Salmonella in the flock both during rearing (the last vaccination always preceded this sampling occasion) and at arrival for processing. These observations suggest that the continuity of immunostimulation during rearing may also be important. A higher overall number of immunizations (in-hatchery and during grow-out) was associated with a reduced probability of detecting Salmonella in the flock arriving for processing.
There was a higher probability of detecting Salmonella in the flock at the time of delivery to the farm and in the crops of the birds during rearing if the birds received Marek's disease live vaccine by injection at age 1 day compared to the in-ovo vaccinates. Perhaps the stress experienced by the 1-day-old birds upon injection increased their susceptibility to intestinal colonization with Salmonella, which resulted in a higher proportion of Salmonella crop-carriers in rearing. However, the delivery by injection was implemented only by certain participating broiler complexes, and the associations observed may be confounded by other differences between the complexes implementing and not implementing this practice. For example, the degree of the hatcheries' contamination with Salmonella may differ.
Higher probabilities of detecting Salmonella upon arrival at the farm and on the broiler carcasses post-chilling were observed in the flocks that received an antibiotic in ovo. The decision to administer an antibiotic could have been driven by awareness of a potentially poor ‘chick quality’ of the hatching flock, and the underlying factors may have confounded the associations observed.
Delivery of any vaccine to the sampled flock in the grow-out house via drinking water was associated with a higher probability of detecting Salmonella in the house litter on the day of the flock's harvest. This effect was relatively high (OR 9·0), although there were few flocks (n=8) to which a vaccine was delivered via drinking water. It might be that the water deprivation the broilers are subjected to before vaccination via drinking water enhances Salmonella shedding in faeces. It might also be that the usage of disinfectant-free water to administer the vaccine (to prevent its inactivation) leads to higher bacterial counts in the grow-out environment.
In conclusion, the content and design of the routine vaccination programme for conventional grow-out broilers, despite targeting infections other than Salmonella, may impact the burden of Salmonella in the flock. Beneficial effects are observed if a flock receives live Eimeria oocyst preparation on the first day of life, higher dosages of live viral vaccines in the late stage of embryonic development and on the first day of life, and higher total numbers of immunizations on the first day of life and during the production lifespan. These effects manifest as early as the time of the flock's arrival at the farm for rearing, which is normally also within the first day of life. The effects can be detected throughout the entire production lifespan, including for the broiler carcasses at the end of processing.
There are two major limitations in interpreting the results of this study. First, it was not possible to evaluate whether maternal immunity to infections other than Salmonella affects the probabilities of Salmonella in grow-out broilers, and how this may interfere with the immunity acquired by the birds. If maternal effects exist, they could alter the susceptibility to Salmonella colonization in newly hatched broilers, when susceptibility is at its highest. Second, all Salmonella isolates obtained were serotyped. Seventy serotypes were encountered. Over half of the isolates were Salmonella Kentucky, and about 40% were of nine other serotypes. Multiple serotypes were encountered at each of the sampling points: upon arrival for rearing and during rearing, in the litter prior to bird placements and after the harvests, and on the carcasses in processing. Therefore the dominant sources of Salmonella for broiler flocks in this study could not be defined and the effects of vaccination practices on Salmonella acquired vertically vs. those acquired during rearing or at later stages of the production cycle could not be differentiated. The sources of Salmonella may be better defined in other production scenarios and, depending on how these sources differ from those for the studied flocks, the associations with the vaccination practices may differ. For example, in a production scenario with no vertical transmission of Salmonella to grow-out broilers, the immunostimulatory effects of vaccinations in the late stage of embryonic development and on the first day of life may be irrelevant or less important. On the other hand, the consistency of preventive effects of Eimeria oocyst administration on the first day of life suggests that this practice may be beneficial irrespective of the sources of Salmonella for broiler flocks.
However, the results of the present report yield a starting point for a discussion of how routine vaccination programmes for grow-out broilers may be adjusted to enhance the control of Salmonella in flocks. The role of Eimeria in the ecology of Salmonella in broiler flocks requires in-depth investigation.
ACKNOWLEDGEMENTS
This pilot analysis was conducted within the project funded by the Epidemiological Approaches for Food Safety, USDA NRICGP 32.1, 2002-02235. We thank Mrs Terry Doler and Mrs Mary Ann Ballard for laboratory support and logistics of the field work. We also thank Dr Karen Dazo-Galarneau, Dr Michael Rybolt, Dr David Smith, Dr Tyler McAlpin and the many student workers for participating in collection and laboratory processing of the samples from broiler flocks.
We thank Dr Michael Thrusfield for contributing a point to the discussion. V.V.V. is grateful to Dr Martin Miller for reading through the paper. Thanks are also due to the farmers for granting access to the flocks. The willingness to cooperate shown by the managerial personnel of participating broiler companies is greatly appreciated.
DECLARATION OF INTEREST
None.



