The study of vitamin D has been of great interest to the researchers for more than a century due to diverse spectrum of clinical and biochemical manifestations. There is emerging evidence from studies to show that vitamin D is not only required for bone health but also for the body to perform other physiological functions including cell differentiation, proliferation, insulin production and immune functions(Reference Holick1). Low 25-hydroxyvitamin D (25(OH)D) levels are associated with several disorders including diabetes, CVD, hypertension, cancer and even depression(Reference Vimaleswaran, Cavadino and Berry2–Reference Pludowski, Holick and Pilz4).
Mild to moderate vitamin D deficiency has been identified as one of the risk factors for type 2 diabetes mellitus (T2DM), as it may affect glucose homoeostasis through increasing insulin resistance (IR) and reducing insulin secretion from β-cells of the pancreas(Reference Dhas, Mishra and Banerjee5). Studies conducted in the west have shown that diabetes and prediabetes are associated with low serum concentrations of 25(OH)D(Reference Scragg, Holdaway and Singh6,Reference Hutchinson, Figenschau and Almås7) . A meta-analysis conducted based on observational studies concluded that there appears to be a consistent relationship between low 25(OH)D levels, T2DM and the metabolic syndrome (MetS)(Reference Pittas, Lau and Hu8).
Previous reports suggest that vitamin D deficiency is present in more than 90 % of Indians and other Asian countries, whereas it is about 40 % in individuals in developed countries(Reference Wahl, Cooper and Ebeling9). Very few population-based studies have been conducted to assess the prevalence of vitamin D deficiency across the spectrum of glucose intolerance in India(Reference Dutta, Maisnam and Shrivastava10,Reference Modi, Ahmed and Chandwani11) or other developed countries(Reference Guan, Zhen and Tang12,Reference Pittas, Dawson-Hughes and Li13) . Hence, this study was carried out to assess the prevalence of vitamin D deficiency in an urban south Indian population in individuals with different grades of glucose tolerance.
Materials and methods
The study participants were recruited from the Chennai Urban Rural Epidemiology Study (CURES) in whom a 10-year follow-up was conducted between 2012 and 2013. The methodology of the CURES has been published elsewhere(Reference Anjana, Shanthi Rani and Deepa14,Reference Deepa, Pradeepa and Rema15) . Of the 3589 individuals followed-up, 645 individuals were lost to follow-up (18 %) and 534 individuals died (14·9 %). From the remaining 2410 individuals, 1500 individuals were randomly selected for this study. The details of their recruitment are described elsewhere(Reference Jayashri, Venkatesan and Rohan16). The exclusion criteria for this study included those who had T2DM, diabetes secondary to other diseases, for example, chronic pancreatitis, those aged <20 years or >80 years or those taking vitamin D supplements.
The study was approved by the Institutional Ethics Committee of the Madras Diabetes Research Foundation, Chennai, Tamil Nadu, India, and written informed consent was obtained from all the participants. For each individual, a detailed questionnaire was administered to collect information about demographic and socio-economic parameters, medical history, family history diabetes and dietary pattern and behavioural factors. Anthropometric details such as height, weight and waist were measured using standardised techniques(Reference Harrison, Buskirk, Lindsay Carter, Lohman, Roche and Martorell17). Blood pressure (BP) was measured using a mercury sphygmomanometer and was recorded in the sitting position in the right arm. Two readings were taken 5 min apart, and the mean of the two was recorded as the BP.
A fasting venous blood sample was collected after an overnight fast of at least 10 h for the estimation of biochemical investigations for recruited study participants. The sample was centrifuged to extract serum for the estimations of glucose, lipids and 25(OH)D, and the whole blood was used for the estimation of HbA1c. Biochemical analyses were performed in a laboratory certified by the National Accreditation Board for Testing and Calibration Laboratories and the College of American Pathologists. Serum insulin and serum 25(OH)D concentrations were estimated using the electrochemiluminescence using a Roche e601Cobas immunoassay analyzer (Roche Diagnostics). The intra- and inter-assay CV for vitamin D assay were 3·6 and 6·4%, respectively.
Definitions
Normal glucose tolerance (NGT): 2-h post-load glucose was <140 mg/dl (7·8 mmol/l) and fasting plasma glucose <110 mg/dl (6·1 mmol/l)(Reference Alberti and Zimmet18).
Impaired fasting glucose: Fasting plasma glucose ≥ 110 mg/dl (6·1 mmol/l) and <126 mg/dl (7·0 mmol/l) and 2 h post-glucose value <140 mg/dl (7·8 mmol/l)(Reference Alberti and Zimmet18).
Impaired glucose tolerance: 2-h post-glucose ≥ 140 mg/dl (7·8 mmol/l) but <200 mg/dl (11·1 mmol/l) and fasting value <126 mg/dl (7·0 mmol/l)(Reference Alberti and Zimmet18).
Prediabetes: Individuals with impaired fasting glucose or impaired glucose tolerance or both.
T2DM: Fasting plasma glucose ≥ 126 mg/dl (7·0 mmol/l) or 2 h post-load glucose level ≥200 mg/dl (11·1 mmol/l), or a past medical history (self-reported diabetes under treatment by a physician), or drug treatment for diabetes(Reference Alberti and Zimmet18).
Vitamin D deficiency: Defined as serum 25(OH)D levels <20 ng/ml(Reference Holick, Binkley and Bischoff-Ferrari19)
Vitamin D insufficiency: Defined as serum 25(OH)D levels ranging between 20 and 29·9 ng/ml(Reference Holick, Binkley and Bischoff-Ferrari19)
Vitamin D sufficiency: Defined as serum 25(OH)D levels ≥30 ng/ml(Reference Holick, Binkley and Bischoff-Ferrari19)
BMI was calculated using the formula: weight (in kg) divided by height (in m2)
Obesity: Generalised obesity (BMI ≥ 25 kg/m2) and abdominal obesity (waist circumference ≥ 90 cm in males and ≥80 cm in females) were defined using WHO Asia Pacific guidelines(20).
Hypertension: Diagnosed in subjects who were on antihypertensive medications or had a systolic BP ≥ 140 mmHg and/or a diastolic BP ≥ 90 mmHg(Reference Chobanian, Bakris and Black21).
IR: IR was calculated using the Homeostasis Model Assessment formula: fasting insulin (mIU/ml) × fasting glucose (mmol/l)/22·5)(Reference Matthews, Hosker and Rudenski22).
MetS: The MetS was defined according to the National Cholesterol Education Program ATP III criteria modified for waist according to WHO Asia Pacific guidelines for obesity. The MetS was defined as the presence of any three of the following abnormalities: abdominal obesity, defined as waist circumference of ≥90 cm for men and ≥80 cm for women; high BP (systolic BP ≥ 130 mmHg or diastolic BP ≥ 85 mmHg); elevated fasting glucose (fasting plasma glucose level ≥ 100 mg/dl (5·6 mmol/l); (TAG ≥1·7 mmol/l)) or low HDL-cholesterol (<1·03 mmol/l for males, <1·3 mmol/l for females)(Reference Alberti, Zimmet and Shaw23,Reference Deepa, Farooq and Datta24) .
Statistical analysis
Statistical analyses were performed with SPSS statistical package (version 22.0; SPSS Inc.). Continuous variables are reported as mean values and standard deviations. Categorical variables are reported in percentages. One-way ANOVA with post hoc Tukey honestly significant difference or Student’s t test was used to compare groups for continuous variables, and the χ 2 test was used to compare proportions between two groups. Univariate logistic regression analysis was performed to look at the association of glucose tolerance, age and sex with vitamin D deficiency in our population. Logistic regression models were used to measure the association of vitamin D deficiency with various factors like diabetes, prediabetes, dysglycaemia, abdominal and generalised obesity, hypertension, the Mets and IR. P values of <0·05 were considered as statistically significant.
Results
Basic characteristics of the study participants
A total of 1500 participants were included in the study, which comprised 900 NGT, 300 prediabetes and 300 T2DM individuals. The study population included 45 % of males and the mean age was 46 (sd 12) years. Individuals with T2DM were older (T2DM: 54 (sd 11) years; prediabetes: 48 (sd 12) years; NGT: 43 (sd 11) years; P < 0·001) and had a higher systolic BP (T2DM: 132 (sd 19) mmHg; prediabetes: 129 (sd 20) mmHg; NGT: 123 (sd 18) mmHg; P < 0·001) compared with individuals with prediabetes and NGT. In addition, individuals with T2DM and prediabetes had a higher BMI (T2DM: 27 (sd 5) kg/m2; prediabetes: 27 (sd 5) kg/m2; NGT: 26 (sd 5) kg/m2; P < 0·001) and waist circumference (T2DM: 90 (sd 10) cm; prediabetes: 90 (sd 11) cm; NGT: 86 (sd 11) cm; P < 0·001) compared with NGT subjects. Similar trend was observed with hypertension (T2DM: 73 %; prediabetes: 29 %; NGT: 23 %; P < 0·001) when compared with individuals with NGT. There was an increasing trend in fasting insulin (T2DM: 11 (sd 7) µIU/ml; prediabetes: 8 (sd 6) µIU/ml; NGT: 8 (sd 5) µIU/ml; P < 0·001), total cholesterol (T2DM: 5 (sd 1) mmol/l; prediabetes: 5 (sd 1) mmol/l; NGT: 5 (sd 1) mmol/l; P < 0·001) and TAG levels (T2DM: 2 (sd 2) mmol/l; prediabetes: 2 (sd 1) mmol/l; NGT: 1 (sd 0·9) mmol/l; P < 0·001) with decreasing glucose tolerance. Among the T2DM participants, the mean duration of diabetes was 7 (sd 6) years and 65 % of them had a family history of diabetes. Overall, 94 % followed a non-vegetarian meal pattern (T2DM: 91 %; prediabetes: 93 %; NGT: 95 %; P = 0·005).
Table 1 describes the basic clinical and biochemical characteristics of the study population with sufficient, insufficient and deficient serum 25(OH)D levels. Individuals with vitamin D deficiency had higher BMI (deficient: 27 (sd 5) mmHg; sufficient: 25 (sd 5) mmHg; P < 0·001), HbA1c (deficient: 6 (sd 2) %; sufficient: 6 (sd 1)%; P < 0·001), Homeostasis Model Assessment IR (deficient: 2 (sd 2); sufficient: 2 (sd 2); P < 0·001) when compared with vitamin D sufficient groups. There were no significance observed in BP and lipid parameters.
Table 1. Basic clinical and biochemical characteristics of participants with sufficient, insufficient and deficient serum 25-hydroxyvitamin D (25(OH)D) levels
(Mean values and standard deviations; numbers and percentages)
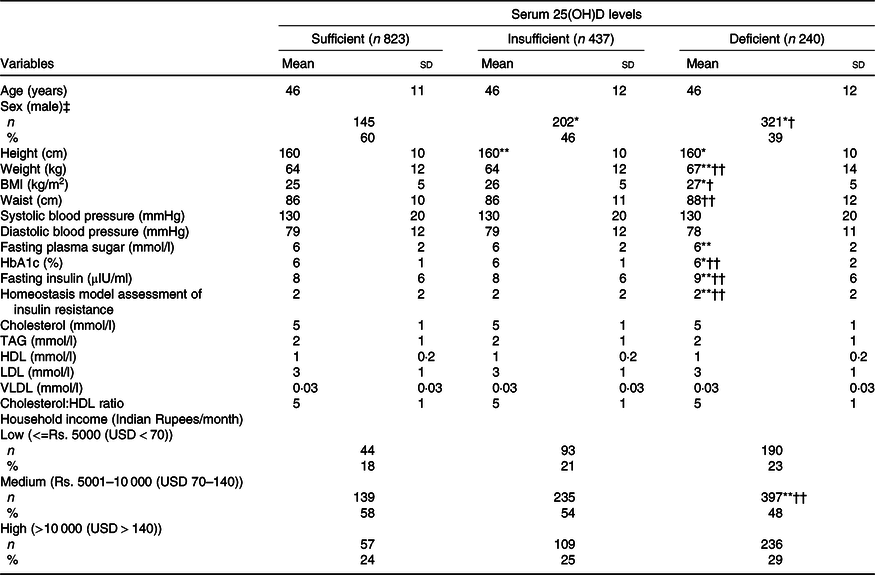
*P < 0·001 and ** P < 0·05 compared with individuals with sufficient serum 25(OH)D levels.
† P < 0·001 and †† P < 0·05 compared with individuals with insufficient serum 25(OH)D levels.
‡ χ 2.
Mean serum 25-hydroxyvitamin D levels and prevalence of vitamin D deficiency
Fig. 1(a) shows the mean values of 25(OH)D levels in participants with different degrees of glucose tolerance. The overall 25(OH)D level for the entire study population was 20 ng/ml. The mean levels of 25(OH)D were 21, 19 and 18 ng/ml in individuals with NGT, prediabetes and T2DM participants, respectively (ANOVA, P < 0·001). Individuals with prediabetes and T2DM had significantly lower levels of 25(OH)D compared with individuals with NGT. The distribution of 25(OH)D levels in the entire study participants (n 1500) showed that only 16 % (n 240) had normal 25(OH)D levels, 29 % (n 437) had insufficient 25(OH)D levels and 55 % (n 823) had deficient 25(OH)D levels. Fig. 1(b) shows the prevalence of vitamin D deficiency in individuals with different degrees of glucose tolerance. The highest prevalence of vitamin D deficiency was observed among individuals with T2DM (63 %) followed by prediabetes (58 %) and NGT (51 %) (trend χ 2 14·24, P < 0·001). An increasing trend of vitamin D deficiency was observed with increasing glucose tolerance. If serum 25(OH)D levels <30 ng/ml were used for defining vitamin D deficiency, we found the overall prevalence of vitamin D deficiency to be 84 %, with the highest prevalence observed among diabetic individuals (90 %) followed by prediabetes (87 %) and NGT (81 %).
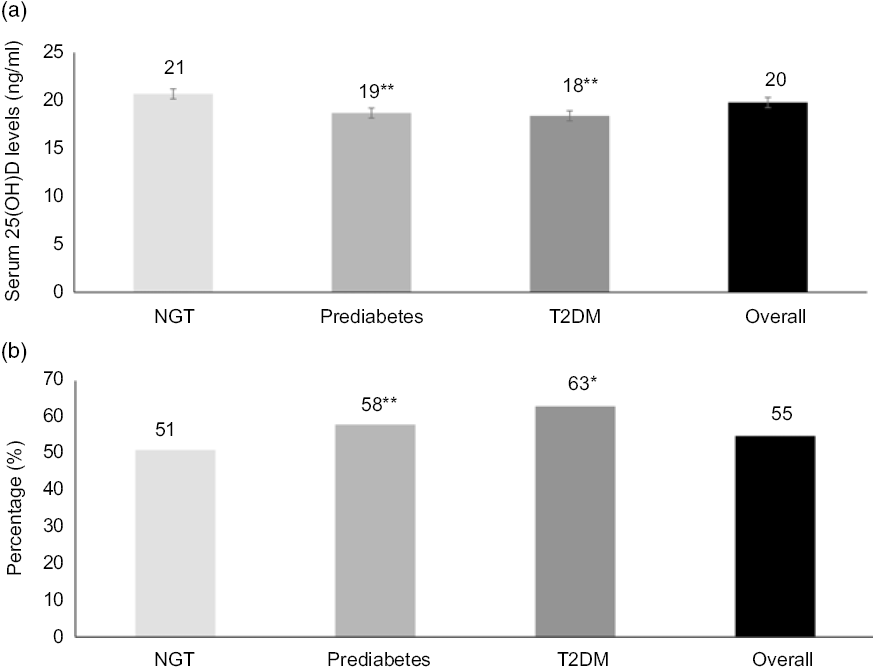
Fig. 1. Mean serum levels of 25-hydroxyvitamin D (25(OH)D) (a) and prevalence of vitamin D deficiency (b) in different degrees of glucose tolerance. * P < 0·001 and ** P < 0·05 compared with normal glucose tolerance (NGT). T2DM, type 2 diabetes mellitus.
Table 2 provides the prevalence and association of age and sex with vitamin D deficiency in the study population (n 1500). The mean serum 25(OH)D levels were lower in women compared with men (women v. men: 19 (sd 10) v. 22 (sd 11); P < 0·001). There was no significant difference of 25(OH)D levels in different age groups (trend χ 2 −0·83, P = 0·4).
Table 2. Association of age and sex with vitamin D deficiency (n 1500)
(Mean values and standard deviations; odds ratios and 95 % confidence intervals)

* Adjusted for each other and further adjusted for BMI, systolic blood pressure, serum TAG, household income, dietary pattern and diabetes duration and medication.
† P < 0·001.
Fig. 2 presents the prevalence of obesity, hypertension, MetS and IR among individuals with sufficient, insufficient and deficient serum 25(OH)D levels. There was a significant increase in generalised obesity (P < 0·001), abdominal obesity (P < 0·001), the MetS (P = 0·02) and IR (P = 0·003) among individuals with vitamin D deficiency compared with those with sufficient 25(OH)D levels. The prevalence of vitamin D deficiency was 80 and 66 % among participants with generalised and abdominal obesity, respectively, 35, 45 and 38 % among those with hypertension, MetS and IR, respectively.
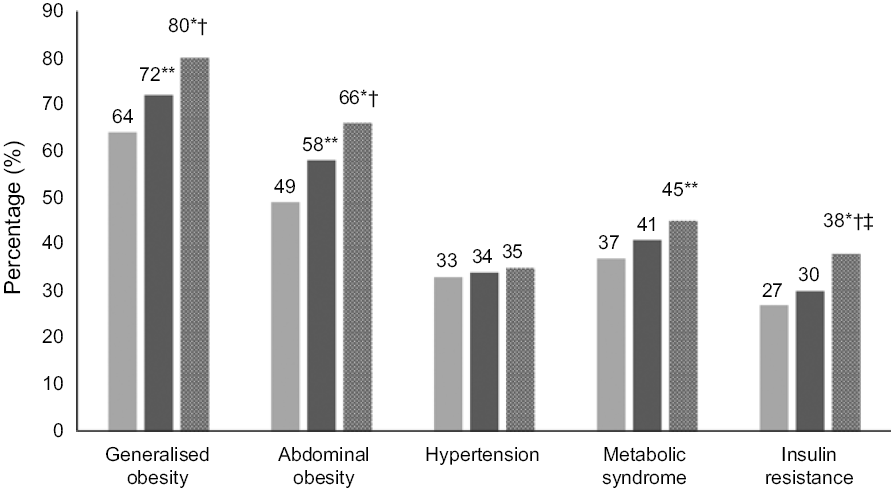
Fig. 2. Prevalence of obesity, hypertension, the metabolic syndrome and insulin resistance among individuals with sufficient, insufficient and deficient serum 25-hydroxyvitamin D levels.  , With vitamin D sufficiency (n 240);
, With vitamin D sufficiency (n 240);  , with vitamin D deficiency (n 823);
, with vitamin D deficiency (n 823);  , with vitamin D insufficiency (n 437). * P < 0·001 and ** P < 0·05 compared with sufficiency group. † P < 0·05 compared with insufficiency group. ‡ Data available in 1440/1500 individuals.
, with vitamin D insufficiency (n 437). * P < 0·001 and ** P < 0·05 compared with sufficiency group. † P < 0·05 compared with insufficiency group. ‡ Data available in 1440/1500 individuals.
Association of vitamin D deficiency with diabetes, prediabetes and other factors
Multiple logistic regression analysis was done using vitamin D deficiency as the dependent variable. Individuals with diabetes and prediabetes had 1·6 times and 1·3 times risk for developing vitamin D deficiency even after adjusting for confounding factors including age, female sex, abdominal obesity, hypertension, family history of diabetes, dietary pattern and income (diabetes OR 1·6 (95 % CI 1·1, 2·2), P = 0·005; prediabetes OR 1·3 (95 % CI 1, 1·8), P = 0·04). In addition, logistic regression models were used to measure the association of vitamin D deficiency with various factors (Fig. 3). It was observed that the dysglycaemia (unadjusted OR: 1·9, P < 0·001; adjusted OR: 1·5, P = 0·001), abdominal obesity (unadjusted OR: 1·8, P < 0·001; adjusted OR: 1·5, P < 0·001), generalised obesity (unadjusted OR: 1·9, P < 0·001; adjusted OR: 1·6, P < 0·001), glycaemic control (unadjusted OR: 1·8, P = 0·007; adjusted OR: 1·4, P = 0·03) and IR (unadjusted OR: 1·5, P = 0·02; adjusted OR: 1·5, P = 0·002) had higher OR for vitamin D deficiency even after adjusting for age, sex, income, dietary pattern and duration of diabetes. However, there was no significant association observed between hypertension, the MetS and vitamin D deficiency.
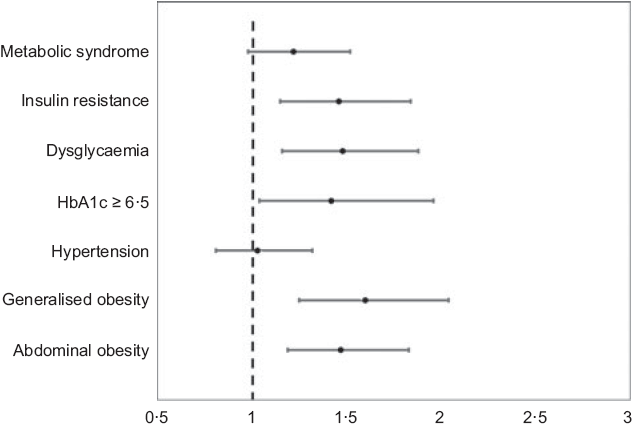
Fig. 3. Association of vitamin D deficiency with various factors. * Adjusted for age, sex, income, vegetarianism and duration of diabetes.
Discussion
This is the first study from southern India to our knowledge, to determine the levels of serum 25(OH)D among individuals with NGT, prediabetes and T2DM. We report the overall prevalence of vitamin D deficiency to be 55 % based on the serum 25(OH)D cut-off value of <20 ng/ml(Reference Holick, Binkley and Bischoff-Ferrari19). A cross-sectional study conducted in south Indian adult urban and rural population residing in Tirupati, South India, reported that two-thirds of the population had low levels of 25(OH)D (54 % – vitamin D insufficiency (<20 ng/ml) and 15 % – vitamin D deficiency(<10 ng/ml))(Reference Harinarayan, Ramalakshmi and Venkataprasad25). Thus, a total of 69 % had serum 25(OH)D < 20 ng/ml, which is higher than the prevalence reported in our study.
The highest prevalence of vitamin D deficiency was observed among diabetic individuals (63 %) followed by prediabetes (58 %) and NGT (51 %). Modi et al. (Reference Modi, Ahmed and Chandwani11) had reported 85 % prevalence of vitamin D deficiency in a clinic-based cross-sectional, observational study at two tertiary level hospitals located in South India and North India and had reported the prevalence of vitamin D deficiency to be 84 % among individuals with diabetes, 77 % among those with prediabetes and 87 % among individuals with NGT. In this study, the investigators considered vitamin D deficiency as serum 25(OH)D < 30 ng/ml. When this cut-off was used in our study, we found a similar overall prevalence of vitamin D deficiency (84 %), where 90 % vitamin D deficiency was observed among diabetic individuals, followed by prediabetes (87 %) and NGT (81 %).
There are many studies, which have reported on prevalence of vitamin D deficiency in healthy populations in both Western population(Reference Daly, Gagnon and Lu26–Reference Alkerwi, Sauvageot and Gilson29) as well as Indian population(Reference Harinarayan, Ramalakshmi and Venkataprasad25,Reference Hovsepian, Amini and Aminorroaya30–Reference Marwaha, Tandon and Garg32) . However, there are no data, which reports on the prevalence of vitamin D deficiency in individuals with glucose tolerance. Of the 11 247 Australian adults aged ≥25 years studied in the Australian Diabetes, Obesity and Lifestyle (AusDiab) study, 4 % had vitamin D deficiency (vitamin D < 25 ng/ml)(Reference Daly, Gagnon and Lu26). Another study conducted in Isfahan, in the central part of Iran among 1111 healthy individuals (243 men and 868 women) aged between 20 and 80 years, reported the prevalence of vitamin D deficiency to be 50·8 %(Reference Hovsepian, Amini and Aminorroaya30). The prevalence of vitamin D deficiency is more or less same even among the healthy NGT population in South India (51 %) compared with these studies.
A study conducted in 1052 women attending a gynaecology clinic in Agra, North India reported the prevalence of vitamin D deficiency with 25(OH)D level <20 ng/ml to be 64 % and those with the level of vitamin D < 30 ng/ml to be 98%(Reference Garg, Agarwal and Agarwal33). In our study, we observed prevalence of vitamin D deficiency in 55 % with 25(OH)D levels <20 ng/ml and in 84% with 25(OH)D levels <30 ng/ml.
Many studies have reported a higher prevalence of vitamin D deficiency among women than men. A study carried out in a tertiary care hospital in Secunderabad(Reference Chowdary, Nellutla and Prasad Reddy34) had reported higher prevalence of vitamin D deficiency in females than males. Modi et al. (Reference Modi, Ahmed and Chandwani11) also reported similar results. Harinarayan et al. (Reference Harinarayan, Ramalakshmi and Prasad35) who studied healthy urban and rural adults also reported higher prevalence of vitamin D deficiency among women compared with men in both urban (62 % men v. 75 % women) and rural areas (44 % men v. 70 % women). In our study, also similar findings were found and the vitamin D deficiency was 1·6 times higher in women when compared with men. In contrast, Marwaha et al. (Reference Marwaha, Tandon and Garg32) reported that there was no significant difference in prevalence of vitamin D deficiency between both sex.
It has been reported that low 25(OH)D levels play an important role in the pathogenesis of T2DM. Several physiological mechanisms have been put forward including the effect of vitamin D on insulin secretion and a direct effect of Ca and vitamin D on insulin action(Reference Palomer, González-Clemente and Blanco-Vaca36,Reference Danescu, Levy and Levy37) . There is evidence to show that vitamin D therapy improves glucose tolerance and IR(Reference Parekh, Sarathi and Shivane38,Reference Von Hurst, Sonehouse and Coad39) . There are studies, both in the West and Indian subcontinent, that have reported that vitamin D deficiency is associated with T2DM(Reference Modi, Ahmed and Chandwani11,Reference Scragg, Sowers and Bell40–Reference Nur-Eke, Özen and Çekin44) and prediabetes(Reference Dutta, Maisnam and Shrivastava10,Reference Nur-Eke, Özen and Çekin44–Reference Bhatt, Misra, Gulati and Singh48) . The recent Hitachi Health Study(Reference Akter, Kuwahara and Matsushita41) conducted in Japanese adults concluded that higher circulating vitamin D was associated with a lower risk of T2DM, and this association was stronger among individuals with prediabetes. A study conducted in South India among 4628 patients with T2DM had shown that 71·4 % were vitamin D deficient, 15 % were vitamin D insufficient and 13·6 % were found to have normal 25(OH)D levels(Reference Palazhy, Viswanathan and Muruganathan49). Among the participants of the Asian Indian Diabetic Heart Study/Sikh Diabetes Study, 83% of T2DM participants was vitamin D deficient compared with 68% among normoglycaemic controls(Reference Braun, Been and Blackett43). In the present study, we observed that 63% of T2DM individuals had vitamin D deficiency. Additionally, a significantly higher proportion of individuals with T2DM had vitamin D deficiency compared those with prediabetes and NGT. Similar observations have been reported in T2DM patients from the area of Athens and Pireaus(Reference Kostoglou-Athanassiou, Athanassiou and Gkountouvas50) and Saudi Arabia(Reference Darraj, Badedi and Poore51).
Lower serum 25(OH)D levels were positively associated with prediabetes in the third National Health and Nutrition Examination Survey conducted in the USA(Reference Shankar, Sabanayagam and Kalidindi46). The present study shows that 58 % of the individuals with prediabetes were vitamin D deficient compared with those with NGT (51 %). While studies conducted in Mysuru(Reference Srinath, Shashidhara and Rajeev Reddy47) and North India(Reference Bhatt, Misra, Gulati and Singh48) reported a higher prevalence of vitamin D deficiency compared with our study (73 and 69 %, respectively), a study conducted in West Bengal(Reference Dutta, Maisnam and Shrivastava10) reported a lower prevalence of vitamin D deficiency (44 %) among individuals with prediabetes, all using the same cut-off for vitamin D deficiency (serum 25(OH)D < 20 ng/ml).
Vitamin D deficiency may also be a risk factor for IR(Reference Sahasrabuddhe, Pitale and Gupta52) as well as the MetS(Reference Ford, Ajani and McGuire53). However, associations of vitamin D with IR have been inconsistent(Reference Gulseth, Gjelstad and Tierney54–Reference Kayaniyil, Vieth and Retnakaran56). Analysis of 712 subjects after evaluating serum 25(OH)D levels and assessing insulin sensitivity by means of the homoeostasis model of IR reported that serum 25(OH)D was significantly correlated with IR and β-cell function in their multiethnic sample(Reference Kayaniyil, Vieth and Retnakaran56). A study conducted in 2005 also reported the association of vitamin D deficiency with the MetS among US adults(Reference Ford, Ajani and McGuire53). In our study also, we found similar association of vitamin D deficiency with IR and the MetS. Several studies reported that the association between low 25(OH)D levels and the MetS was more pronounced in overweight and obese people than in normal-weight individuals(Reference Lu, Yu and Pan57).
Over the past decade, various studies have reported an association between vitamin D deficiency and obesity. A systematic review and meta-analysis on vitamin D deficiency and obesity concluded that vitamin D deficiency was associated with obesity irrespective of age, latitude, cut-offs to define vitamin D deficiency and the Human Development Index of the study location(Reference Pereira-Santos, Costa and Assis58). A landmark study conducted by researchers of the Framingham Heart Study measured serum 25(OH)D levels in 3890 third-generation participants without CVD and diabetes reported lower 25(OH)D levels with greater BMI that could not be accounted for in variations in physical activity or diet. The study also reported an inverse relationship between serum 25(OH)D and subcutaneous and visceral adiposity even among lean individuals(Reference Cheng, Massaro and Fox59). A retrospective study done in 170 individuals in Turkey also reported an association of vitamin D deficiency (serum 25(OH)D < 25 ng/ml) with obesity(Reference Durmaz, Demir and Ozkan60). In our study, we observed an association of vitamin D deficiency with abdominal as well as generalised obesity. For individuals with abdominal obesity, the risk for vitamin D deficiency was 1·5 times higher than those without abdominal obesity, while it was 1·6 times more in those with generalised obesity.
The increasing prevalence of vitamin D deficiency in our country could be attributed to high melanin skin content, lack of sunlight exposure, clothing habits (well covered even when out in the sun), inadequate dietary consumption of foods rich in vitamin D due to predominant vegetarian dietary habits and limited availability of fortified foods(Reference Harinarayan and Joshi61). In addition, increase in the number of hours spent indoors due to modernisation and changing work culture especially in urban Indians could be one of the reasons for high prevalence of vitamin D deficiency(Reference Babu and Calvo62). Some studies have shown that atmospheric pollution plays a role in reducing the efficiency of serum 25(OH)D photosynthesis as pollution scatters short UVB wavelengths(Reference Agarwal, Mughal and Upadhyay63). There is a report of high incidence of vitamin D deficiency rickets in toddlers living in areas of high atmospheric pollution in Delhi, India (28·35° N)(Reference Marwaha and Dabas64,Reference Tiwari and Puliyel65) .
The strengths of the study are that study participants were recruited from a population-based study in an ethnic group with a high prevalence of T2DM. Moreover, careful inclusion and exclusion criteria were used. Individuals were classified into different grades of glucose tolerance, namely, NGT, prediabetes and T2DM using oral glucose tolerance test. One of the limitations of the study is that being a cross-sectional design, we are unable to demonstrate a cause and effect relation between serum 25(OH)D with T2DM. Moreover, we do not have the details of sunlight exposure in these individuals.
In summary, our study reports the following findings: Firstly, serum 25(OH)D levels lowered with increasing severity of glucose tolerance. Secondly, the mean serum 25(OH)D levels were lower in women compared with men. Finally, the prevalence of vitamin D deficiency significantly increased with abdominal obesity, generalised obesity, the MetS and IR. Further prospective studies are needed to confirm the association between serum 25(OH)D levels and severity of glucose tolerance.
Acknowledgements
The authors thank the participants for their cooperation.
The authors also acknowledge the Research Society for the Study of Diabetes in India (RSSDI) for the financial support for the study through their research grant (project no. RSSDI/HQ/Grants/2014/250). This is the 156th publication from the CURES.
R. P., R. J. and V. M. conceived the study; R. J. was involved in laboratory analysis; R. P. and R. J. were involved in interpretation of the data and wrote the first and subsequent drafts of the manuscript. U. V. performed the statistical analyses; V. M., R. M. A., M. D. and C. S. S. designed the CURES and critically reviewed the manuscript. All authors contributed to the revision of the manuscript and approved the final submitted version.
The authors declare that there are no conflicts of interest.








