Staphylococcus aureus is a common colonizer of the upper respiratory tract of healthy individals but can cause a wide range of infections in both humans and animals [Reference Lowy1]. Much attention worldwide has focused on S. aureus strains resistant to methicillin (MRSA) which contribute significantly to morbidity and mortality of hospitalized patients. Recently, community-acquired MRSA (caMRSA) infections have been reported in people not considered at risk for such infections, causing in the main skin and soft tissue infections and necrotizing pneumonia particularly in children and adolescents [Reference Klevens2]. CaMRSA strains are usually defined by the presence of types IV, V and VI SCCmec cassette and susceptibility to the majority of other non-β-lactam antibiotics [Reference Vandenesch3]. These strains sometimes produce Panton–Valentine leukocidin (PVL) but the this is not a universal characteristic.
Several animals have been reported to be reservoirs of caMRSA strains especially pigs and horses [Reference Witte4]. Indeed, the highest risk groups for colonization or infection with animal MRSA strains are pig farmers and veterinarians who typically show higher prevalence of MRSA carriage. Typing of MRSA isolates from pigs and humans occupationally exposed to pigs has shown close clonal relatedness and commonly classified as sequence type (ST) ST398 [Reference Witte4]. In the Czech Republic, an upward trend has been observed in the incidence of MRSA bacteraemia in hospitals with an almost threefold increase over the last 6 years; the average prevalence of MRSA blood isolates reached 12% in 2006 [5]. Although caMRSA strains were detected, the reported prevalence is very low. Out of 1336 S. aureus isolates collected mainly from skin and soft-tissue infections and referred to the National Reference Laboratory for Staphylococci from 2004 to 2006, only 11 were PVL positive [Reference Machová6]. A single MRSA strain of animal origin was detected in 115 Staphylococcus species isolates from piglets sampled in a farm within a survey of antimicrobial resistance [Reference Bardon7]. The strain was not characterized in detail by molecular typing.
The objective of this study was to determine the prevalence of caMRSA in veterinary professionals participating in Vetfair 2008 (a specialized exhibition of veterinary products) held in Hradec Králové, Czech Republic.
Sample collection
To enrol volunteers the investigators addressed about 1000 veterinary professionals (clinicians and technicians) at Vetfair. All attendees were eligible and information was obtained regarding the animal MRSA risk and sampling procedure; 280 volunteers were screened. Each participant completed a questionnaire designed to collect data on occupational exposure to animals (animal species and frequency of contacts, work experience duration and workplace address), recent hospital stay and close contact with health-care professionals, if any. The respondents were screened for staphylococcal carriage from bilateral nasal swab specimens collected with cotton-tipped swabs (Venturi Transystem®, Italy).
MRSA identification, characterization and typing
Swabs were transported in Amies transport medium without charcoal and cultured in tryptone soya broth (Oxoid, UK) with 2·5% NaCl, 3 mg/l cefoxitin (Sigma-Aldrich, USA) and 10 mg/l aztreonam (USPC Inc., USA). After 24 h the broth was subcultured to chromogenic MRSA medium (ORSAB, Oxoid) and Columbia agar (Oxoid) with 6·5% NaCl. S. aureus strains were putatively identified morphologically and by positive tube coagulase test (ITEST Plus Ltd, Czech Republic) and confirmed by the ID32 Staph kit (bioMérieux, France). Oxacillin resistance was confirmed by the MRSA Screen latex agglutination test (Denka Seiken Co. Ltd, Japan) and PCR detection of the mecA gene [Reference Melter8]. Antimicrobial susceptibility of the isolates was determined with the CLSI reference broth microdilution method [9] for mupirocin, erythromycin, clindamycin, trimethoprim–sulfamethoxazole, rifampicin, ciprofloxacin, gentamicin, tobramycin, teicoplanin, vancomycin, fusidic acid, chloramphenicol, quinupristin–dalfopristin and tetracycline. The presence of PVL-encoding genes lukS and lukF was detected by specific PCR [Reference Lina10] and SCCmec types by a multiplex PCR [Reference Oliveira and De Lencastre11]. The spa gene type [Reference Harmsen12] and multilocus sequence types [Reference Enright13] of isolates were determined as described.
The main characteristics of the study volunteers are summarized in Table 1. Two hundred sixty-one (93·2%) were veterinary professionals and most, i.e. 248 (88·6%) reported daily contact with animals. Only two [0·7%, 95% confidence interval (CI) 0·087–2·56] of all participants carried MRSA strains and both reported daily contact with cows, but no regular contact with pigs, horses and sheep or recently hospitalized humans. One isolate was susceptible to all tested antibiotics except erythromycin while the other was resistant to multiple antibiotics including mupirocin, erythromycin, clindamycin, rifampicin, gentamicin, chloramphenicol, quinupristin–dalfopristin, tobramycin and tetracycline. The isolates were unrelated by spa typing and multilocus sequence typing; the erythromycin-resistant isolate was ST30, spa type t012 while the multi-resistant isolate was ST45, spa type t026. Both isolates carried a type IV SCCmec element and none was positive for PVL genes.
Table 1. Main characteristics of the 280 study subjects
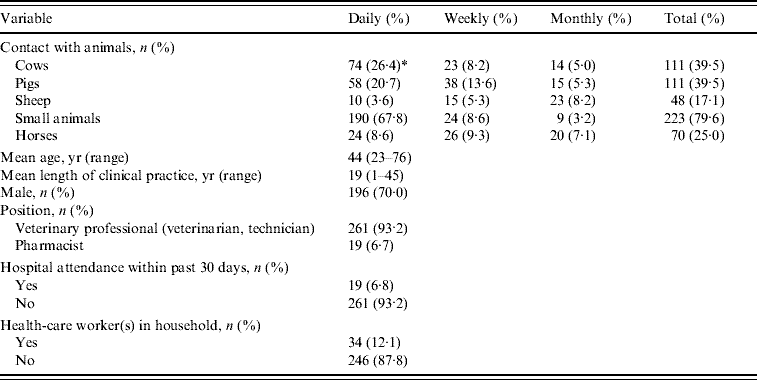
* Two MRSA carriers; genetic profile of isolates: ST30-MRSA-IV spa type t012 and ST45-MRSA-IV spa type t026. Both isolates were Panton–Valentine leukocidin negative.
A high prevalence of caMRSA carriage among veterinarians and pig farmers has been documented [Reference Witte4, Reference Wulf14, Reference Hanselman15]. The latter two studies performed at international veterinarian conferences in the USA and Denmark reported 6·5% and 12·5% of the attendees, respectively, to be colonized with MRSA [Reference Wulf14, Reference Hanselman15]. Characterization of MRSA carriage isolates in the USA study revealed two predominant clones, ST8-MRSA-IV and ST5-MRSA-II. ST8 was found in persons with high occupational exposure to animals, from the USA, UK and Denmark while colonization with ST5 strains was reported primarily in individuals in contact with small animals, from the USA and Germany. In the Danish study, all of the caMRSA strains belonged to closely related spa types corresponding to ST398 and MRSA carriage was significantly associated with extensive exposure to pigs.
Our study does not corroborate the results reported from other countries. Molecular typing did not reveal any caMRSA strains of prevalent animal MRSA clones. ST30-MRSA-IV isolates are emerging community-aquired human pathogens in Europe while ST45 was previously found in both methicillin-susceptible S. aureus (MSSA) and MRSA strains. ST45 represents one of the major lineages of MRSA widely distributed in European hospitals and isolates within this clone carry various types of SCCmec cassette. The wide geographical spread of ST45-MRSA carrying SCCmec type IV (designated as the Berlin clone) and the presence of ST45 genetic background in MSSA strains suggests multiple aquisitions of this SCCmec element by MSSA strains [Reference Wannet16]. Although the Berlin clone is mostly responsible for hospital-aquired MRSA infections, the representatives of this clone were recovered from healthy carriers in Israel and a nosocomial outbreak caused by ST45-MRSA-IV strain previously identified in the community has been reported [Reference Regev-Yochay17].
The rate of MRSA colonization (0·7%) corresponded with the pooled caMRSA colonization prevalence (0·76%) obtained in the meta-analysis of three studies performed in communities outside health-care facilities [Reference Salgado, Farr and Calfee18]. This low colonization rate and the absence of clones circulating in the community make it difficult to link the carriage of MRSA in veterinary personnel with occupational risk. As we are unaware of any special precautions being taken by Czech veterinarians to prevent MRSA carriage, a possible explanation could be a low exposure to animal MRSA as a result of the implied low prevalence of these strains in the Czech Republic. To date, the import of pigs to the Czech Republic has been limited to breeding animals which have been strictly quarantined (S. Mikulášek, personal communication) thus preventing incidental transmission of strains from the countries where animal MRSA are prevalent. As the use of antibiotics on animal husbandries is controlled by veterinarians, the selection of MRSA strains is likely to be lowered. A potential limitation of the study for MRSA prevalence is the recruitment of individuals for sampling. The voluntary nature of participation could introduce a sampling bias as the persons familiar with the unfavourable consequences of MRSA colonization might not have been willing to participate in screening in order to avoid being identified as MRSA positive. On the other hand, our survey disclosed the presence of epidemic caMRSA lineages in Czech residents. As caMRSA becomes established in the country, enhanced sampling from skin and soft-tissue infections and routine screening for MRSA colonization at admission to hospitals is advised in order to reduce the risk of introduction of caMRSA into hospitals.
DECLARATION OF INTEREST
None.



