Evidence supports that isoenergetic meals with a high proportion of dietary energy from protein are more satiating( Reference Belza, Ritz and Sorensen 1 , Reference Astrup 2 ) and stimulate diet-induced thermogenesis (DIT)( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 ) to a greater extent than energy intake from carbohydrate (CHO) and fat; consequently, intake of high-protein meals may lead to weight loss or reduce body-weight gain over time( Reference Larsen, Dalskov and van Baak 4 – Reference Weigle, Breen and Matthys 6 ). Proteins have unique characteristics related to their content of amino acids, structure and rate of absorption and digestion. Therefore, it is speculated that different proteins have diverse metabolic effects( Reference Gilbert, Bendsen and Tremblay 7 , Reference Bendtsen, Lorenzen and Bendsen 8 ), and some studies have suggested that they differ in their effects on appetite( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 , Reference Bendtsen, Lorenzen and Bendsen 8 – Reference Pal and Ellis 10 ) and energy expenditure (EE)( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 , Reference Mikkelsen, Toubro and Astrup 11 ).
In many Western countries, dairy products are a major source of dietary protein, and two recent meta-analyses have shown that intake of dairy products combined with energy restriction, but not combined with ad libitum diets, may favour weight loss( Reference Abargouei, Janghorbani and Salehi-Marzijarani 12 , Reference Chen, Pan and Malik 13 ). Casein and whey are the major proteins found in dairy products. They differ in the way they are digested and absorbed. Whey is absorbed rapidly and induces a high transient rise in the levels of plasma amino acids, whereas casein coagulates in the acidic environment in the stomach, delaying gastric emptying with a subsequent slower and prolonged rise in the levels of plasma amino acids( Reference Boirie, Dangin and Gachon 14 ). Moreover, whey and casein differ in their amino acid composition, and some studies have found that whey is more satiating than casein( Reference Hall, Millward and Long 9 , Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 15 ), but the results have been inconsistent( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 , Reference Bendtsen, Lorenzen and Bendsen 8 , Reference Lorenzen, Frederiksen and Hoppe 16 ). Likewise, there is no clear evidence that one dairy protein is superior to the other when their effects are examined on EE( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 , Reference Bendtsen, Lorenzen and Bendsen 8 , Reference Lorenzen, Frederiksen and Hoppe 16 , Reference Alfenas, Bressan and Paiva 17 ).
The absorption rate of proteins can be manipulated through exogenous hydrolysis, and the absorption rate of hydrolysed casein has been found to be more rapid than that of intact casein and to approach the absorption rate of whey( Reference Calbet and Holst 18 , Reference Koopman, Crombach and Gijsen 19 ). Hydrolysis of proteins, especially casein, may consequently affect metabolic parameters. A recent study in mice has demonstrated that consumption of hydrolysed casein reduced body weight and fat mass, partly through increased β-oxidation in white adipose tissue, compared with consumption of intact casein( Reference Lillefosse, Tastesen and Du 20 ). Moreover, it has been observed that hydrolysed casein tend to induce a lower respiratory quotient (RQ) than intact casein( Reference Lillefosse, Tastesen and Du 20 ). To our knowledge, the satiating effects of casein and its hydrolysate on appetite and EE have not been previously compared in human subjects, while few studies have investigated the satiating effects of whey and its hydrolysate. From these studies, it has been concluded that hydrolysed and intact whey induce similar effects on appetite( Reference Akhavan, Luhovyy and Brown 21 , Reference Anderson, Tecimer and Shah 22 ), which is supported by similar absorption rates found by Calbet & Holst( Reference Calbet and Holst 18 ). However, this finding is not consistent, as Morifuji et al. ( Reference Morifuji, Ishizaka and Baba 23 ) found that hydrolysed whey is absorbed more rapidly than intact whey. Therefore, whether whey, casein and its hydrolysate have different effects on appetite regulation and EE, as well as the potential mechanisms underlying such possible differences remain to be elucidated.
The objective of the present study was to compare the effects of hydrolysed casein, intact casein and intact whey on EE and appetite regulation, and thereby to investigate the influence of amino acid composition and the rate of absorption. On the basis of the expected differences in absorption rates, we hypothesised that whey and hydrolysed casein would be more satiating and have a greater effect on EE shortly after protein consumption, whereas intact casein would be more satiating and have a greater thermogenic effect several hours after protein consumption. Moreover, we hypothesised that whey would have a more satiating effect than hydrolysed casein due to its higher protein quality. Finally, we expected that consumption of hydrolysed casein would result in greater lipid oxidation than consumption of intact casein, as observed previously in mice( Reference Lillefosse, Tastesen and Du 20 ).
Subjects and methods
Subjects
A total of thirty-six overweight to moderately obese (BMI 27–35 kg/m2) men and women aged 22–40 years were recruited for the study by advertisements at local educational institutions and through web pages from October 2012 to July 2013. All subjects participated in a medical screening, and thirty-three subjects were selected for the study based on the following criteria: healthy; no food allergies; non-vegetarians; non-smokers; no drug abuse; low-to-moderate alcohol consumption; physically active < 10 h/week; no use of medication (except for contraceptives); no blood donation 3 months before the study; no participation in other clinical studies 4 weeks before the study; Hb level >8 mmol/l; fasting blood glucose concentration < 6·1 mmol/l; non-pregnant and non-lactating women. Moreover, all subjects had a stable weight at baseline ( ± 3 kg within the previous 3 months), and were instructed not to change their dietary patterns and activity levels throughout the study period. Of the total subjects, seven dropped out between the screening period and the initiation of the study as they could not fit the experimental visits into their schedules, and two were excluded at their first visit due to illness. Finally, twenty-four subjects completed the study. The baseline characteristics of the study participants are presented in Table 1.
Table 1 Characteristics of the study subjects at baseline (Mean values and standard deviations, n 24)
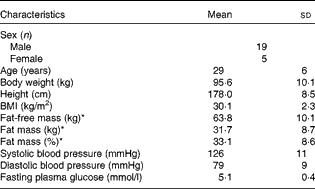
* Body composition was determined by dual-energy X-ray absorptiometry.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki II, and all procedures involving human subjects were approved by the Danish National Committee on Health Research Ethics. Written informed consent was obtained from all subjects after verbal and written information about the study procedures. The study was registered at ClinicalTrials.gov as NCT01677273.
Experimental design
The present study was a randomised, double-blind, three-way cross-over trial. All men were tested with at least 2 weeks between the visits, whereas women had to be in the same phase of their menstrual cycle at each visit. The subjects received the following three dietary treatments in a random and balanced order: (1) hydrolysed casein (HC, Hyvital Casein CMA 500; Friesland Campina Domo) with a degree of hydrolysis of 37 %; (2) intact casein (IC, Miprodan® 30; Arla Foods Amba); (3) intact whey (IW, Lacprodan® SP-9225 Instant; Arla Foods Ingredients).
Each intervention visit lasted 40 h, during which the subjects spent 25 h in a respiration chamber (Fig. 1). The subjects were asked to consume a standardised meal (17 % of energy (E%) from protein, 50 E% from CHO and 33 E% from fat) at home no later than 20.00 hours and to arrive at the metabolic research unit at 22.00 hours on day 0. They slept in the respiration chamber (with doors opened) to acclimatise to the environment. At 07.30 hours on day 1, the subjects were awakened and weighed after emptying their bladder in order to confirm weight stability during the study. Moreover, a Venflon catheter (Venflon™ Pro I.V. Cannula; Becton Dickinson) was placed in an antecubital vein for blood sampling at the following time points after 09.30 hours on day 1: 0, 15, 30, 60, 90, 120, 180 and 240 min. At 08.30 hours on day 1, the respiration chamber was closed to ensure that the subjects were in a steady state when measurements were started at 09.30 hours. The chamber remained closed until 09.30 hours on day 2. During the stay in the respiration chamber, subjective appetite assessments, biochemical measures, respiratory gas exchange measures and 24 h urine collection were obtained as described in detail below. Blood samples for biochemical measures were collected through airtight locks. On day 2, the subjects were served breakfast at 09.30 hours, and appetite was assessed until the intervention visit ended with an ad libitum lunch meal at 12.30 hours.
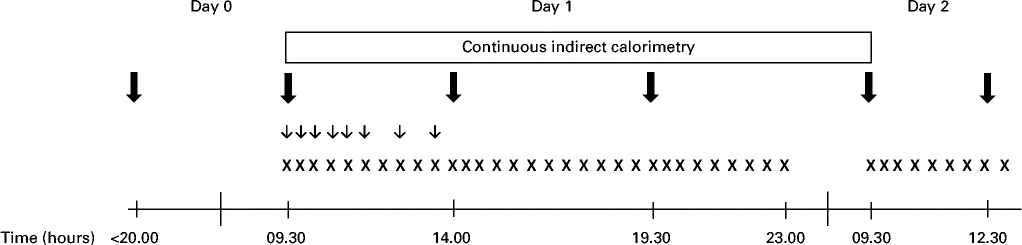
Fig. 1 Experimental design. All twenty-four subjects made three visits during the study period. ![]() , Meals/proteins;
, Meals/proteins; ![]() , biochemical measures;
, biochemical measures; ![]() , visual analogue scales.
, visual analogue scales.
Physical examination
Physical examination conducted at the screening visit included measurements of body weight, height and blood pressure. Body weight was recorded to the nearest 0·1 kg (Lindeltronic 8000S; Lindells), and height was measured to the nearest 0·5 cm with a wall-mounted stadiometer. Body composition was determined by dual-energy X-ray absorptiometry (Lunar Radiation Company). Systolic and diastolic blood pressure measurements were recorded by an automatic device (A & D Instruments Limited) after a 5–10 min rest in a supine position. All measurements were made with subjects wearing only underwear and after a 12 h fast.
Diets
All meals served included a protein shake containing one of the three dietary treatments of interest: HC; IC; IW. Energy density, macronutrient composition and fibre content were similar between the three dietary treatments. Daily energy requirements (ER) were calculated based on fat-free mass( Reference Cunningham 24 ), with a physical activity factor of 1·4 representing a sedentary lifestyle.
The subjects received 3 g/MJ required/d of added protein (HC, IW or IC) in each shake (corresponding to approximately 30 g of protein per shake in a diet containing 10 MJ). Each shake contained 26 E% from protein, where HC, IW or IC contributed to 22·3 E% in the respective shakes. The amino acid composition of the three proteins is presented in Table 2. Unfortunately, it was not possible to use a casein hydrolysate manufactured from the intact casein provided, and analyses showed that the amount of some of the potentially important amino acids deviated between HC and IC. Consequently, single free amino acids (leucine, phenylalanine, proline, tryptophan and tyrosine) were added to HC in accordance with a predetermined cut-off limit of ± 10 % deviation between the proteins. On day 1, the subjects were served three meals at 09.30, 14.00 and 19.30 hours (Fig. 1), contributing to 20, 40 and 40 % of ER, respectively. For breakfast, the subjects were served a protein shake (26 E% from protein, 50 E% from CHO and 24 E% from fat), but for lunch (18 E% from protein, 57 E% from CHO and 25 E% from fat) and dinner (17 E% from protein, 58 E% from CHO and 25 E% from fat), the subjects were provided with additional standardised foods to meet their ER. The subjects were not allowed to drink water ad libitum, but 200 ml of water were served with all meals and, in addition, three times during day 1. On day 2, the subjects were served a protein shake for breakfast (identical to day 1) and an ad libitum lunch meal (15 E% from protein, 55 E% from CHO and 30 E% from fat) at 12.30 hours, during which period the subjects were asked to eat until they felt comfortably satiated.
Table 2 Amino acid composition of hydrolysed casein (HC), intact casein (IC) and intact whey (IW)*
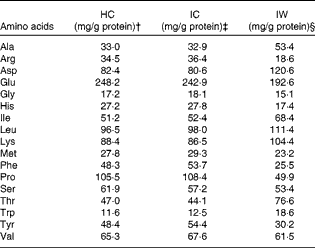
* Single free amino acids (Leu, Phe, Pro, Trp and Tyr) were added to HC to match the amino acid composition of IC.
† 80·2 % protein.
‡ 87·9 % protein.
§ 86·2 % protein.
Energy expenditure and substrate oxidation
EE was calculated from respiratory gas exchange measured in two identical respiration chambers( Reference Astrup, Thorbek and Lind 25 ). The respiration chambers are rooms measuring 14·7 m3 equipped with a bed, desks, chairs, telephone, television, computer and toilet facilities. The room temperature was kept constant at 22·5°C. Gas exchange was calculated from measured airflow and concentrations of O2 and CO2 at the outlet of the chamber as well as in the fresh air going in. Urine was quantitatively collected for 24 h in containers with HCl, and protein oxidation was calculated from the amount of urinary N multiplied by 6·25.
Measurements of 24 h total EE, RQ and substrate oxidation were made starting from 09.30 hours on day 1 until 09.30 hours on day 2, and presented as postprandial effects after the breakfast meal on day 1 (09.30–14.00 hours) during daytime (09.30–01.00 hours), during night-time (01.00–06.00 hours) and over the total 24 h period. BMR was assessed from 08.00 to 09.00 hours on day 2, and sleeping metabolic rate was determined from 01.00 to 06.00 hours on day 2. The subjects were told to avoid physical activity, but spontaneous physical activity was measured by a microwave radar based on the Doppler principle (Sisor Mini-Radar; Static Input System). Substrate oxidation was calculated from 24 h urinary N, O2 consumption and CO2 production. CHO and lipid oxidation were calculated according to the method described by Elia & Livesey( Reference Elia and Livesey 26 ).
Appetite assessment and energy intake
Visual analogue scales were used to assess subjective appetite sensations as well as palatability of meals. The scales were based on a series of questions presented individually on sheets in a visual analogue scale booklet. They consisted of a 100 mm horizontal line with words anchored at each end, expressing the most positive and the most negative ratings of hunger, satiety, fullness and prospective food consumption, and desire to eat something fatty, salty, sweet or savoury, and, finally, the palatability of the test meals. On day 1, visual analogue scales were filled out every 30 min as well as after each meal (09.40 hours (10 min), 14.15 hours (285 min) and 19.40 hours (610 min)) from 09.30 to 23.00 hours. On day 2, they were filled out every 30 min and after each meal (09.40 hours and after the ad libitum meal) from 09.30 to 12.30 hours.
Energy intake was assessed from an ad libitum lunch meal served at 12.30 hours on day 2. The meal consisted of spaghetti Bolognese (15 E% from protein, 55 E% from CHO and 30 E% from fat). The subjects were instructed to eat until they felt comfortably satiated. The time spent was self-determined, but registered for the analysis of data.
Biochemical measures
Blood plasma or serum samples were analysed to measure the concentrations of insulin, glucose, total glucagon-like peptide-1 (GLP-1) and NEFA.
Blood samples were drawn into 2 ml sodium fluoride–oxalate tubes and 2 ml serum clot activator tubes (Vacuette; Hettich) for analyses of glucose and insulin concentrations, respectively. For analysis of GLP-1 concentration, blood samples were collected into 6 ml EDTA tubes, to which 300 μl dipeptidyl peptidase IV inhibitor were added. For analysis of NEFA concentration, blood samples were collected into 4 ml EDTA tubes. The glucose, GLP-1 and NEFA samples were immediately centrifuged for 10 min at 4°C at 2500 rpm, pipetted into 2 ml cryotubes and stored at − 80°C until further analysis. Before centrifugation, insulin samples were allowed to coagulate for 20–30 min. Serum insulin concentration was quantified by a solid-phase, two-site chemiluminescent immunometric assay (Siemens Healthcare Diagnostics) on an Immulite 1000 analyser. Concentrations of glucose and NEFA were analysed by ABX Pentra 400 (Horiba ABX) and total GLP-1 concentration was analysed by RIA (antiserum no. 89390)( Reference Orskov, Rabenhoj and Wettergren 27 ).
Finally, 24 h urinary N was determined by the Dumas combustion method, using a VarioMax CN analyser (Elementar).
Statistical analyses
Power calculations were based on previous studies examining protein-induced changes in EE( Reference Mikkelsen, Toubro and Astrup 11 , Reference Hochstenbach-Waelen, Veldhorst and Nieuwenhuizen 28 , Reference Hochstenbach-Waelen, Westerterp-Plantenga and Veldhorst 29 ). With a sensitivity of 0·80 and a significance level of 0·05, power calculation indicated a sample size of twenty-four when the minimal detectable difference was set to 0·22 with a within-subject standard deviation of 0·25.
Data are presented as means with their standard errors, unless otherwise specified.
Repeated-measures analyses were conducted using linear mixed models to examine the differences between proteins in relation to EE, RQ, substrate oxidation, biochemical measures and subjective appetite. All analyses included adjustment for age, sex and order of intervention, and subjects were included as random effects. Moreover, in the analysis of EE, RQ and substrate oxidation, respiration chambers were additionally included as random effects, and analyses were also controlled for spontaneous physical activity. A two-way ANOVA was used to examine the differences (means with their standard errors) in BMR and ad libitum energy intake between the dietary treatments. Furthermore, associations between insulin and glucose, insulin and GLP-1, insulin and lipid oxidation and NEFA and lipid oxidation were explored using Pearson's correlation coefficients.
Repeated-measures analyses were also used to analyse the data on EE, RQ, substrate oxidation, biochemical measures and subjective appetite in the postprandial period after the breakfast meal on day 1 (09.30–14.00 hours). Moreover, data on EE, RQ and substrate oxidation were analysed during daytime (09.30–01.00 hours), during night-time (01.00–06.00 hours) and over the total 24 h period. BMR values were analysed from 08.00 to 09.00 hours on day 2, and data on subjective appetite were additionally analysed during daytime (09.30–23.00 hours) and after the breakfast meal on day 2 (09.30–12.45 hours).
Finally, peak magnitude and time to peak were analysed to determine the levels of glucose, insulin and GLP-1, and nadir magnitude and time to nadir were analysed to determine the levels of glucose and NEFA.
All statistical models were checked for normality and variance homogeneity, and all data that were non-normally distributed were transformed. Moreover, all multiple pairwise comparisons were Bonferroni-adjusted.
Statistical analyses were performed using the STATA software, version 11.2 (StataCorp LP, 2011), and statistical significance was set at P< 0·05.
Results
Energy expenditure
The results revealed that 24 h EE did not differ from 24 h energy intake (10 396 (sem 255) kJ/24 h) and between the dietary treatments (Table 3), which indicates that the subjects remained in energy balance during all the visits. Likewise, there was no difference between the dietary treatments in relation to either the postprandial EE after consumption of the breakfast meal on day 1, daytime EE, sleeping metabolic rate, or BMR after adjustment for spontaneous physical activity (Table 3).
Table 3 Energy expenditure (Mean values with their standard errors)
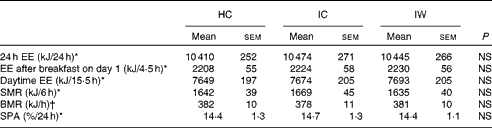
HC, hydrolysed casein; IC, intact casein; IW, intact whey; EE, energy expenditure; SMR, sleeping metabolic rate; SPA, spontaneous physical activity.
* Data were analysed by a repeated-measures analysis using a mixed linear model.
† BMR was analysed by a two-way ANOVA (n 24).
Respiratory quotient and substrate oxidation
Total protein oxidation in relation to total EE over 24 h was similar between the dietary treatments (HC 22·2 (sem 0·7) %, IC 21·5 (sem 0·8) % and IW 22·1 (sem 0·7) %).
There was no effect observed for the time × treatment interaction on the RQ at either time interval studied (after breakfast: P= 0·42; daytime: P= 0·69; 24 h: P= 0·58; night-time: P= 0·22); however, an effect of treatment was observed during daytime (P= 0·048) and after the breakfast meal (P= 0·029) on day 1. The mean postprandial RQ value was 0·02 units higher after consumption of HC than after consumption of IW (P= 0·036) during daytime (Fig. 2(a)), as well as during the time after the breakfast meal (0·02 units, P= 0·018) on day 1 (Fig. 2(b)), when the food was provided, and these results remained significant after adjustment for 24 h energy balance. There was no difference in RQ between the dietary treatments during night-time and when calculated over the total 24 h period.
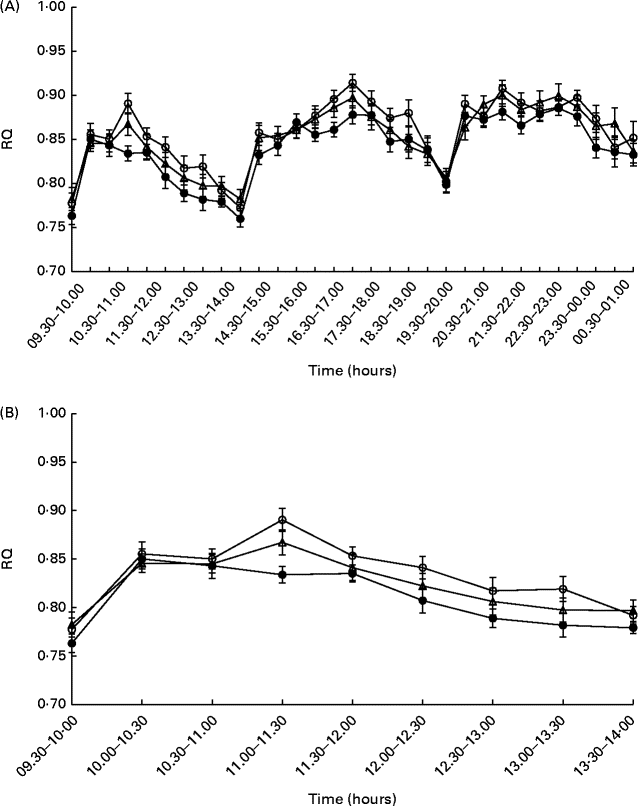
Fig. 2 Postprandial respiratory quotient (RQ) values during daytime (A) and after the breakfast meal (B) on day 1 (n 24). Values are means, with their standard errors represented by vertical bars. Repeated-measures analyses showed no effect for the time × treatment interactions (after breakfast: P= 0·42; daytime: P= 0·69), but the RQ value was higher for hydrolysed casein (![]() ) than for intact whey (
) than for intact whey (![]() ) during daytime (P= 0·036), as well as during the time after the breakfast meal on day 1 (P= 0·018).
) during daytime (P= 0·036), as well as during the time after the breakfast meal on day 1 (P= 0·018). ![]() , Intact casein.
, Intact casein.
Accordingly, there was no effect observed for the time × treatment interaction on either lipid (after breakfast: P= 0·50; daytime: P= 0·72; 24 h: P= 0·53; night-time: P= 0·16) or CHO oxidation (after breakfast: P= 0·46; daytime: P= 0·70; 24 h: P= 0·53; night-time: P= 0·16) at all time intervals studied. However, an effect of treatment was observed on lipid oxidation during daytime and after the breakfast meal on day 1 (after breakfast: P= 0·008; daytime: P= 0·014). Postprandial lipid oxidation was 13 % higher after ingestion of IW than after ingestion of HC during daytime (P= 0·036) and 15 % higher after ingestion of the breakfast meal on day 1 (P= 0·015). There was no difference between the HC and IC treatments or between the IW and IC treatments (Fig. 3). Accordingly, an effect of treatment was observed on CHO oxidation after the breakfast meal on day 1 (P= 0·041). It was 18 % lower after ingestion of IW than after ingestion of HC, but did not differ between the HC and IC treatments or between the IW and IC treatments (Fig. 3). There was no difference in substrate oxidation during night-time and during the 24 h period between the dietary treatments.
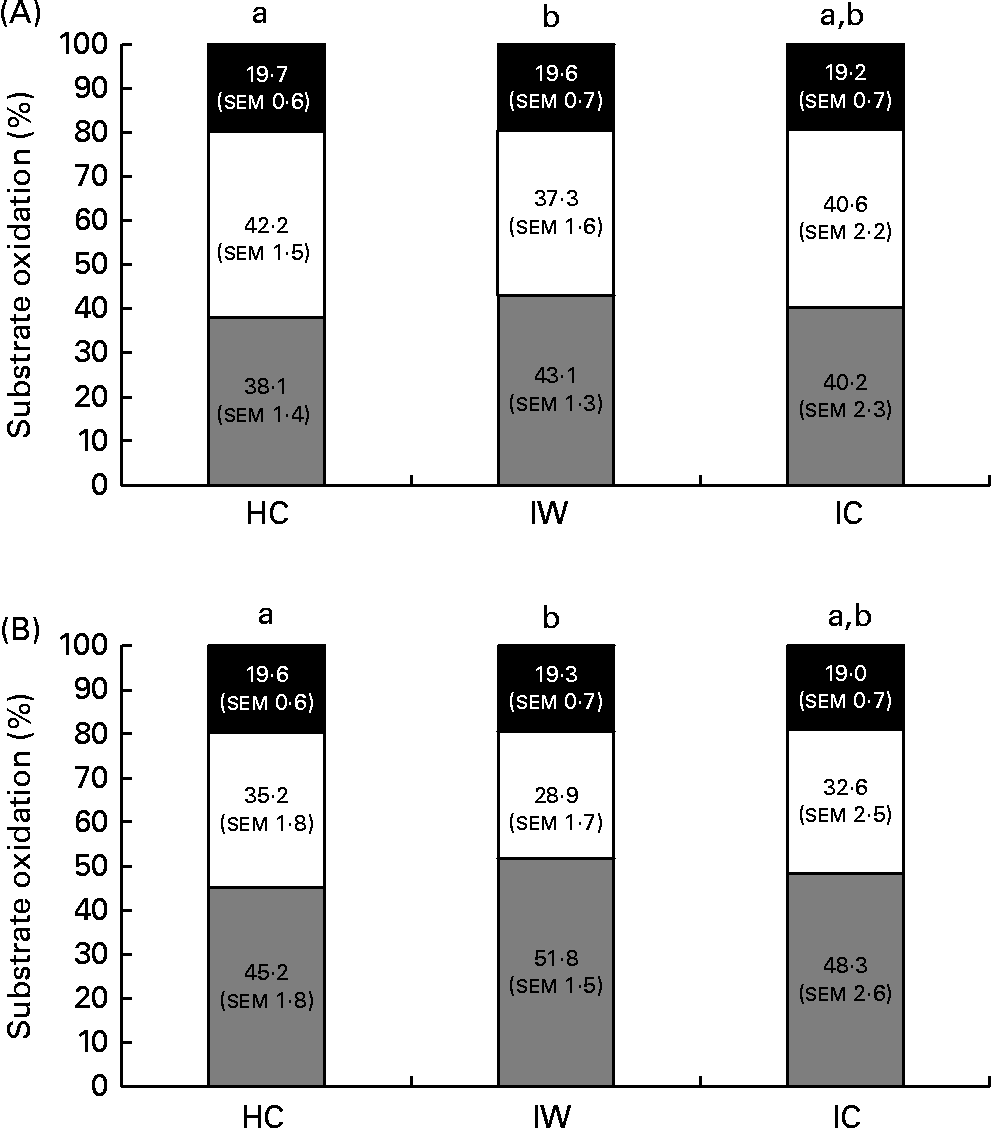
Fig. 3 Substrate oxidation during daytime (A) and after the breakfast meal (B) on day 1 (n 24). There was no difference in protein oxidation between the dietary proteins. Repeated-measures analyses showed that intact whey (IW) stimulated larger lipid oxidation and smaller carbohydrate oxidation than hydrolysed casein (HC) during daytime as well as during the time after the breakfast meal on day 1. There was no difference between the HC and intact casein (IC) treatments or between the IW and IC treatments. Values are means, with their standard errors. a,bMean values with unlike letters were significantly different (P< 0·05). ![]() , Lipid; □, carbohydrate; ■, protein.
, Lipid; □, carbohydrate; ■, protein.
Biochemical measures
There were no significant differences in baseline values for serum insulin and plasma glucose, NEFA and GLP-1 concentrations. Likewise, no effects of the time × treatment interaction were observed for postprandial serum insulin, plasma glucose or NEFA concentrations (Fig. 4). However, the overall postprandial plasma glucose concentration was higher after ingestion of IC than after ingestion of HC (P= 0·033), with no difference being observed between the HC and IW treatments and between the IW and IC treatments. Moreover, the difference in plasma glucose concentration was not explained by the difference in serum insulin concentration, which was similar after ingestion of all the three dietary treatments. Accordingly, there were no significant differences observed between the dietary treatments in relation to peak magnitude and time to peak for glucose and insulin concentrations, as well as no significant differences between the dietary treatments in relation to nadir magnitude and time to nadir were observed for glucose concentration. On the contrary, nadir magnitude for NEFA concentration was lower after consumption of HC than after consumption of IW (P< 0·001) and IC (P= 0·003), with no significant difference in time to nadir. Furthermore, the mean postprandial concentration of NEFA was higher after consumption of IW than after consumption of HC (P= 0·003) and IC (P< 0·0001), with no difference being observed between the HC and IC treatments (Fig. 4). No overall effect of treatments was observed for GLP-1 concentration, but a time × treatment interaction effect was found (P= 0·012). At 15 min, GLP-1 concentration was higher after ingestion of IC than after ingestion of HC and IW (P< 0.05) with no difference being observed between the HC and IW treatments. GLP-1 concentration was higher after consumption of HC than after consumption of IW at 60 and 90 min (P= 0·042 and P= 0·015, respectively) and higher after consumption of HC than after consumption of IC at 90 min (P= 0·012). It did not differ between the IW and IC treatments (Fig. 4), and there were no significant differences observed between the dietary treatments in relation to peak magnitude and time to peak for GLP-1 concentration.
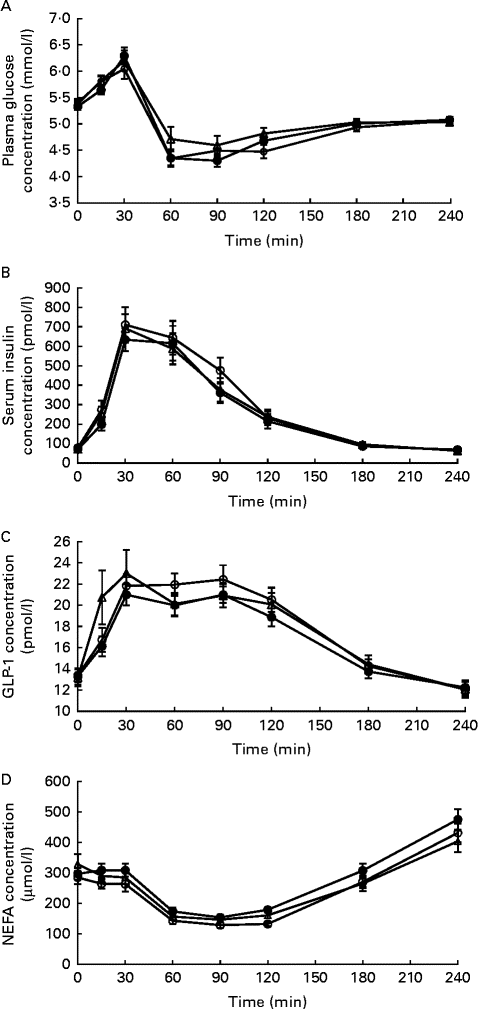
Fig. 4 Response of (A) glucose, (B) insulin, (C) glucagon-like peptide 1 (GLP-1) and (D) NEFA concentrations after ingestion of the breakfast meal on day 1 (0–240 min; n 24). Glucose concentrations were higher after ingestion of intact casein (IC, ![]() ) than after ingestion of hydrolysed casein (HC (
) than after ingestion of hydrolysed casein (HC (![]() ); P= 0·033); however, there was no difference between the HC and intact whey (IW,
); P= 0·033); however, there was no difference between the HC and intact whey (IW, ![]() ) treatments or between the IW and IC treatments. No difference was observed in insulin concentrations between the dietary treatments. For GLP-1, there was a significant effect of the time × treatment interaction. At 15 min, GLP-1 concentration was higher after ingestion of IC than after ingestion of HC and IW (P< 0·05), with no difference being observed between the HC and IW treatments. However, at 60 and 90 min, GLP-1 concentration was higher after ingestion of HC than after ingestion of IW and IC (P< 0·05 and P< 0·01, respectively), but did not differ between the IW and IC treatments. There were no significant differences observed at any other time points. The concentration of NEFA was higher after ingestion of IW than after ingestion of HC (P= 0·003) and IC (P< 0·0001), with no difference being observed between the HC and IC treatments.
) treatments or between the IW and IC treatments. No difference was observed in insulin concentrations between the dietary treatments. For GLP-1, there was a significant effect of the time × treatment interaction. At 15 min, GLP-1 concentration was higher after ingestion of IC than after ingestion of HC and IW (P< 0·05), with no difference being observed between the HC and IW treatments. However, at 60 and 90 min, GLP-1 concentration was higher after ingestion of HC than after ingestion of IW and IC (P< 0·05 and P< 0·01, respectively), but did not differ between the IW and IC treatments. There were no significant differences observed at any other time points. The concentration of NEFA was higher after ingestion of IW than after ingestion of HC (P= 0·003) and IC (P< 0·0001), with no difference being observed between the HC and IC treatments.
Serum insulin concentration was correlated with plasma GLP-1 (r 0·3042, P< 0·0001) and glucose (r 0·3519, P< 0·0001) concentrations. Moreover, NEFA and insulin concentrations were positively (r 0·4894, P< 0·0001) and negatively (r − 0·1813, P= 0·0002) correlated, respectively, with lipid oxidation.
Appetite
There were no effects observed for the time × treatment interaction and for treatment on satiety, hunger, fullness and prospective food consumption during daytime, as well as during the time after the breakfast meal on day 1 or day 2. Thirst was also not affected differently by the three dietary treatments at any time intervals studied. However, IC elicited a lower desire to eat savoury foods than IW during daytime (P= 0·006), and tended to elicit a lower desire to eat savoury foods after ingestion of the breakfast meal on day 1 (P= 0·075). There was no difference observed between the HC and IC treatments or between the HC and IW treatments, as well as between any of the treatments after the breakfast meal on day 2. IC also elicited a lower desire to eat sweet foods than HC after ingestion of the breakfast meal on day 1 (P= 0·015) and day 2 (P= 0·021). Moreover, IW tended to induce a lower desire to eat sweet foods than HC after ingestion of the breakfast meal on day 2 (P= 0·087). However, there was no difference observed between the dietary treatments during daytime. Likewise, there was no difference observed between the dietary treatments in relation to the desire to eat salty or fatty foods at any of the time intervals studied.
Moreover, there was no difference in energy intake observed between the dietary treatments after the ad libitum lunch meal on day 2 (HC: 3571 (sem 218) kJ; IW: 3780 (sem 216) kJ; IC: 3667 (sem 180) kJ), and palatability assessed after the ad libitum meal did not depend on the dietary treatments.
The palatability of the test meals was rated lower after consumption of HC than after consumption of IW and IC (P< 0·01), and an effect of the time × treatment interaction was found for feelings of comfort (P= 0·038). After breakfast, the subjects felt less comfortable after consumption of HC than after consumption of IW at 10, 30 and 60 min (P< 0·01), and tended to feel less comfortable at 150 min (P= 0·075). Moreover, immediately after the breakfast meal on day 1 (10 min), the subjects felt less comfortable after consumption of HC than after consumption of IC (P= 0·003). There was no difference in feelings of comfort observed between the IW and IC treatments during daytime, as well as during the time after the breakfast meal on day 1; however, the subjects tended to feel less comfortable after consumption of HC than after consumption of IW (P= 0·093).
Discussion
The present study showed that whey, casein and hydrolysed casein induced similar effects on appetite regulation and on 24 h total and postprandial EE after ingestion of high-protein mixed meals. However, we showed that the RQ value was lower after consumption of whey than after consumption of hydrolysed casein, also after adjustment for energy balance. This might indicate higher lipid oxidation after ingestion of whey than after ingestion of hydrolysed casein, which was supported by the response of NEFA concentration.
Proteins are more satiating than CHO and fat( Reference Astrup 2 , Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 , Reference Westerterp 30 ), and Belza et al. ( Reference Belza, Ritz and Sorensen 1 ) recently showed that proteins stimulate satiety in a dose-dependent manner. However, little is known about the effects of different protein sources. Proteins differ in their amino acid composition, which may influence their effects on satiety. Casein and whey are both complete proteins, but whey has a higher content of branch-chained amino acids and contains the components glycomacropeptide and α-lactoglobulin, which have previously been found to stimulate satiety( Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 31 – Reference Hursel, van der Zee and Westerterp-Plantenga 33 ). Despite this finding, there is no clear evidence that whey is more satiating than casein or vice versa( Reference Bendtsen, Lorenzen and Bendsen 8 ). In the present study, no difference was observed between the whey, casein and hydrolysed casein treatments, which is supported by several human studies( Reference Lorenzen, Frederiksen and Hoppe 16 , Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 32 , Reference Bowen, Noakes and Trenerry 34 ). However, the results have been inconsistent. Hall et al. ( Reference Hall, Millward and Long 9 ) found that whey stimulates satiety compared with casein during 180 min following ingestion. Accordingly, they observed a higher plasma concentration of the appetite-regulating hormones cholecystokinin, GLP-1 and glucose-dependent insulinotropic polypeptide after consumption of whey. This finding is partly supported by Veldhorst et al. ( Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 15 ), who demonstrated a decreased feeling of hunger after consumption of whey compared with casein at a protein dose of 10 E%. However, they observed no difference at a dose of 25 E%. They suggested that the inconsistent results may be explained by the difference in protein concentration, as the concentration of certain amino acids may need to be above a particular threshold to promote a relatively stronger hunger suppression or greater satiety. Their results suggested that certain proteins will reach these threshold concentrations at lower doses than other proteins. At high-protein loads, it may not be possible to distinguish between different complete proteins as the concentration of all amino acids rapidly increases above this threshold. In the present study, the percentage of energy from proteins varied from 17 to 26 E%, suggesting that the protein load may be too high to detect a difference between the proteins. However, other factors may play a role. For example, Acheson et al. ( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 ) found that casein is more satiating than whey at a protein content of 50 E%. They continued their measurements for 330 min, which indicated that timing of measurements may also play an important role. In support, Boirie et al. ( Reference Boirie, Dangin and Gachon 14 ) showed that plasma amino acid concentrations are higher after consumption of whey than after consumption of casein at 100 min and higher after consumption of casein than after consumption of whey at 300 min. This suggests that the effects of casein may not be fully developed when appetite measures are obtained shortly (180 min) after protein consumption as in the study by Hall et al. ( Reference Hall, Millward and Long 9 ), and that the concentration of amino acids after consumption of whey may have returned to baseline levels when the measurements are continued for 330 min. Accordingly, we expected that whey and hydrolysed casein would have a greater satiating effect than casein shortly after protein consumption, and that casein would be more satiating several hours after consumption of proteins. Moreover, we expected that gastric emptying and the rate of absorption would influence the effect on EE in a similar way.
We observed no differences between the dietary proteins in relation to postprandial or 24 h total EE. In support of this finding, Lorenzen et al. ( Reference Lorenzen, Frederiksen and Hoppe 16 ) also did not find a difference in DIT between whey and casein; however, the results have been inconsistent( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 , Reference Alfenas, Bressan and Paiva 17 ). Previously it has been shown that when whey and casein are served in mixed meals, it may mask the differences in kinetics, mostly due to a slower absorption rate of whey( Reference Dangin, Guillet and Garcia-Rodenas 35 ). Therefore, this may explain the reason why we observed no differences in appetite regulation and EE between the dietary proteins. Moreover, it can be speculated that the differences in kinetics may be masked by other proteins present in the shakes/meals. However, they only make up a very small amount of total protein (2·7 E% of 26 E% in the shakes) and were also similar between the dietary treatments, why it is unlikely to affect the present results. However, other factors may play important roles such as those suggested by Acheson et al. ( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 ) who found that consumption of whey induced a larger increase in DIT and cumulative EE at 330 min than consumption of casein, although the proteins were served as shakes that additionally contained fat and CHO. The authors suggested that the higher DIT observed following consumption of whey may be explained by the beneficial effect on protein synthesis. Boirie et al. ( Reference Boirie, Dangin and Gachon 14 ) showed that protein synthesis was 2-fold more rapid, measured at 40–140 min, after consumption of whey than after consumption of casein. In contrast to the present study and the study by Lorenzen et al. ( Reference Lorenzen, Frederiksen and Hoppe 16 ) where casein was served as a caseinate, in the study by Acheson et al. ( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 ), casein was served as micellar casein. Caseinates are absorbed more rapidly than the micellar form, which may explain why the difference in absorption rates between proteins used in the present study may be very small.
Furthermore, comparison of studies may be hampered by the use of different methodologies, timings of measurements, energy contents and proportions of protein in the meals served. The majority of studies investigating the effects of protein on EE have measured the thermogenic effect after one single meal using ventilated hood systems, whereas the present study used respiration chambers. It can be speculated that the ‘dead space’ of the chambers (14·7 m3) may be too large to allow the detection of small and transient changes in EE. However, respiration chambers were also used in the study by Acheson et al. ( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 ), but these chambers may have very different response times. Moreover, as DIT is only 10 % of the total EE, it can be speculated that a rather large meal is necessary to induce a detectable increase in postprandial EE. The meal size in the study by Acheson et al. ( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 3 ) was similar to the breakfast meal in the present study (20 % of ER), but the protein content was much greater (50 E%).
To our knowledge, no human studies have compared the effects of casein and its hydrolysate on EE or appetite regulation; however, a recent study in mice has shown that intact casein and hydrolysed casein induce similar effects on EE( Reference Lillefosse, Tastesen and Du 20 ). The authors also examined the effect on the RQ and showed that hydrolysed casein tends to induce a lower RQ than intact casein during light periods (07.30–19.00 hours), indicating that the absorption rate may play an important role. In the present study, no difference in RQ was found between hydrolysed casein and intact casein; however, we found that hydrolysed casein induced a slightly higher mean RQ value (0·86 (sem 0·01) v. 0·84 (sem 0·01), respectively) than whey during daytime when food was provided, and these results remained significant after adjustment for energy balance. A higher RQ value suggests higher CHO and lower lipid oxidation, but could also indicate higher amino acid oxidation, which would drive the RQ value to 0·8( Reference Weir 36 ). A limitation of the present study was that there was no continuous measurement of protein oxidation. Only the data on the average 24 h protein oxidation were obtained from 24 h N excretion. However, data on the RQ and substrate oxidation were analysed over rather long postprandial time intervals ( ≥ 4·5 h post-meal consumption), and based on previous findings( Reference Boirie, Dangin and Gachon 14 , Reference Calbet and Holst 18 ), we expected that amino acids, from all the three dietary proteins, would be absorbed within this time period.
Furthermore, data on the RQ and substrate oxidation were supported by the response of plasma NEFA concentrations. NEFA concentrations were positively correlated with lipid oxidation and have previously been associated with a lower RQ value( Reference Toubro, Sorensen and Hindsberger 37 ) and lower glucose oxidation( Reference Roden, Price and Perseghin 38 ). In accordance with a lower RQ value after consumption of whey, the response of NEFA concentration was increased compared with hydrolysed casein. Furthermore, insulin is known to suppress lipolysis( Reference Nurjhan, Campbell and Kennedy 39 ). Therefore, it can be speculated that insulin may have a modulating effect on lipolysis and consequently lipid oxidation. In the present study, insulin was weakly correlated with lipid oxidation estimated from the RQ; however, we observed no differences in postprandial response of insulin concentration between the dietary proteins. Data on the differences in the insulinotropic effects of whey and casein in healthy human subjects are sparse; however, some studies have suggested a beneficial effect of whey( Reference Holmer-Jensen, Mortensen and Astrup 40 , Reference Gunnerud, Holst and Ostman 41 ). Moreover, it has been previously demonstrated that the response of insulin concentration is associated with the availability of plasma amino acids( Reference Morifuji, Ishizaka and Baba 23 , Reference Gunnerud, Ostman and Bjorck 42 ), with rapidly absorbed proteins inducing a greater response of insulin concentration than slowly absorbed proteins. Accordingly, Deglaire et al. ( Reference Deglaire, Fromentin and Fouillet 43 ) showed a higher insulin response after ingestion of hydrolysed casein than after ingestion of casein, especially during the initial postprandial hours when plasma amino acids were higher.
In contrast, Akhavan et al. ( Reference Akhavan, Luhovyy and Brown 21 ) investigating the effects of intact and hydrolysed whey on glucose and insulin concentrations found that intact whey stimulates a lower postprandial glucose concentration than hydrolysed whey, despite similar insulin concentrations. This indicates that intact proteins regulate blood glucose levels in a non-insulinotropic manner. A higher blood glucose concentration following consumption of hydrolysed whey may stimulate a higher RQ value, which was not measured. This also suggests that gastric emptying may affect the magnitude and timing of postprandial blood glucose and insulin increases and that intact whey is emptied more slowly than hydrolysed whey because whey, but not hydrolysed whey, stimulates the secretion of gut peptides that are involved in glycaemic control and gastric emptying. This is though not a consistent finding. Calbet & Holst( Reference Calbet and Holst 18 ) showed that whey and casein, as well as their hydrolysates, stimulate the secretion of incretins. Likewise, we showed that at some time points, GLP-1 concentrations were higher after consumption of hydrolysed casein than after consumption of intact casein and intact whey. In contrast, there was no overall difference in GLP-1 concentration between the dietary proteins, which is in accordance with the data on insulin and appetite regulation. Data from previous studies investigating the effect of different protein sources are inconsistent( Reference Hall, Millward and Long 9 , Reference Calbet and Holst 18 , Reference Nilsson, Stenberg and Frid 44 ). For example, Hall et al. ( Reference Hall, Millward and Long 9 ) observed an increase in GLP-1 concentration after consumption of whey than after consumption of casein in accordance with a greater satiating effect, whereas Calbet & Holst( Reference Calbet and Holst 18 ) showed no difference in GLP-1 response between whey, casein and their hydrolysates. Finally, we observed a higher mean glucose concentration after consumption of casein than after consumption of hydrolysed casein, although there was no difference in insulin concentration. This may, however, be a matter of chance finding due to multiple testing.
The strength of the present study was the randomised cross-over design, where the subjects were kept under controlled settings for 40 h, including the measurements. Moreover, proteins were served in mixed meals, which reflect real-life situations, and measurements were continued for a total 24 h period. However, the study has also some obvious limitations. As mentioned previously, protein oxidation was not assessed as a function of time, and fat and CHO oxidation were not independently tested but estimated from the RQ values. Moreover, gastric emptying and differences in absorption rates were not measured. Finally, as shown in Fig. 4, a blood sample taken at 45 min would have been advantageous as all biochemical measures were likely to reach peak or nadir magnitude between 30 and 60 min. Therefore, the lack of the blood sample taken at this time period limits the analyses of peak and nadir magnitudes.
Conclusion
In conclusion, data from the present study suggest that repeated meals with whey, hydrolysed casein and casein induce similar effects on appetite regulation and postprandial and total EE over 24 h, which may be explained by similar absorption rates when proteins are served as high-protein mixed meals. However, we found that whey resulted in a slightly lower RQ value than hydrolysed casein, indicating a potentially higher lipid oxidation and lower CHO oxidation. Future studies are needed to investigate whether the effects on substrate oxidation can be verified and whether they persist over time, and thereby can contribute to a beneficial effect on body weight. Moreover, future studies should focus on the effect of the relative amount of protein in the meals served, and the effect of serving protein in mixed meals v. the effect of serving proteins alone, as this may influence gastric emptying and the rate of absorption.
Acknowledgements
The authors are grateful to the laboratory and kitchen staff at the department for their assistance, especially Jane Jørgensen, Søren Andresen, John Lind, Lene Stevner, Yvonne Fatum and Charlotte Kostecki. They especially thank Sisse Gomes, Jan la Cour Lindboe, Sofia Wannbäck and Mostafa Jafar for their help during the practical work.
The present study was sponsored by a grant from the Ministry of Science, Technology and Innovation, Denmark; Arla Foods Ingredients Group P/S, Denmark; and the Danish Dairy Research Foundation, Denmark. Proteins were supplied by FrieslandCampina Domo EMEA, The Netherlands; Arla Foods Amba, Denmark; and Arla Foods Ingredients, Denmark. The sponsors were not involved in the design of the study, in the collection, analysis and interpretation of the data or in the writing of this article.
The authors’ contributions are as follows: L. Q. B., J. K. L., B. L. and A. A. designed the research; L. Q. B., J. K. L. and S. G. conducted the research; L. Q. B., S. G., J. J. H., S. R. and C. R. analysed the data; L. Q. B. and A. S. drafted the manuscript; J. K. L., S. G., B. L., S. R., C. R., J. J. H. and A. A. co-edited and revised the manuscript critically for important intellectual content; L. Q. B. had the primary responsibility for the final content. All authors read and approved the final manuscript.
A. A. is currently the member of the advisory board for Global Dairy Platform, USA. He is the member of the Arla A/S – Copenhagen University steering group. Over the past 5 years, his research has received funding from Arla Foods A/S and the Danish Dairy Association, and from international dairy interests contributing to a collaborative grant coordinated by Global Dairy Platform. The rest of the authors declare that there are no conflicts of interest.









