Vitamin D is important for normal bone growth and development in children. Vitamin D deficiency is associated with lower bone mineral density as well as elevated parathyroid hormone concentrations( Reference Cheng, Tylavsky and Kröger 1 ) and in severe cases with rickets( Reference Wharton and Bishop 2 ). Sun exposure is the main contributor of vitamin D for most people in northern latitudes during summer months. But from October to March, dietary vitamin D intake and the amount of vitamin D stored in body tissues become increasingly important due to the large solar zenith angle and thereby insufficient UVB radiation to initiate vitamin D synthesis( Reference Webb 3 , Reference Webb, Kline and Holick 4 ). Since very few foods contain a naturally high amount of vitamin D, low levels of serum 25-hydroxyvitamin D (s-25(OH)D) are prevalent during winter in northern latitudes, especially in countries where fortification is rare( Reference Spiro and Buttriss 5 ). In Denmark, fortification is almost absent and recommendations on vitamin D supplements include only children aged 0–2 years or children with dark skin( 6 ). In a recent paper on vitamin D status in Danish school children, 28% had s-25(OH)D≤50 nmol/l in September–November after the summer peak( Reference Petersen, Damsgaard and Dalskov 7 ), and the proportion of children with deficient (<30 nmol/l( 8 , 9 )) and insufficient (≤50 nmol/l( 8 , 9 )) vitamin D status has been shown to increase throughout winter( Reference Ní Chaoimh, McCarthy and Hourihane 10 , Reference Andersen, Brot and Jakobsen 11 ). The exact long-term implications of these seasonal fluctuations in s-25(OH)D are still unknown( 12 ). Previously, it has been calculated that to have a winter s-25(OH)D of ~50 nmol/l in northern latitudes, the previous summer concentration should be ~100 nmol/l( Reference Andersen, Brot and Jakobsen 11 ). To identify children at risk of low winter vitamin D status and find strategies to improve levels, it is of strong relevance to investigate factors that influence autumn vitamin D status.
A number of biological factors such as age, sex and BMI have been associated with s-25(OH)D in various populations of children( Reference Petersen, Damsgaard and Dalskov 7 , Reference Gordon, DePeter and Feldman 13 – Reference Kumar, Muntner and Kaskel 15 ). In addition to biological factors, behaviours such as dietary intake, physical activity and sun-related habits may influence s-25(OH)D. Intake of vitamin D among young children in most European countries is low at 2–3 µg/d( Reference Spiro and Buttriss 5 ). This is far from the Estimated Average Requirement of 7·5 µg/d set in the Nordic countries( 9 ) and of 10 µg/d set by the US Institute of Medicine( 8 ). Many of the studies investigating the influence of dietary intake on s-25(OH)D in children were performed in countries with widespread food fortification or use of supplements, and these reported positive associations between s-25(OH)D and dietary intake( Reference Ní Chaoimh, McCarthy and Hourihane 10 , Reference Bjarnadottir, Kristjansdottir and Hrafnkelsson 14 , Reference Hayek, Pham and Finch 16 , Reference Öhlund, Silfverdal and Hernell 17 ). In contrast, a Danish study showed a positive association between intake and status in adults but not in children( Reference Madsen, Rasmussen and Mejborn 18 ). Thus, the influence of dietary intake on vitamin D status in countries without mandatory fortification is not clear.
Some studies have reported associations between s-25(OH)D and various sun behaviours in children living in northern latitudes( Reference Petersen, Damsgaard and Dalskov 7 , Reference Hayek, Pham and Finch 16 , Reference Madsen, Rasmussen and Mejborn 18 ). However, a study with almost 600 children aged 2–18 years did not find associations between adherence to sun exposure guidelines and s-25(OH)D in children but did find associations in adults( Reference Hansen, Tjønneland and Køster 19 ). Children’s physical activity may be a marker of sun exposure if the activities are mainly performed outside and it has previously been reported that moderate-to-vigorous physical activity (MVPA)( Reference Petersen, Damsgaard and Dalskov 7 ) and cardiorespiratory fitness( Reference Bjarnadottir, Kristjansdottir and Hrafnkelsson 14 ) are positively associated with s-25(OH)D among school children. Likewise, sedentary behaviour may be a marker of limited sun exposure due to fewer outdoor activities, and screen time has been associated with vitamin D deficiency among children and adolescents( Reference Kumar, Muntner and Kaskel 15 , Reference Absoud, Cummins and Lim 20 ). However, few of the studies looking at determinants of vitamin D status in children assessed physical activity and sedentary behaviour.
When transferring from pre-school to school, various habits having a potential association with vitamin D status may change. For instance, fewer outdoor activities are expected to take place during school hours compared with pre-school, where most children play outside on sunny days. Sun-protective behaviour may also change with greater child independence and expected reduced focus on sun protection in school (from 6 years of age in most Northern European countries) compared with pre-school (covering typically ages 2–5 years). It has previously been shown that even though older school children know more about sun protection than their younger peers, they protect themselves the least from the sun( Reference Reinau, Meier and Gerber 21 ). Similar differences could be hypothesized when comparing children in pre-school and school. To our knowledge, no study has assessed determinants of vitamin D status in children while taking pre-school and school (referred to collectively as ‘school type’ hereafter) into account.
Thus, the aim of the present study was to explore determinants of s-25(OH)D during autumn in 4–8-year-old Danish children (55°N) not consuming vitamin D-fortified foods or supplements. Second, the aim was to explore differences in sun behaviours between pre-school and school children.
Methods
Study design and participants
The present study used baseline data from the ODIN Junior study (Food-based solutions for optimal vitamin D nutrition and health through the life cycle), which was a randomized, placebo-controlled trial with changes in s-25(OH)D as primary outcome( Reference Mortensen, Damsgaard and Hauger 22 ). A total of 130 Danish children living in the greater area of Copenhagen (55°N) were recruited in summer 2014 by use of the Danish Civil Registration System. Children were included if they were 4–8 years old and of white Danish/European origin. They were excluded prior to the study if they had diseases or intake of medicine known to affect vitamin D or Ca metabolism, or if they received vitamin D-containing supplements≥4 d/week in the last 8 weeks or any vitamin D-containing supplements in the last 4 weeks before examinations. Baseline examinations were performed during 33 d from 29 September to 31 October 2014.
Blood sampling and analysis
A 25ml venous blood sample was taken after a 2–4 h fasting period. It was centrifuged and stored as previously described( Reference Mortensen, Damsgaard and Hauger 22 ). Serum 25-hydroxyergocalciferol (s-25(OH)D2) and serum 25-hydroxycholecalciferol (s-25(OH)D3) were analysed by LC–MS/MS at University College Cork, Ireland. Total s-25(OH)D was calculated as the sum of s-25(OH)D2 and s-25(OH)D3. Intra- and inter-assay CV was<5% and<6%, respectively.
Anthropometry
Weight and height were measured by standard procedures( Reference Mortensen, Damsgaard and Hauger 22 ), and sex- and age-adjusted Z-scores for BMI were calculated with WHO AnthroPlus software( 23 ). We measured body composition with bioelectrical impedance analysis (Quantum III; RJL Systems, Inc., Clinton Township, MI, USA). Fat mass index (FMI) and fat-free mass index (FFMI) were calculated as fat mass and fat-free mass in kilograms divided by the square of height in metres, respectively.
Dietary vitamin D intake
Dietary vitamin D intake was estimated using an FFQ that has previously been validated( Reference Kiely, Collins and Lucey 24 ). The FFQ was administered by nutrition researchers and contained fourteen questions covering eight food items: milk, yoghurt, cheese, cereals, bread, egg, meat and fish( Reference Mortensen, Damsgaard and Hauger 22 ). The chosen food items contribute 95% of the vitamin D intake in Danish 4–8-year-olds according to the recent national Danish dietary survey( Reference Pedersen, Fagt and Groth 25 ). The Danish food composition database( 26 , 27 ) was used for vitamin D composition values, supplemented by UK food composition tables( 28 ) if a suitable Danish value was not available.
Sun behaviour
Children’s sun behaviours were reported by the parents using questions previously used among young children in large population surveys( Reference Pichora and Marrett 29 , Reference Klostermann and Bolte 30 ). Parents were asked: ‘When your child is outside during summer at a sunny day, how often does your child: seek shade and avoid direct sunlight? protect the skin with shirt and long pants? use a sun hat or a cap? use sunscreen?’ For each of the four questions, parents selected one of the following categories: ‘always’, ‘often’, ‘sometimes’, ‘rarely’ and ‘never’. Some categories had very few observations and were merged in data analyses, thus giving three categories for each question. In addition, parents estimated outdoor time from the questions: ‘How much time does your child usually spend outside during the summer season: on weekdays? on weekends?’ Categories were: <15 min, 15–30 min, 30–60 min, 1–4 h or >4 h. Since nearly all children were estimated to be outside for >1 h/d, categories were dichotomized to ≤4 h/d and >4 h/d. Moreover, parents were asked about their child’s usual sunscreen’s sun protection factor (SPF) during summer, which was categorized into SPF<30 or SPF≥30, and asked about number of sunscreen applications daily, which was dichotomized into <2 or ≥2 times/d. Vacations abroad the last 6 months were also reported and categorized as ‘vacations to southern latitudes the last month’ (yes, no).
Physical activity and parental education
Parents reported children’s physical activity and sedentary behaviour by a questionnaire with reference to the last 7 d. The number of days per week with MVPA for 1 h (or intervals summing up to 1 h) were reported to evaluate if the official WHO( 31 ) and Scandinavian( 9 ) recommendation of being active for 1 h/d was fulfilled. Moreover, parents reported the total number of hours spent in MVPA daily, including both leisure-time and pre-school/school hours. In addition, they reported daily hours of ‘screen time’ (i.e. watching television or using a computer, tablet, games console, smartphone, etc.). Parents’ self-reported education was used to determine the highest level of education obtained in the household, categorized as either short higher education or less (≤14 years) or medium/long higher education (≥15 years).
Power calculation
The original power calculation was based on the expected slope of the relationship between total vitamin D intake and s-25(OH)D, the primary outcome( Reference Mortensen, Damsgaard and Hauger 22 ). To see a dose–response relationship of 1·0–1·5 at a significance level of 0·05 with 90% power, a total of 105 completing children were needed. To take account of 15–20% potential dropouts and insufficient blood samples, 130 children were included in the trial and in the present cross-sectional study. Of the 130 children included, 125 (including six pairs of siblings) had a successful blood sample drawn and were included in the analyses of s-25(OH)D determinants.
Statistical analyses
Model assumptions were inspected visually with residual and normal probability plots. Descriptive data are presented as means and standard deviations, medians and interquartile ranges, or numbers and percentages as appropriate. Differences in characteristics between children attending pre-school and school (school type) were tested with ANOVA for continuous variables (Wilcoxon rank-sum test for non-normally distributed variables) and the χ 2 test for categorical variables.
Linear mixed models were used to identify factors associated with s-25(OH)D. The basic model included s-25(OH)D as dependent variable, a biological or behavioural explanatory variable, as well as age and sex as fixed effects to account for biological variation, and siblings as random effect to account for dependency within pairs of siblings. The potential biological or behavioural determinants of s-25(OH)D were tested one by one, and associations were expressed as the mean adjusted difference (β) and 95% confidence interval. Additional analyses were adjusted for level of parental education, travels to southern latitudes during the last month (yes, no) and week of measurement, respectively, to rule out a potential influence of these covariates on the results.
Moreover, when a significant association was found in the basic models, we made additional adjustments for variables which were associated with the explanatory determinant. This was done to identify behaviours which could potentially explain the observed associations between s-25(OH)D and the identified determinant. The included variables were identified by bivariate correlation analyses with the use of Pearson’s correlation coefficient, Spearman’s correlation coefficient (r s), the χ 2 test, ANOVA or the Kruskal–Wallis test, as appropriate, depending on the distribution and nature of the variable.
Lastly, we included an interaction term between school type (pre-school and school) and the various behaviours in the basic model to explore differences in associations according to school type. If the interaction term was significant at P<0·05, results were stratified by school type.
As the present study was an exploratory one, we did not adjust for multiple comparisons. All statistical analyses were carried out using the statistical software package Stata version 14.0. P<0·05 was considered statistically significant.
Results
Participant characteristics
Children had a mean age of 6·6 (sd 1·5) years and most were normal weight (Table 1). Mean s-25(OH)D was 56·8 (sd 12·5) nmol/l. Prevalence of deficiency and insufficiency according to the Institute of Medicine cut-offs (<30 and 30–50 nmol/l, respectively( 8 )) were 2% and 30%, respectively.
Table 1 Characteristics of 4–8-year-old Danish children, overall and stratified by school type (pre-school and school); baseline data from the ODIN Junior study (Food-based solutions for optimal vitamin D nutrition and health through the life cycle) at 55°N, September–October 2014

FMI, fat mass index; FFMI, fat-free mass index; MVPA, moderate-to-vigorous physical activity; s-25(OH)D, serum 25-hydroxyvitamin D; BW, body weight; SPF, sun protection factor.
* Differences according to school type tested with ANOVA for continuous variables (Wilcoxon rank-sum test for non-normally distributed variables) and the χ 2 test for categorical variables. Significant P values are indicated in bold font.
† Based on highest parental education in the household.
‡ Blood samples obtained from 125 children (pre-school, n 44; school, n 81).
Median vitamin D intake was 1·9 (interquartile range 1·2–2·8) µg/d (Table 1), and fish contributed most to vitamin D intake (41%) followed by consumption of meat (21%). None of the children reached the Estimated Average Requirement of 7·5 µg/d recommended in the Nordic countries( 9 ) and only 10% reached our previously estimated dietary requirement of ~4 µg/d( Reference Mortensen, Damsgaard and Hauger 22 ).
Differences between pre-school and school children
Table 1 shows various characteristics of the children stratified by school type. School children had a higher FFMI than pre-school children (P<0·001), but s-25(OH)D did not differ between school types (P=0·105; Table 1). However, vitamin D intake in relation to body weight was lower in school children compared with pre-school children (P=0·012; Table 1). Children attending pre-school reported more minutes of MVPA than children in school (P=0·024), tended to adhere more to the recommendation of 1 h of MVPA daily (P=0·051) and had more outdoor time during weekdays compared with school children (P<0·001; Table 1). As expected, pre-school children had a more sun-protective behaviour than school children since more children in pre-school used a sun hat always/often compared with school children (P=0·026), used a sunscreen with SPF≥30 (P=0·032) and had sunscreen applied ≥2 times/d (P=0·038; Table 1).
Associations between potential behavioural determinants of serum 25-hydroxyvitamin D
Various sun behaviours were associated in the children: frequency of using protective clothing was positively associated with seeking shade (r s=0·276, P=0·002), using a hat (r s=0·234, P=0·007) and using sunscreen (r s=0·231, P=0·008). Moreover, use of sunscreen was positively associated with both use of a hat (r s=0·248, P=0·004) and sunscreen SPF (r s=0·241, P=0·006).
The amount of MVPA was higher among children spending >4 h/d outside during weekdays v. children being outside ≤4 h/d (P=0·010) and among children seeking shade more often (P=0·015) and using a hat more often (P=0·003). Children being active 6–7d/week v. those being active 1–5d/week had a higher vitamin D intake in relation to body weight (P=0·013). No other associations were observed between the various behavioural factors.
Biological determinants of serum 25-hydroxyvitamin D
Neither sex nor age was associated with s-25(OH)D (Table 2). Among the anthropometric variables, FFMI was positively associated with s-25(OH)D (P=0·014), whereas BMI-for-age Z-score tended to associate positively with s-25(OH)D (P=0·053; Table 2). Since the positive association between FFMI and s-25(OH)D could be confounded by physical activity (which may affect FFMI positively; and, if it is performed outside, may affect s-25(OH)D positively), we adjusted the model for the physical activity variables one by one. However, none of these adjustments changed the association (P=0·011–0·015). In support of this, we did not find that FFMI was associated with any of the physical activity variables in bivariate correlation analyses (P>0·65; data not shown).
Table 2 Biological and behavioural determinants of s-25(OH)D in 4–8-year-old Danish children; baseline data from the ODIN Junior study (Food-based solutions for optimal vitamin D nutrition and health through the life cycle) at 55°N, September–October 2014

s-25(OH)D, serum 25-hydroxyvitamin D; FMI, fat mass index; FFMI, fat-free mass index; BW, body weight; MVPA, moderate-to-vigorous physical activity; SPF, sun protection factor; Ref., referent category.
* Linear mixed models were used to identify factors associated with s-25(OH)D. Age and sex were included as fixed effects, and siblings as random effects. β is the estimate of change in s-25(OH)D per unit increase in the variable or change in category of variable. Significant P values are indicated in bold font.
† β value for every 0·1 unit increase in vitamin D intake/kg BW.
Behavioural determinants of serum 25-hydroxyvitamin D
Children who only sometimes or rarely/never sought shade had ~7 nmol/l higher s-25(OH)D than children always/often seeking shade on sunny summer days (P=0·018 and P=0·028, respectively; Table 2). Adjustment for use of protective clothing, which was associated with seeking shade, did not affect the result. Use of sunscreen with SPF≥30 tended to associate negatively with s-25(OH)D (P=0·056; Table 2).
Among the physical activity variables, children adhering to the recommendation of being active for 1 h/d on 6–7d/week had 5·6 nmol/l higher s-25(OH)D than children being less active (P=0·014; Table 2). Although dietary vitamin D intake was not associated with s-25(OH)D, neither when expressed in µg/d nor in µg/kg per d (P>0·24; Table 2), we tested if the higher vitamin D intake seen among the most active children was a mediator in the association between physical activity and s-25(OH)D. However, the β estimates and P values did not change notably. In addition, we tested if the association was mediated by a higher amount of outdoor time in the most active children, but this was not the case either. None of the other physical activity variables, outdoor time or screen time was associated with s-25(OH)D (Table 2).
Children from households with ≥15 years of education, children not being on vacation to southern-latitude countries within the last month and children with blood sampled in week 44 v. week 40 tended to have lower s-25(OH)D (β estimates from −5·3 to −7·7, all P<0·10). Adjusting the basic models for each of these three variables did not change the results except for the following. The borderline negative association for sunscreen SPF≥30 became negatively associated with s-25(OH)D when adjusted for parental education (P=0·045) and when adjusted for week of measurement (P=0·028). In addition, the observed association between seeking shade and s-25(OH)D strengthened further when adjusted for vacations south within the last month (P=0·006 for children seeking shade sometimes and P=0·008 for children seeking shade rarely/never, v. often/always).
Behavioural determinants of serum 25-hydroxyvitamin D in pre-school and school children
When investigating the interaction term school type× ‘behaviour’, we found a significant interaction term in the model for use of a sun hat (P interaction=0·019). When analysing pre-school and school children separately, school children using a hat rarely/never had 12·1 (95% CI 2·5, 21·7) nmol/l higher s-25(OH)D compared with school children always/often using a hat (P=0·014). Thus, the association observed in school children with a higher s-25(OH)D when using a sun hat more rarely was significantly different from that seen in pre-school children (Fig. 1). Use of a sun hat did not associate with outdoor time in pre-school children. Thus, the lack of association between sun hat use and s-25(OH)D in pre-school children did not seem to be explained by more frequent use of a sun hat with more time spent outside.
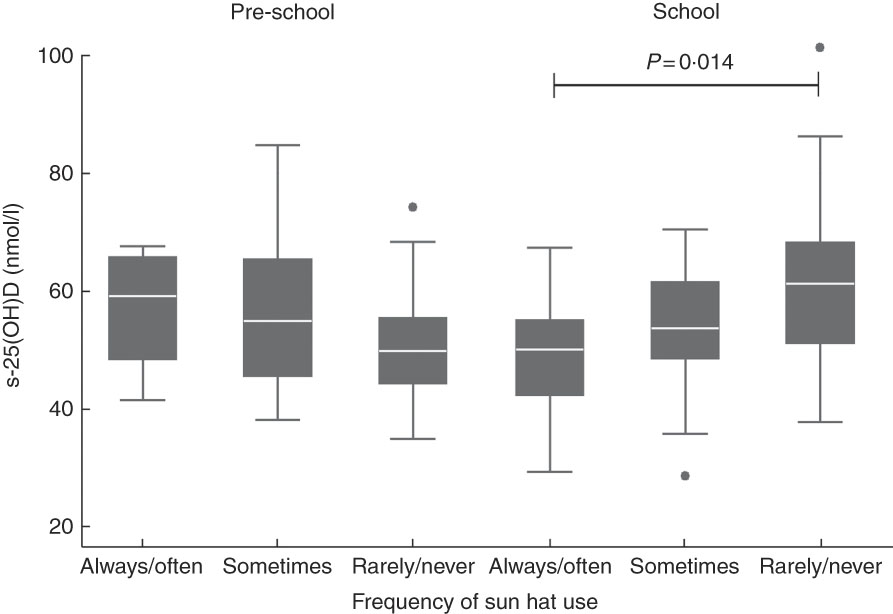
Fig. 1 Box-and-whisker plots for frequency of use of a sun hat on sunny summer days in relation to serum 25-hydroxyvitamin D (s-25(OH)D) in 4–8-year-old Danish children, stratified by school type (pre-school, n 44 v. school, n 81); baseline data from the ODIN Junior study (Food-based solutions for optimal vitamin D nutrition and health through the life cycle) at 55°N, September–October 2014. The bottom and top edge of the box represent the first and third quartiles (interquartile range); the line within the box represents the median; the ends of the bottom and top whiskers represent the minimum and maximum values; and the dots represent outliers. The interaction term between school type and use of a sun hat was significant (P interaction=0·019). When analysing pre-school and school children separately by linear mixed models with s-25(OH)D as dependent variable and with age and sex as fixed effects, school children using a sun hat rarely/never had 12·1 (95% CI 2·5, 21·7) nmol/l higher s-25(OH)D compared with school children always/often using a sun hat (P=0·014)
Discussion
In the current cross-sectional analysis of the ODIN Junior study among Danish 4–8-year-olds, 30% of the children had s-25(OH)D≤50 nmol/l during autumn, the same prevalence as reported in a large study in 8–11-year-old children living at this northern latitude( Reference Petersen, Damsgaard and Dalskov 7 ). We have previously reported that all children in the ODIN Junior study who did not receive vitamin D supplements became vitamin D insufficient during winter and nearly half became deficient (<30 nmol/l)( Reference Mortensen, Damsgaard and Hauger 22 ). Thus, it is highly relevant to identify factors affecting serum vitamin D status during autumn in countries with minimal fortification. Physical activity as well as seeking shade were determinants of s-25(OH)D in our analyses, whereas dietary intake in children not consuming vitamin D-containing supplements did not determine s-25(OH)D. Children in pre-school had more outdoor time and physical activity than school children, but also a more sun-protective behaviour. The behaviour of wearing a sun hat determined s-25(OH)D in school children only.
Others have also reported associations between sun behaviours and vitamin D status among children. For instance, a positive association between s-25(OH)D and outdoor stay in light clothes( Reference Madsen, Rasmussen and Mejborn 18 ), walking during school hours between classrooms( Reference Petersen, Damsgaard and Dalskov 7 ) and a calculated sun exposure index based on various sun-related habits in pre-schoolers( Reference Hayek, Pham and Finch 16 ) has previously been shown in children in northern latitudes. Contrary, the StatusD study did not find associations between adherence to four sun exposure guidelines and s-25(OH)D in almost 600 children aged between 2 and 18 years but did find associations in adults in relation to frequency of seeking shade and use of protective clothing( Reference Hansen, Tjønneland and Køster 19 ). In our study we asked about sun behaviours at any given sunny summer day, whereas the StatusD study asked about behaviours only on a day off between 12.00 and 15.00 hours, and this may not capture the true sun exposure of children. In the Irish BASELINE birth cohort study, sun-related habits were not associated with s-25(OH)D among 2-year-old children( Reference Ní Chaoimh, McCarthy and Hourihane 10 ). However, it is likely that children of this age are only rarely exposed to the sun due to adherence to sun-safe recommendations whereas children aged 4–8 years are more likely to have outdoor activities on sunny days. The Danish summer of 2014, after which our study took place, was more warm, dry and sunny than usual( 32 ), and this may have affected the extent of outdoor activities of the children. Almost 20% of the children in the present study stated that they always/often sought shade on sunny summer days. We found that this behaviour was associated with a lower vitamin D status and with a strengthening of the association when taking into account that some children had been on a sunny vacation. At the end of winter these children who often seek shade will most likely have insufficient vitamin D status since their s-25(OH)D during autumn only just exceeded the sufficiency cut-off (51·2 (sd 10·7) nmol/l). Thus, even though seeking shade is a very effective sun-safe behaviour to avoid sunburn and reduce the risk of skin cancer, it may also lower vitamin D status to near insufficient levels if it is practised to a large extent on sunny summer days. However, giving advice on more time in the sun would not only be difficult but could also be unethical and counteract official recommendations of sun-protective behaviour. Thus, instead, this knowledge on an association between seeking shade and s-25(OH)D could be used to identify children at risk and strategies could focus on fortified foods and/or winter vitamin D supplementation. We also found a tendency towards a negative effect on s-25(OH)D with use of sunscreen having SPF≥30, which became significant when taking either parental education or week of measurement into account. The association with s-25(OH)D is somewhat surprising, since application of sunscreen with SPF 15 is generally thought to reduce the cutaneous vitamin D synthesis by almost 100%, if it is used properly( Reference Webb 3 ), and only three children used sunscreen with SPF<15. However, it can be questioned how often sunscreen is applied to the proper extent and whether it is applied on all sun-exposed skin.
Our analyses showed that frequent use of a sun hat among school children was associated with a lower vitamin D status compared with school children who more rarely used a sun hat, whereas this was not the case in pre-school children. One explanation could be that even though school children may have fewer opportunities for outdoor play during the day, they also have less sun-protective behaviour than the pre-school children, and thus each individual sun-protective behaviour may influence s-25(OH)D to a larger extent. In contrast, children in pre-school may generally use more than one sun-safe behaviour simultaneously due to a higher degree of adult awareness and control. The finding in the school-based OPUS Study that children walking outside between classrooms during school days had a higher vitamin D status than children who less frequently moved between classrooms( Reference Petersen, Damsgaard and Dalskov 7 ) also supports that vitamin D status of school children may be affected by apparently small and insignificant sun behaviours. This may be due to general ‘unsafe’ sun behaviour with, for instance, fewer sunscreen applications and use of sunscreen with a lower SPF. Surprisingly, children in pre-school who always or often used a sun hat had a vitamin D status of ~60 nmol/l, which was higher than that of pre-school children who used a sun hat less frequently, although not significantly different, and close to the s-25(OH)D status of school children who rarely/never use a hat (Fig. 1). It can be hypothesized that the high amount of outdoor time when in pre-school makes a sun hat an ineffective sun protection since only the head and maybe the shoulders are covered. However, the interaction with school type should be interpreted with caution due to the limited number of school children (n 9) with frequent sun hat use.
We did not find any association between outdoor time and s-25(OH)D, which is similar to a Swedish study in pre-schoolers( Reference Öhlund, Silfverdal and Hernell 17 ). However, both in our study and the Swedish study nearly all children were estimated to be outside for >1 h/d, thus giving little variation in outdoor time. Synthesis of vitamin D in the skin requires only a very short stay in the sun: only ~15 min/d during summer at latitude 55°N will produce the equivalent of ingesting 25 µg/d( Reference Webb 3 ). In addition, the formation of previtamin D3 in the skin has an upper limit( Reference Webb 3 ). Thus, it may not be surprising that outdoor time did not determine s-25(OH)D. When investigating associations between the various behaviours we found that children having a higher amount of MVPA also spent more time outdoors during weekdays. Thus, physical activity may be a marker for outdoor time to some extent. Others have reported that objectively measured MVPA in 8–11-year-olds( Reference Petersen, Damsgaard and Dalskov 7 ) or cardiorespiratory fitness in 7-year-olds( Reference Bjarnadottir, Kristjansdottir and Hrafnkelsson 14 ) was positively associated with s-25(OH)D. However, none of these studies investigated if the associations were due to more outdoor time. One study in English children estimated outdoor exercise and found that exercise outside of 30 min/d was associated with a higher s-25(OH)D( Reference Absoud, Cummins and Lim 20 ). We found that children who adhered to the advice of 1 h of MVPA daily for 6–7d/week had higher vitamin D status than children who were less active. It could be hypothesized that this was due to more outdoor stay even though this was not identified as a mediator in the association or a determinant itself. Even though others have found negative associations between television time( Reference Absoud, Cummins and Lim 20 ) and screen time( Reference Kumar, Muntner and Kaskel 15 ) and s-25(OH)D in children, we did not find this. This could be explained by the fact that the amount of sedentary behaviour in the present study was not associated with outdoor time (data not shown), or that the amount of screen time in the present study was not as high as reported in other groups of children, for instance among US children( Reference Kumar, Muntner and Kaskel 15 ).
Similar to our findings, others have reported a positive association between FFMI and s-25(OH)D( Reference Valtueña, Gracia-Marco and Huybrechts 33 , Reference Foo, Zhang and Zhu 34 ). This association may explain our finding of a tendency towards a positive association between s-25(OH)D and BMI-for-age Z-score. This is in contrast to the more often reported negative association between obesity and vitamin D status( Reference Drincic, Armas and van Diest 35 ) but similar to a study among Swedish pre-school children( Reference Öhlund, Silfverdal and Hernell 17 ). Our finding could be explained by the fact that most children were normal weight with low fat mass, making it less likely that vitamin D is diluted in the fat depots to a large extent, which is a commonly used theory for the negative association between BMI and s-25(OH)D( Reference Drincic, Armas and van Diest 35 ).
The lack of association between dietary intake of vitamin D and s-25(OH)D is similar to other studies conducted in late summer/autumn in northern latitude countries where fortification is rare( Reference Petersen, Damsgaard and Dalskov 7 , Reference Andersen, Brot and Jakobsen 11 , Reference Madsen, Rasmussen and Mejborn 18 ). On the contrary, studies in which fortification or supplementation is prevalent often find positive associations( Reference Ní Chaoimh, McCarthy and Hourihane 10 , Reference Bjarnadottir, Kristjansdottir and Hrafnkelsson 14 , Reference Hayek, Pham and Finch 16 , Reference Öhlund, Silfverdal and Hernell 17 ). This can probably be explained by the high vitamin D intakes seen in these studies, for instance up to ~19 µg/d among Irish 2-year-olds( Reference Ní Chaoimh, McCarthy and Hourihane 10 ) and ~31 µg/d among Canadian 2–5-year-olds( Reference Hayek, Pham and Finch 16 ). Similarly, among Icelandic 7-year-olds intake of fish-liver oil and vitamin D supplements was prevalent and could explain the positive association with s-25(OH)D in that study( Reference Bjarnadottir, Kristjansdottir and Hrafnkelsson 14 ). In the present study, none of the children consumed vitamin D supplements as this was an exclusion criterion for the randomized controlled trial( Reference Mortensen, Damsgaard and Hauger 22 ). With neither mandated nor common fortification this gave a low intake of vitamin D, thus making sun behaviour much more influential on s-25(OH)D. An increased amount of fortified foods could potentially increase intake as well as status among children, as previously shown in a family-based, randomized winter trial at same northern latitude( Reference Madsen, Rasmussen and Andersen 36 ).
We found a tendency towards a lower s-25(OH)D in children of parents with the longest educational background. This is in agreement with findings in young Canadian children( Reference Houghton, Szymlek-Gay and Gray 37 ). It could be speculated that the parents with long education in our study adhered to a high degree with sun-safe recommendations since others have previously shown that parents’ intention to let their child tan was inversely associated with level of parental education( Reference Morris, McGee and Bandaranayake 38 ). However, we could not see any associations between sun behaviour among the children and education of the parents (data not shown). In contrast, others have found a positive association between parental education and child s-25(OH)D status( Reference Petersen, Damsgaard and Dalskov 7 , Reference Weng, Shults and Leonard 39 ) and thus the link between educational background and child vitamin D status is still not completely clear.
To our knowledge, the present study is one of very few assessing dietary intake as well as physical activity and sun-related behaviours and their association with vitamin D status in the same children. The age span of 4–8 years permitted us to investigate the influence of school type whereas most other studies in children are performed in either pre-school children or school children. The LC–MS/MS method used to measure s-25(OH)D is considered the gold standard( Reference Arneson and Arneson 40 ) and has been used in only few of the previous studies on determinants of vitamin D status in children. Our sun behaviour questions have been used previously among young children in large population surveys( Reference Pichora and Marrett 29 , Reference Klostermann and Bolte 30 ). The dietary intake of vitamin D was estimated using a previously validated FFQ specially developed for quantifying dietary intakes of vitamin D and Ca( Reference Kiely, Collins and Lucey 24 ). This method is suitable for food items not eaten on a daily basis, such as fish, which was the main vitamin D source in the children. However, it should also be noted that the method is suitable when ranking children according to their vitamin D intake, and data on absolute amounts of vitamin D intake should be interpreted with caution( Reference Thorisdottir, Gunnarsdottir and Steingrimsdottir 41 ). Limitations of the study include the relatively small sample size and multiple tests, which increase the risk of chance findings; thus the results should be interpreted with caution. Moreover, even though the questions to assess sun behaviour were previously used in large surveys, they are not validated, and answers could also be subject to recall bias or social desirability bias. Since our study participants were all Caucasian children with highly educated parents, generalizations to other groups may be difficult. In line with this, others have previously reported that ethnicity is a significant determinant of s-25(OH)D in children( Reference Petersen, Damsgaard and Dalskov 7 , Reference Kumar, Muntner and Kaskel 15 , Reference Absoud, Cummins and Lim 20 , Reference Rockell, Green and Skeaff 42 ). In addition, we cannot rule out the effect of a potential tracking of vitamin D status from infancy as has been reported previously among 6-year-old Icelandic children( Reference Thorisdottir, Gunnarsdottir and Steingrimsdottir 41 ). Lastly, genetic factors have also been suggested to regulate vitamin D status( Reference Wang, Zhang and Richards 43 ) and we cannot know if this modified the response to sun exposure, for instance.
Conclusions
In conclusion, autumn vitamin D status in 4–8-year-old Danish children was associated with sun behaviours and physical activity habits, whereas their dietary vitamin D intake was low and of no influence. Also, FFMI was positively associated with vitamin D status. Special attention should be given to children who always avoid the sun and protect their skin from the sun in other ways, since they may store less vitamin D for the winter. Keeping the risk of sunburn and skin cancer in mind, strategies could include fortification and/or winter vitamin D supplementation, as well as promoting adherence to the physical activity recommendations. The transition from pre-school to school seems to be accompanied by decreased outdoor time but also less sun-safe behaviour, which may make individual sun behaviours in school children more influential on vitamin D status compared with pre-school children. This knowledge is relevant for lowering the risk of inadequate vitamin D status among children during winter. However, additional studies with larger sample sizes are warranted to further investigate determinants of vitamin D status in pre-school and school.
Acknowledgements
Financial support: This project received funding from the European Commission under its Seventh Framework Programme (FP7/2007-2013) under Grant Agreement 613977 for the ODIN Integrated Project (Food-based solutions for optimal vitamin D nutrition and health through the life cycle; http://www.odin-vitd.eu/). The European Commission had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: C.Mø. and C.T.D. designed the study, C.Mo., C.M.ø, C.T.D. and H.H. carried out the study and formulated the research questions, C.Mo. analysed the data and wrote the paper, C.Mo., C.Mø., C.T.D., H.H. and M.K. contributed in manuscript preparation. C.Mo. had primary responsibility for the final article. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Committees on Biomedical Research Ethics for the Capital Region of Denmark (H-3-2014-022). Written informed consent was obtained from all custody holders of the children. The trial was registered as NCT02145195 at http://www.clinicaltrials.gov.





