Introduction
The success of reintroductions or introductions of species for conservation (i.e. survival and increase of the reintroduced/introduced populations) depends on several factors, such as the number of individuals released (Berger, Reference Berger1990; Beck et al., Reference Beck, Rapaport, Stanley Price, Wilson, Olney, Mace and Feistner1994; Saltz, Reference Saltz1998; Wolf et al., Reference Wolf, Garland and Griffith1998; Fischer & Lindenmayer, Reference Fischer and Lindenmayer2000; Clark et al., Reference Clark, Huber and Servheen2002), their age and sex (Komers & Curman, Reference Komers and Curman2000; Apollonio et al., Reference Apollonio, Bassano, Mustoni, Festa-Bianchet and Apollonio2003), sanitary status (Cunningham, Reference Cunningham1996; Mathews et al., Reference Mathews, Moro, Strachan, Gelling and Buller2006), origin (from the wild or from captive-breeding programmes; Fischer & Lindenmayer, Reference Fischer and Lindenmayer2000; Sarrazin & Legendre, Reference Sarrazin and Legendre2000; Vickery & Mason, Reference Vickery and Mason2003; Jule et al., Reference Jule, Leaver and Lea2008), number of releases (Saltz, Reference Saltz1998), habitat suitability (Wolf et al., Reference Wolf, Garland and Griffith1998; Owen-Smith, Reference Owen-Smith, Festa-Bianchet and Apollonio2003) and connectivity between metapopulations (Wolf et al., Reference Wolf, Garland and Griffith1998; Festa-Bianchet, Reference Festa-Bianchet2002). Population persistence is more likely when the number of founders is large (Fischer & Lindenmayer, Reference Fischer and Lindenmayer2000), the rate of population increase is high (Griffith et al., Reference Griffith, Scott, Carpenter and Reed1989) and the effect of competition is low (Burgman & Lindenmayer, Reference Burgman and Lindenmayer1998).
Reintroduction programmes should incorporate feasibility studies and preparatory activities (e.g. removing causes of former extinction) as well as a monitoring period after release (IUCN, 2012). Reintroduction methods and post-release ranging behaviour may be assessed through monitoring, the latter depending on the response to the new environment (Stanley Price, Reference Stanley Price1989).
The use of previous results to design further operations is a central concept of adaptive management. The monitoring period that should follow reintroductions is often neglected or documented only in grey literature (Sarrazin & Barbault, Reference Sarrazin and Barbault1996), or the project duration is insufficient to complete the monitoring (Beck et al., Reference Beck, Rapaport, Stanley Price, Wilson, Olney, Mace and Feistner1994). In the case of the Apennine chamois Rupicapra pyrenaica ornata in Italy, apart from a short article (Lovari et al., Reference Lovari, Artese, Damiani, Mari and Soorae2010) there is no information available on its post-release behaviour or the factors that determine the success of conservation actions, despite the large number of introductions/reintroductions that have been attempted since the early 1990s.
The selection and availability of individuals of appropriate age and sex are the main concerns in introductions/reintroductions. Given the lack of information on the consequences of various release strategies, arbitrary approaches are used to select individuals for population restoration (Caughley, Reference Caughley1994; Sarrazin & Barbault, Reference Sarrazin and Barbault1996). Adults are usually preferred (Komers & Curman, Reference Komers and Curman2000; Sarrazin & Legendre, Reference Sarrazin and Legendre2000) because young individuals may suffer high post-release mortality (Gogan & Barrett, Reference Gogan and Barrett1987), and populations with mature animals grow faster (Komers & Curman, Reference Komers and Curman2000). In reintroductions of polygynous species the number of females is usually maximized to increase the production of offspring (Sigg et al., Reference Sigg, Goldizen and Pople2005). Adult females are preferred to subadult females because primiparous mammals are often less fecund than older ones and they are more susceptible to environmental stress, which could further decrease fecundity (Bronson, Reference Bronson1989). In ungulates young males are the dispersing individuals (Greenwood, Reference Greenwood1980), to decrease the risk of inbreeding. Reintroduced individuals may come from captive-breeding programmes or be translocated from natural populations. Captive-bred individuals may suffer higher mortality compared to wild-caught individuals (Ginsberg, Reference Ginsberg, Olney, Mace and Feistner1994) and display behavioural limitations when released into the wild, because of lack of familiarity with the local habitat and ecosystem (Curio, Reference Curio1996). The availability of individuals for release may vary among species but wild-caught animals are often preferred (Bright & Morris, Reference Bright and Morris1994).
In the first months after release translocated animals may exhibit an altered ranging behaviour because they need to locate resources (Owen-Smith, Reference Owen-Smith, Festa-Bianchet and Apollonio2003) and become familiar with the new environment (Michallet & Toïgo, Reference Michallet and Toïgo2000; Dolev et al., Reference Dolev, Saltz, Bar-David and Yom-Tov2002). Explorative movements can increase the risk of mortality (Banks et al., Reference Banks, Norrdahl and Korpimäki2002), affecting the success of reintroduction.
The Apennine chamois is protected under national and international legislation (Appendix II of the Bern Convention, Annex II* and Annex IV of the EU Habitats and Species Directive, Appendix II of CITES, and as an ‘especially protected species’ under Italian hunting law). It is categorized as Vulnerable (criterion D1+2) on the IUCN Red List, with four small populations in as many national parks.
In the Holocene the Apennine chamois ranged along the Apennines, from the Sibillini Mountains to the Pollino massif (Masini & Lovari, Reference Masini and Lovari1988), but by the early 1990s the species survived only in the Abruzzo, Lazio and Molise National Park. In 2008 there were three extant populations of several hundred animals, following two reintroductions in the early 1990s (Mari & Lovari, Reference Mari and Lovari2006), in Majella National Park and the Gran Sasso–Monti Laga National Park. Thus, the species was not present in our study area in historical times. A hard release (i.e. immediate release of animals to the wild, without previous confinement or acclimatization) was carried out in the Sibillini Mountains in 2008 as part of several conservation projects (Reintroduction Programme and LIFE09 NAT/IT/000183 COORNATA) for this threatened species and was preceded by a feasibility study (WWF, 1997). A captive population (n = 18) was kept in large enclosures in four national parks in the Central Apennines.
We investigated the post-release phase of a conservation introduction of Apennine chamois to an area of the Central Apennines, focusing on the sex, age and origin (captive-bred or wild) of translocated individuals, to gather data to inform future releases.
Study area
Our study area covered 850 ha in the Mount Bove area of the Sibillini Mountains National Park (70,000 ha) in the Central Apennines, Italy (Fig. 1). At low altitudes, beech Fagus sylvatica (on the northern slopes), oak Quercus spp. and European hop hornbeam Ostrya carpinifolia (on the southern slopes) are dominant, and at higher elevations grasslands of Festuco-Trifolietum thalii alternate with rocky walls.
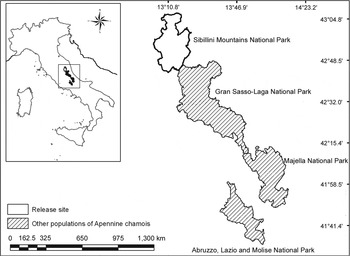
Fig. 1 Sibillini Mountains National Park, Italy, where 16 Apennine chamois Rupicapra pyrenaica ornata were released during 2008–2010. Other populations of the species exist in the Abruzzo, Lazio and Molise National Park, Majella National Park and the Gran Sasso–Monti Laga National Park. The rectangle on the inset indicates the location of the main map in Italy. Coordinates are in WGS84 system.
The climate is high-mountainous at 1,350–1,850 m (mean annual temperature 7−9 °C; mean annual precipitation 1,300–1,500 mm) and sub-alpine at 1,850–2,300 m (mean annual temperature < 5−6 °C; mean annual precipitation 1,300–1,500 mm).
Wild boar Sus scrofa (c. 1,700), roe deer Capreolus capreolus (c. 900), red deer Cervus elaphus (c. 90) and Canis lupus (c. 25–30) are present in the Park. Chamois kids are vulnerable to predation by golden eagles Aquila chrysaetos (four nesting pairs) and possibly the red fox Vulpes vulpes (Couturier, Reference Couturier1938; Bertolino, Reference Bertolino2003).
Methods
During 2008–2010 16 Apennine chamois (10 females, 6 males; Table 1) were released into the Sibillini Mountains National Park, increasing the population there by 41%, to 55. Eight of the animals (two females < 3 years old; three females ≥ 3 years old; one male < 4 years old; two males ≥ 4 years old) were caught in the wild, in the core distribution area of the Apennine chamois (Abruzzo Lazio and Molise National Park; Fig. 1). The others were caught in three fenced wildlife areas in Majella National Park (4.2 ha; Fig. 1; one female < 3 years old; one female ≥ 3 years old; one male < 4 years old), Gran Sasso–Monti Laga National Park (4 ha; Fig. 1; one female < 3 years old; one female ≥ 3 years old; one male < 4 years old), and Sibillini Mountains National Park (3 ha; Fig. 1; one female < 3 years old; one male < 4 years old). One to three animals were released at a time because of the low availability of founders. In each release group the animals were all wild or all captive-bred. Capture and release occurred in late summer/early autumn, well before the reproductive season.
Table 1 The origin and age of the 16 Apennine chamois Rupicapra pyrenaica ornata released in the Sibillini Mountains National Park, Italy (Fig. 1), during 2008–2010.
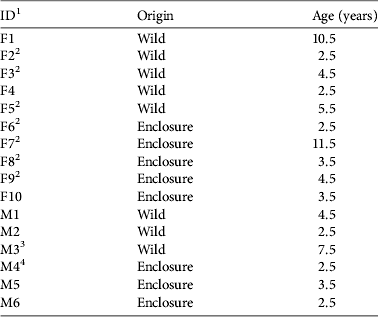
1 F, female; M, male
2 Had kid during first year post release
3 Died 11 weeks post release
4 Died 10 weeks post release
All individuals were fitted with global positioning systems (GPS) or VHF radio-collars and translocated by road or helicopter to the Sibillini Mountains National Park. Both types of collars were equipped with mortality sensors. The methods of capture and handling were in accordance with the legislation on animal care in Italy.
Our sampling regime involved 42 fixes per individual per month, evenly distributed over daylight hours, for chamois fitted with VHF transmitters. At least three bearings (loudest signal method; Springer, Reference Springer1979) were used to locate chamois and there was a minimum time period of 4 hours between two consecutive fixes. GPS transmitters were programmed to record locations every 7 hours and to transmit data by GSM (global system for mobile communications).
Radio-tracking data were analysed using Animal Movement v. 2.0 beta for ArcView v. 3.2 (Hooge & Eichenlaub, Reference Hooge and Eichenlaub1997). In the first 20 weeks after release the spatial behaviour of chamois was analysed in terms of (1) hourly standardized inter-fix distance (i.e. the linear distance between successive fixes), as a measure of their mobility (i.e. dispersal behaviour in the new site); (2) maximum distance from the release site; and (3) overlap of successive weekly home ranges (minimum convex polygon).
Results
Two males (aged 7.5 and 2.5 years) died during the first 20 weeks after release (Table 1). The last radiolocation for both individuals was far from the release site (M3: 8.4 km; M4: 8.9 km), apparently indicating dispersal.
Individuals caught in the wild moved (inter-fix distance) significantly more than those from captivity during the first few weeks after release (Mann–Whitney U Test; week 1: U = 5.0, P = 0.029, N w = 5, N e = 8; week 2: U = 6.0, P = 0.045, N w = 5, N e = 8; week 4: U = 5.0, P = 0.003, N w = 8, N e = 8; week 19: U = 3.0, P = 0.004, N w = 7, N e = 7; Fig. 2a). Males moved significantly more than females (Mann–Whitney U Test; week 6: U = 7.0, P = 0.011, N f = 10, N m = 6; week 7: U = 7.0, P = 0.011, N f = 10, N m = 6; week 10: U = 3.0, P = 0.002, N f = 10, N m = 6; week 14: U = 4.0, P = 0.024, N f = 10, N m = 4). There were no significant differences between young (females < 3 years, males < 4 years) and adults (females ≥ 3 years, males ≥ 4 years; Mann–Whitney U Test; U = 14.0–29.0, P = 0.093–1.000, N a = 7–9, N y = 6–7).
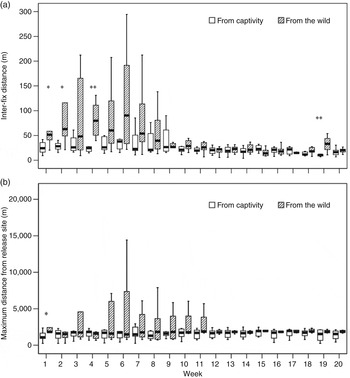
Fig. 2 (a) Inter-fix distance (median values and quartiles) moved, and (b) maximum distance moved from the release site, by 16 captive-bred and wild-caught Apennine chamois (Table 1) during the first 20 weeks following their release in the Sibillini Mountains National Park (Fig. 1). * P < 0.05, ** P < 0.01, Mann–Whitney U test.
Wild-caught individuals moved significantly further from the release site than those from captivity only during the first week after release (Mann–Whitney U Test; week 1: U = 3.0, P = 0.011, N w = 5, N e = 8; other weeks: U = 14.0–31.0, P = 0.209–1.000, N w = 5–8, N e = 7–8), with a high variability during the first 11 weeks (Fig. 2b). No significant differences were found between sexes (Mann–Whitney U Test; U = 11.0–26.0, P = 0.093–1.000, N f = 8–10, N m = 4–6) or between young and adult animals (Mann–Whitney U Test; U = 10.0–28.0, P = 0.093–0.958, N a = 7–9, N y = 5–7).
We calculated the percentage of overlap between individual home ranges in successive weeks: individuals caught in the wild shifted their home ranges significantly more than those from captivity (Mann–Whitney U Test; week 1–2: U = 4.0, P = 0.048, N w = 4, N e = 8; week 10–11: U = 8.0, P = 0.037, N w = 7, N e = 7; other weeks: U = 8.0–31.0, P = 0.073–0.959, N w = 4–8, N e = 7–8). No significant differences were found between sexes (Mann–Whitney U Test; U = 11.0–25.0, P = 0.260–0.945, N f = 7–10, N m = 4–6) or between young and adult animals (Mann–Whitney U Test; U = 14.0–28.0, P = 0.190–1.000, N a = 6–9, N y = 5–7).
Seven of the 10 females had one kid in the first year after release (Table 1). Of the three females with no kid, two were from the wild and one was from captivity.
Discussion
A conservation introduction aims to preserve a threatened species in an area outside its historical distribution but within an appropriate habitat and eco-geographical area. Reintroduced/introduced populations are small initially and therefore susceptible to the risks faced by all small populations (e.g. environmental fluctuations, demographic stochasticity and inbreeding; Caughley & Sinclair, Reference Caughley and Sinclair1994). To achieve success a primary aim of introductions or reintroductions should be to maximize the initial rate of population increase and thereby shorten the period during which the new population is exposed to risks. In a review Komers & Curman (Reference Komers and Curman2000) showed that a significant proportion of variation in the rate of increase of reintroduced Artiodactyla populations is explained by age and sex structure. In particular, the proportion of socially mature animals is positively correlated to population growth, as is the proportion of males to females (Komers & Curman, Reference Komers and Curman2000). In our study, post-release mortality affected only males, probably on dispersal, and was independent of age or origin. Captive-bred females showed greater reproductive success (and hence higher recruitment) compared to those wild-caught, which may be attributable to better body condition. However, this is a preliminary finding; in reintroduction programmes reproductive success usually improves significantly with time after release (Saltz & Rubenstein, Reference Saltz and Rubenstein1995; Jiang et al., Reference Jiang, Yu, Feng, Zhang, Xia, Ding and Lindsay2000; Bar-David et al., Reference Bar-David, Saltz, Dayan, Perelberg and Dolev2005).
Nutrition during early development has been shown to have an effect on reproductive physiology and behaviour in adulthood (Curio, Reference Curio1996), and therefore food availability and parental feeding are important for both wild and captive stock.
The age and sex structure of an introduced/reintroduced population influences its success because it determines the spatial behaviour of founders. The first few weeks after release are a period of spatial instability, with animals behaving unpredictably (Kleiman, Reference Kleiman1989). Usually, young individuals exhibit greater dispersion after release than adults (Greenwood, Reference Greenwood1980; Calenge et al., Reference Calenge, Maillard, Invernia and Gaudin2005), and males disperse more than females (Moseby & O'Donnell, Reference Moseby and O'Donnell2003; Lovari et al., Reference Lovari, Artese, Damiani, Mari and Soorae2010). Analysing the spatial behaviour of our introduced individuals during the first 20 weeks after release we found no significant differences between males and females or between young animals and adults. There were significant differences between captive-bred and wild-caught founders, the former showing greater site fidelity. This is a particularly important issue in introductions/reintroductions because it is desirable that individuals remain near each other and close to the release site (i.e. the most suitable area for the new population) rather than dispersing immediately after release. A large post-release dispersion rate may reduce the potential to establish a breeding population (Hardman & Moro, Reference Hardman and Moro2006). This is even more important when founders are released just before the mating season (as in this study) because they may miss mating opportunities.
Griffith et al. (Reference Griffith, Scott, Carpenter and Reed1989) showed how translocations of exclusively wild-caught animals were more likely to succeed than those of exclusively captive-reared animals. In a review Beck et al. (Reference Beck, Rapaport, Stanley Price, Wilson, Olney, Mace and Feistner1994) found that wild populations were established successfully in only 11% of reintroductions of captive-bred animals. The causes of failure are mainly associated with behavioural deficiencies in captive-bred animals, especially in relation to foraging, predator avoidance and social behaviour (Beck et al., Reference Beck, Rapaport, Stanley Price, Wilson, Olney, Mace and Feistner1994). We used a mixed strategy, with wild and captive-bred animals, because the source wild population could not provide enough animals. This appears to be a good compromise, maintaining the animals close to the release area and at the same time increasing genetic variability and reducing risks linked to ignorance of local ecological features. We suggest that this mixed strategy may improve the success of releases for species that are not abundant in the wild.
Acknowledgements
We are greatly indebted to A. Rossetti, F. Mari, N. Felicetti, and the staff of Abruzzo, Lazio and Molise National Park, Majella National Park and Gran Sasso–Monti Laga National Park for their help in the field. A. Fermanelli and F. Perco provided support throughout our work. Two anonymous reviewers provided helpful comments. SL received financial support from the Sibillini Mountains National Park Agency and, from 2010, through the LIFE09 NAT/IT/000183 COORNATA. The Italian Army and the Corpo Forestale dello Stato facilitated the transportation of the animals to the Sibillini Mountains National Park.
Biographical sketches
A. Bocci's research activities include wildlife monitoring and management, spatial behaviour and habitat selection. She has experience in capturing and marking wildlife, census techniques, radiotelemetry, diet analysis of carnivores, and GIS applications. S. Menapace and S. Alemanno are wildlife technicians at the Sibillini Mountains National Park, where they have participated in radiotracking of Apennine chamois since 2009. S. Lovari has been working steadily on the biology of chamois since 1976 and was chairman of the IUCN Caprinae Specialist Group from 1986 to 1998.





