Introduction
Foodborne trematodes (FBT) are defined as trematodes infecting humans, which are transmitted by consumption of foods, globally or locally available (including traditional foods) or those taken rarely or accidentally. In 1995, the World Health Organization (WHO) estimated the total global number of people infected with FBT at more than 40 million (WHO, 1995). A decade later (in 2005), about 56.2 million people were estimated to be infected with FBT, with 7.9 million having severe sequelae and 7158 people dying mostly from cholangiocarcinoma and cerebral infections (Fürst et al., Reference Fürst, Keiser and Utzinger2012a). The most recent estimate extrapolated the total global number of people infected with FBT at 74.7 million as of 2015–2016 with 0.2 million new cases annually and 2 million disability-adjusted life years (Fürst et al., Reference Fürst, Yongvanit, Khuntikeo, Lun, Haagsma, Torgerson, Odermatt, Bürli, Chitnis, Sithithaworn, Utzinger, Yap, Bratschi and Steinmann2019; WHO, 2020). However, there are problems of low sensitivity and specificity of diagnostic tools as well as possible numerous endemic areas so far undetected, and these global estimates of the FBT burden seem to be much underestimated. Thus, the WHO road map for ‘control of FBT diseases 2021–2030’ recommends critical actions, including the development of accurate surveillance and mapping tools and methods, with information on environmental factors involved in infection, and the promotion of application and awareness of preventive chemotherapy (WHO, 2020).
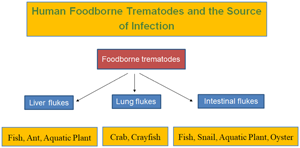
Taxonomically, the FBTs are highly diverse, and at least 99 species (15 liver flukes, 9 lung flukes and 75 intestinal flukes) have been reported from human infections (Chai et al., Reference Chai, Murrell and Lymbery2005; Chai, Reference Chai, Murrell and Fried2007, Reference Chai2019; Fürst et al., Reference Fürst, Keiser and Utzinger2012a; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019, Reference Chai and Jung2020; Cho et al., Reference Cho, Jung, Chang, Sohn, Sinuon and Chai2020). Among them, those of greatest public health significance are Clonorchis sinensis, Opisthorchis spp., Fasciola hepatica, Paragonimus westermani, and several species of intestinal flukes, such as Metagonimus spp., Haplorchis spp. and echinostomes (WHO, 1995; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019).
The origin of FBTs may date back to almost 8000 BCE when humans started to switch from a nomadic, hunter-gatherer lifestyle to a settled, agricultural way of life (Steverding, Reference Steverding2020). However, from the evolutionary point of view, intestinal flukes seem to be the oldest group, which subsequently evolved to parasitize the bile duct and liver (liver flukes) or lungs (lung flukes) in the human body.
The mode of human infection with FBT is closely linked to human behavioural patterns in endemic localities, specifically the methods of food production, preparation and consumption (WHO, 1995). Thus, the epidemiology of FBT infection is determined by ecological and environmental factors related to food and is strongly influenced by poverty, pollution and population growth (WHO, 1995). The infection source, i.e. food, is diverse, including aquatic or semi-terrestrial snails (gastropods and bivalves), fish, crustaceans, water plants, amphibians and reptiles (WHO, 1995; Chai, Reference Chai2019). Rarely insects and mammals may serve as the source of human infection for some species (Chai, Reference Chai2019; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019).
The geographical distribution of FBT is almost worldwide. However, as FBT infections are closely related to food habits, they are predominantly found in East Asian and Western Pacific countries where local people prefer to eat various types of foods raw or under improperly cooked conditions (WHO, 1995). This kind of food habit is in most cases traditional and one of the longstanding customs, so it is very difficult to change within a short period of time. Health education targeting schoolchildren is an important strategy for long-term control of FBT infections. Control of infection sources, including intermediate hosts, is very difficult and practically almost impossible. Mass drug administration (MDA) using an effective anthelmintic, such as praziquantel, may be feasible to lower the infection rate of people in endemic communities. However, if reinfection persists, the prevalence would rise again to the previous level. Thus, control of FBT infections by infrequent MDA may be insufficient. Repeated MDA with sustained health education of young people in endemic communities is an ideal control strategy.
In this review, the authors briefly summarized the current status of FBT occurring around the world. The taxonomy and phylogeny of FBT, their life cycles, mode of transmission, epidemiology and geographical distribution, global disease burden, pathogenicity and clinical manifestations, diagnostic problems, anthelmintics used, and control strategies are briefly reviewed.
Liver flukes
At least 15 species of liver flukes are known to cause human infections. They include C. sinensis, Opisthorchis viverrini, O. felineus, Metorchis conjunctus, M. bilis, M. orientalis, Amphimerus sp., Amphimerus noverca, A. pseudofelineus, Pseudamphistomum truncatum, P. aethiopicum, Dicrocoelium dendriticum, D. hospes, Fasciola hepatica and F. gigantica (Table 1) (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). They can be divided into small liver flukes (C. sinensis, Opisthorchis spp., Metorchis spp., Amphimerus spp., Pseudamphistomum spp. and Dicrocoelium spp.) and large liver flukes (F. hepatica and F. gigantica) according to the size of the adult worms. The first intermediate host is freshwater or brackish water snails, and the second intermediate host is freshwater fish (C. sinensis, Opisthorchis spp., Metorchis spp., Amphimerus spp. and Pseudamphistomum spp.), ants (Dicrocoelium spp.) or aquatic vegetation (Fasciola spp.) (Table 1). The morphological similarity of opisthorchiid eggs to heterophyid as well as lecithodendriid-like fluke eggs frequently poses diagnostic problems in human fecal examinations. Molecular techniques using internal transcribed spacers (ITS) and mitochondrial cytochrome c oxidase 1 (cox1) have been used to differentiate the eggs as well as larvae and adults of opisthorchiid and heterophyid flukes (Duflot et al., Reference Duflot, Setbon, Midelet, Brauge and Gay2021).
Table 1. Liver flukes infecting humans with biological, clinical characteristics and geographical distribution

a The geographical distributions of liver flukes are mostly referred from Chai and Jung (Reference Chai, Jung, Toledo and Fried2019).
Species involved
Clonorchis sinensis
Clonorchis sinensis (Cobbold, 1875) Looss, 1907 (the Chinese liver fluke) was first found in the bile passages of a Chinese carpenter in Calcutta, India, and an endemic focus was discovered in south China (Beaver et al., Reference Beaver, Jung and Cupp1984). Eggs of this fluke were also found in the coprolites of human mummies (estimated date; 1647) in South Korea (Seo et al., Reference Seo, Guk, Kim, Chai, Bok, Park, Oh, Kim, Yi, Shin, Kang and Shin2007) and China (date back to the 5th century BCE) (Ye and Mitchell, Reference Ye and Mitchell2016). This fluke infects the bile duct of humans and animals and can cause inflammation of the bile duct and gall bladder which leads to obstruction of the bile duct, cholangitis and cholecystitis (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). In severe cases, liver cirrhosis and cholangiocarcinoma may develop.
Phylogenetic relationships of small liver flukes have been studied using analysis of nuclear (18S rDNA, ITS region and 28S rDNA) and mitochondrial genes (cox1, cox2, cox3, nd1, ATPase subunit 6 and others) (Thaenkham et al., Reference Thaenkham, Blair, Nawa and Waikagul2012; Besprozvannykh et al., Reference Besprozvannykh, Tatonova and Shumenko2018; Saijuntha et al., Reference Saijuntha, Sithithaworn, Kiasopit, Andrews, Petney, Toledo and Fried2019, Reference Saijuntha, Sithithaworn, Petney and Andrews2021; Duflot et al., Reference Duflot, Setbon, Midelet, Brauge and Gay2021). Within the genus Clonorchis, there is only a single valid species, C. sinensis. ITS2 can clearly separate C. sinensis, O. viverrini, O. felineus, Amphimerus sp. and Metorchis spp. from the Heterophyidae and Cryptogonimidae flukes (Thaenkham et al., Reference Thaenkham, Blair, Nawa and Waikagul2012; Besprozvannykh et al., Reference Besprozvannykh, Tatonova and Shumenko2018).
Freshwater snails take the role of the first intermediate host for C. sinensis, which includes Parafossarulus manchouricus, P. anomalospiralis and Bithynia fuchsiana (Chen et al., Reference Chen, Lu, Hua and Mott1994). At least 113 species of freshwater fish, including Pseudorasbora parva, Carassius spp., Cyprinus spp., Zacco spp. and Puntungia herzi, are known to serve as the second intermediate hosts and the source of human and animal infections (Chen et al., Reference Chen, Lu, Hua and Mott1994; Chai et al., Reference Chai, Murrell and Lymbery2005; Rim, Reference Rim2005). Shrimps were also reported to be a second intermediate host (Chen et al., Reference Chen, Lu, Hua and Mott1994).
The mode of infection is related to traditional food habits, in particular, the consumption of raw or improperly cooked freshwater fish (Chai et al., Reference Chai, Murrell and Lymbery2005). In South Korea, the major type of fish dish responsible for C. sinensis infection is the sliced raw freshwater fish with red pepper sauce (Chai et al., Reference Chai, Murrell and Lymbery2005). In southern China and Hong Kong, the major type of fish dish responsible is the morning congee (rice gruel) with slices of raw freshwater fish (Chen et al., Reference Chen, Lu, Hua and Mott1994), whilst in the Guangdong Province of China, half-roasted or undercooked fish is commonly linked to infection (Chen et al., Reference Chen, Lu, Hua and Mott1994).
The endemic areas are located mostly in the Far East and East Asia (Chen et al., Reference Chen, Lu, Hua and Mott1994; Chai et al., Reference Chai, Murrell and Lymbery2005; Rim, Reference Rim2005). In South Korea, a national survey in 2012 reported an egg positive rate of 1.9%, and the number of infected people was estimated to be about 1 million (Korea Association of Health Promotion, 2013). In China, a total of 24 major endemic localities were reported, with 12.5 million infected people nationwide (Chen et al., Reference Chen, Lu, Hua and Mott1994; Hong and Fang, Reference Hong and Fang2012; Lai et al., Reference Lai, Hong, Su, Liang, Hide, Zhang, Yu and Lun2016). In Taiwan, the prevalence of clonorchiasis was high in some localities; however, the current status is unknown (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). In Vietnam, northern parts, especially along the Red River Delta, including Haiphong and Hanoi, are well-known endemic areas, with the number of infected people estimated at about 1 million (Rim, Reference Rim, Steele, Hillyer and Hopla1982a; Chai et al., Reference Chai, Murrell and Lymbery2005; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). In Russia, human infections were reported in the Amur River territory, although the exact prevalence is unknown; however, at least 1 million people are estimated to be infected (Hong and Fang, Reference Hong and Fang2012). The total global number of infected people is estimated at about 20 million (Hong and Fang, Reference Hong and Fang2012), and the number at risk is about 601 million (Garcia, Reference Garcia2016).
The metacercariae excyst in the duodenum, migrate through the ampulla of Vater to the distal biliary ducts, where they begin to lay eggs 3–4 weeks after infection (Beaver et al., Reference Beaver, Jung and Cupp1984). Cholangitis and cholecystitis are the main pathological features in association with secondary bacterial infections (Rim, Reference Rim, Steele, Hillyer and Hopla1982a). The major histopathological features of the involved bile ducts include irregular bile duct dilatation, glandular hyperplasia, mucin-secreting cell metaplasia, cystic degeneration and periductal fibrosis (Rim, Reference Rim, Steele, Hillyer and Hopla1982a; Reference Rim2005; Hong and Fang, Reference Hong and Fang2012). In some patients, biliary cirrhosis and biliary obstruction with blockage of the common bile duct by adult worms or stones, or both, may occur (Beaver et al., Reference Beaver, Jung and Cupp1984). The pathogenesis and pathology depend on the intensity of infection as well as the frequency of continuous reinfection over a period of years and the total length of the infection which can last for 15–20 years or longer (Beaver et al., Reference Beaver, Jung and Cupp1984). The clinical symptoms include jaundice accompanied by transient urticaria and pain at the site of the liver; in heavy infections, weakness, lassitude, epigastric discomfort, paraesthesia, loss of weight, palpitation, tachycardia, diarrhoea and toxaemic symptoms may occur (Rim, Reference Rim, Steele, Hillyer and Hopla1982a). In chronic stages, suppurative cholangitis, biliary stone, pancreatitis, liver cirrhosis and cholangiocarcinoma are important complications (Rim, Reference Rim, Steele, Hillyer and Hopla1982a; Kim, Reference Kim1984b).
C. sinensis is one of the well-known carcinogenic flukes that can cause cholangiocarcinoma of the bile duct (Rim, Reference Rim, Steele, Hillyer and Hopla1982a; Reference Rim2005; Hong and Fang, Reference Hong and Fang2012). The scientific evidence for this was first raised in Hong Kong, an endemic area of clonorchiasis, where it was shown that at least 15% of 200 primary cancers in the liver were induced by infection with C. sinensis (Hou, Reference Hou1956). In South Korea, close correlations were also found between the cholangiocarcinoma incidence and the prevalence of C. sinensis infection in a southern endemic area (Rim, Reference Rim, Steele, Hillyer and Hopla1982a; Kim, Reference Kim1984b).
In known endemic areas, it is relatively easy to diagnose C. sinensis infection through fecal examinations to detect parasite eggs. The daily number of eggs produced per worm was estimated at about 4000 (Rim, Reference Rim, Steele, Hillyer and Hopla1982a); therefore, the egg detectability in fecal examinations is considerably high. However, in areas previously unknown for FBT infections, the eggs should be differentiated from those of other small liver fluke species (such as O. viverrini, O. felineus, Metorchis spp., or Amphimerus spp.) and also of heterophyid (Metagonimus yokogawai, Heterophyes nocens and various others) and lecithodendriid-like trematode species (Caprimorgorchis molenkampi and others) (Lee et al., Reference Lee, Hwang, Chai and Seo1984; Chai and Lee, Reference Chai and Lee2002; Chai, Reference Chai, Murrell and Fried2007). Serological tests, such as enzyme-linked immunosorbent assay (ELISA), and radiologic techniques, including ultrasound and CT, are also helpful for diagnosis (Hong and Fang, Reference Hong and Fang2012). Detection of parasite DNA using varied genetic techniques is another method applicable for the diagnosis of clonorchiasis (Hong and Fang, Reference Hong and Fang2012).
Treatment of C. sinensis infection can be done using a potent anthelmintic, praziquantel (Chai, Reference Chai2013a), or alternatively, albendazole (Table 2). Although such treatment is effective at clearing the infection, fluke-induced bile duct pathology did not recover within 9–12 weeks after praziquantel treatment (Lee et al., Reference Lee, Chai, Yang, Yun, Hong and Lee1988; Chai, Reference Chai2013a). Control strategies include MDA of infected people in endemic areas, environmental sanitation (sterilization of feces and protection of fish ponds from contamination with night-soil), control of snail hosts, and health education to avoid eating raw or improperly cooked freshwater fish (Rim, Reference Rim, Steele, Hillyer and Hopla1982a).
Table 2. Drugs used for the treatment of FBT infections
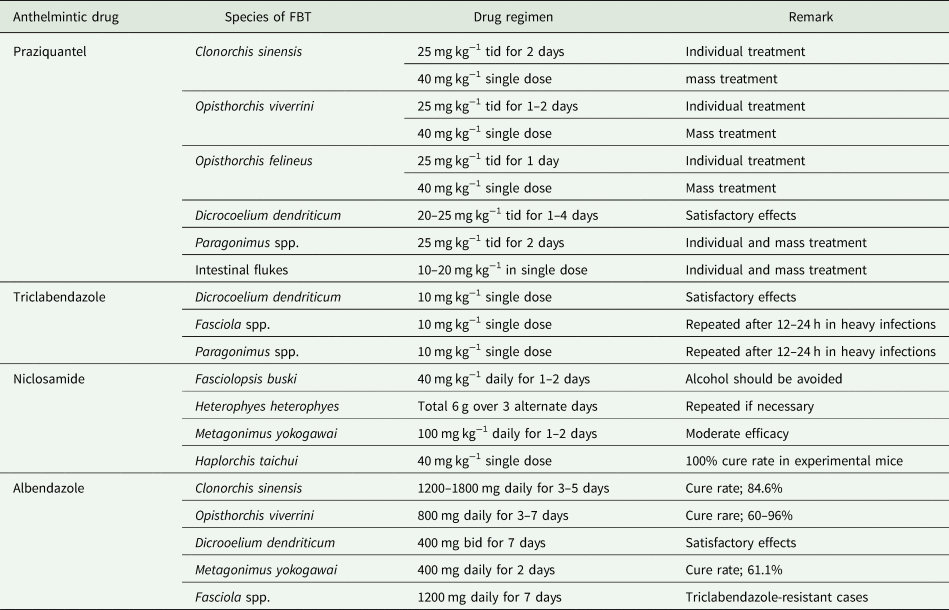
Information obtained from Kumchoo et al. (Reference Kumchoo, Wongsawad, Vanittanakom, Chai and Rojanapaibul2007), Fürst et al. (Reference Fürst, Sayasone, Odermatt, Keiser and Utzinger2012b), Chai (Reference Chai2013a), Garcia (Reference Garcia2016) and Chai et al. (Reference Chai, Jung and Hong2021a).
Opisthorchis viverrini and O. felineus
In the genus Opisthorchis, at least 53 nominal species were described (Nawa et al., Reference Nawa, Doanh and Thaenkham2013). Among them, 30 species were recorded from avian hosts, and the remaining 23 were from molluscs, fish, reptiles and mammals (Nawa et al., Reference Nawa, Doanh and Thaenkham2013).
Opisthorchis viverrini (Poirier, 1886) Stiles and Hassall, 1896 (the cat liver fluke) and Opisthorchis felineus (Rivolta, 1884) Blanchard, 1895 (the cat liver flukes) are known to infect humans (Rim, Reference Rim, Steele, Hillyer and Hopla1982b; Saijuntha et al., Reference Saijuntha, Sithithaworn, Kiasopit, Andrews, Petney, Toledo and Fried2019). O. viverrini was first discovered in the liver of a civet cat (Felis viverrini) brought from India to France (Miyazaki, Reference Miyazaki1991). Its first human infection was reported by Leiper in 1911 from the autopsy of two prisoners in Chiang Mai, Thailand (Wykoff et al., Reference Wykoff, Harinasuta, Juttijudata and Winn1965). O. felineus was first discovered in the liver of a cat (Miyazaki, Reference Miyazaki1991), and human infection was first reported by Winogradoff in 1892 in Tomsk, Siberia (Beaver et al., Reference Beaver, Jung and Cupp1984). Eggs of O. felineus were found in the coprolites of humans and dogs in Russia (Slepchenko, Reference Slepchenko2020). Phylogenetic studies of Opisthorchis spp. have been performed using sequences of nuclear (18S rDNA, ITS region and 28S rDNA) and mitochondrial genes (cox1, cox2, cox3, nd1, ATPase subunit 6 and others) (Besprozvannykh et al., Reference Besprozvannykh, Tatonova and Shumenko2018; Saijuntha et al., Reference Saijuntha, Sithithaworn, Kiasopit, Andrews, Petney, Toledo and Fried2019, Reference Saijuntha, Sithithaworn, Petney and Andrews2021; Duflot et al., Reference Duflot, Setbon, Midelet, Brauge and Gay2021). Molecular genetic investigations showed that O. viverrini is a species complex ‘O. viverrini sensu lato’, containing two evolutionary lineages with many cryptic species (morphologically similar but genetically distinct species) occurring in Thailand and Laos (Saijuntha et al., Reference Saijuntha, Sithithaworn, Petney and Andrews2021).
These flukes can infect the bile duct of humans and animals and can cause hepatobiliary disorders, as in C. sinensis (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). O. viverrini seems to have a higher potential for inducing cholangiocarcinoma than C. sinensis (Chai et al., Reference Chai, Murrell and Lymbery2005; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). Regarding O. felineus, studies are required for a proper understanding of its carcinogenic potential (Maksimova et al., Reference Maksimova, Pakharukova, Kashina, Zhukova, Kovner, Lvova, Katokhin, Tolstikova, Sripa and Mordvinov2017).
The molluscan intermediate host of O. viverrini is freshwater snails, including Bithynia goniomphalos, B. funiculata, B. siamensis and Melanoides tuberculata (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). The second intermediate hosts are various species of freshwater fish, i.e. Hampala dispar, Puntius orphoides, P. brevis, P. gonionotus, P. proctozysron and P. viehoever (Rim, Reference Rim, Steele, Hillyer and Hopla1982b; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019; Chai et al., Reference Chai, Lee, Rim, Sohn and Phommasack2019a). O. viverrini may survive for 10–20 years in the human host (Saijuntha et al., Reference Saijuntha, Sithithaworn, Kiasopit, Andrews, Petney, Toledo and Fried2019).
With regard to O. felineus, the molluscan host is Bithynia leachi species complex (B. leachi, B. troscheli and B. inflata) distributed in Eastern Europe (Mordvinov et al., Reference Mordvinov, Yurlova, Ogorodova and Katokhin2012). The second intermediate hosts are various species of freshwater fish, including the chub (Idus melanotus), tench (Tinca tinca and T. vulgaris), bream (Abramis brama and A. sapa), barbel (Barbus barbus) and carp (Cyprinus carpio) (Rim, Reference Rim, Steele, Hillyer and Hopla1982b).
The geographical distribution of O. viverrini, which is determined in close relationship with the distribution of the snail host, is mostly along the Mekong River basin and Indochina peninsula (Chai et al., Reference Chai, Murrell and Lymbery2005; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). In Thailand, O. viverrini is distributed mainly in the north and northeastern regions (Sithithaworn and Haswell-Elkins, Reference Sithithaworn and Haswell-Elkins2003), however, the prevalence has been declining since the 1990s (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). By contrast, in Laos, mainly along the Mekong River, the prevalence has been reported to be high even after the 1990s, with the prevalence being 51–67% among the riparian villagers (Rim et al., Reference Rim, Chai, Min, Cho, Eom, Hong, Sohn, Yong, Deodato, Standgaard, Phommasack, Yun and Hoang2003; Chai et al., Reference Chai, Han, Guk, Shin, Sohn, Yong, Eom, Lee, Jeong, Ryang, Hoang, Phommasack, Insisiengmay, Lee and Rim2007; Nakamura, Reference Nakamura2017). In Vietnam, endemic areas are located in southern parts, especially along the Mekong River (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). In Cambodia, Phnom Penh Municipality, Takeo, Kratie, Stung Treng and Preah Vihear Provinces were reported to be low-grade endemic areas (Yong et al., Reference Yong, Chai, Sohn, Eom, Jeoung, Hoang, Yoon, Jung, Lee, Sinuon and Socheat2014; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019; Khieu et al., Reference Khieu, Fürst, Miyamoto, Yong, Chai, Huy, Muth and Odermatt2019). In Myanmar, a low prevalence of O. viverrini infection has recently been confirmed in a suburban population around Yangon (Sohn et al., Reference Sohn, Jung, Hong, Lee, Park, Kim, Cho, Htoon, Tin and Chai2019). The number of global people infected with O. viverrini is estimated at over 10 million (Sripa et al., Reference Sripa, Tangkawattana and Brindley2018), with 80 million at risk for Opisthorchis spp. infections (Garcia, Reference Garcia2016).
O. felineus is known to be distributed from the Iberian Peninsula (Portugal and Spain) to Eastern Europe and West Siberia (Mordvinov et al., Reference Mordvinov, Yurlova, Ogorodova and Katokhin2012). Many riverside areas, including the Ob, Irtysh, Ural and Volga Rivers, are the most important endemic localities; the highest prevalence of 20–60% or above was reported in areas of West Siberia (Fedorova et al., Reference Fedorova, Fedotova, Sokolova, Golovach, Kovshirina, Ageeva, Kovshirina, Kobyakova, Ogorodova and Odermatt2018). Human infections were recorded previously in Lithuania (before 1901), Poland (before 1937), Romania (before 1957) and Spain (before 1932) but recently no cases seem to occur in these countries (Mordvinov et al., Reference Mordvinov, Yurlova, Ogorodova and Katokhin2012). However, in the last 50 years, human cases have been reported in some European Union (EU) countries, Eastern Europe, and Kazakhstan (Mordvinov et al., Reference Mordvinov, Yurlova, Ogorodova and Katokhin2012; Pozio et al., Reference Pozio, Armigbacco, Ferri and Morales2013). In Italy, from 2003 to 2011, there were 8 small outbreaks of O. felineus infection involving a total of 211 people through consumption of raw fillet of the tench (T. tinca) fished from two lakes named Bolsena and Bracciano (Pozio et al., Reference Pozio, Armigbacco, Ferri and Morales2013). Thus, O. felineus infection is regarded as an emerging disease in Italy (Pozio et al., Reference Pozio, Armigbacco, Ferri and Morales2013). The worldwide estimated number of people infected with O. felineus is 1.2–1.6 million (Saijuntha et al., Reference Saijuntha, Sithithaworn, Kiasopit, Andrews, Petney, Toledo and Fried2019).
The pathogenesis and pathology as well as clinical symptoms of O. viverrini and O. felineus infections are basically the same as those of C. sinensis infection. Cholangiocarcinoma due to O. viverrini infection accounts for about 15% of all liver cancers and is the second most frequent primary liver cancer worldwide (Saijuntha et al., Reference Saijuntha, Sithithaworn, Kiasopit, Andrews, Petney, Toledo and Fried2019). The highest incidence of this disease occurs in northern Thailand, where 5000 cases of cholangiocarcinoma are diagnosed annually (Sripa et al., Reference Sripa, Brindley, Mulvenna, Laha, Smout, Mairiang, Bethony and Loukas2012; Saijuntha et al., Reference Saijuntha, Sithithaworn, Kiasopit, Andrews, Petney, Toledo and Fried2019). In Laos and Vietnam, the precise incidence of cholangiocarcinoma due to O. viverrini infection has been unknown. However, the global ‘Cholangiocarcinoma Foundation’ recently launched a Vietnam Veterans project regarding developing cholangiocarcinoma among the veterans due to their military service in Vietnam in the 1970s (https://cholangiocarcinoma.org/vietnam-veterans/). In endemic areas of O. felineus infection in eastern Europe and Russia, around 400 cases of cholangiocarcinoma occur every year (Hotez and Alibek, Reference Hotez and Alibek2011). No precise carcinogenic mechanisms of Opisthorchis-induced cholangiocarcinoma have been completely understood (Sripa et al., Reference Sripa, Tangkawattana and Brindley2018). However, a two-stage carcinogenic process was proposed; some kinds of carcinogenic substances, including dimethylnitrosamine (DMN), as an ‘initiator’, and parasites, like O. viverrini and O. felineus, act as a ‘promoter’ (Flavell, Reference Flavell1981; Maksimova et al., Reference Maksimova, Pakharukova, Kashina, Zhukova, Kovner, Lvova, Katokhin, Tolstikova, Sripa and Mordvinov2017). This hypothesis has been further developed into a three-stage (multistage) theory, including initiation, promotion and tumour progression (Sripa et al., Reference Sripa, Tangkawattana and Brindley2018).
The diagnosis of O. viverrini or O. felineus infection can be done by fecal examinations to detect eggs. The daily number of eggs produced per worm of O. viverrini was estimated at 160–900, considerably less than the figure of 4000 as reported for C. sinensis (Rim, Reference Rim, Steele, Hillyer and Hopla1982a, Reference Rim, Steele, Hillyer and Hopla1982b). Serological tests and fecal antigen detection using ELISA (Sripa et al., Reference Sripa, Kaewkes, Intapan, Maleewong and Brindley2010; Teimoori et al., Reference Teimoori, Arimatsu, Laha, Kaewkes, Sereerak, Sripa, Tangkawattana, Brindley and Sripa2017) and radiologic techniques, including ultrasound (Pungpak et al., Reference Pungpak, Viravan, Radomyos, Chalermrut, Yemput, Plooksawasdi, Ho, Harinasuta and Bunnag1997; Kim et al., Reference Kim, Yong, Rim, Chai, Min, Eom, m Sohn, Lim, Choi, Insisiengmay, Phommasack and Insisiengmay2018) and CT (Mairiang and Mairiang, Reference Mairiang and Mairiang2003), are helpful for the diagnosis of opisthorchiasis and related cholangiocarcinoma. Detection of parasite DNA is another method applicable for the diagnosis of opisthorchiasis (Lovis et al., Reference Lovis, Mak, Phongluxa, Soukhathammavong, Sayasone, Akkhavong, Odermatt, Keiser and Felger2009; Sripa et al., Reference Sripa, Kaewkes, Intapan, Maleewong and Brindley2010).
Treatment of O. viverrini and O. felineus infection can be done using a potent anthelmintic, praziquantel (Chai, Reference Chai2013a), or alternatively, albendazole (Table 2). For prevention and control, see C. sinensis section. It is noteworthy that the metacercariae of O. felineus are tolerant to low temperatures, drying and high salt concentration and can be killed only by a high temperature (Pakharukova and Mordvinov, Reference Pakharukova and Mordvinov2016).
Metorchis spp.
Metorchis conjunctus (Cobbold, 1860) Looss, 1899 (the North American liver fluke) is a common parasite of carnivorous mammals, including sledge dogs (causing fatality), in northern Canada (MacLean et al., Reference MacLean, Arthur, Ward, Gyorkos, Curtis and Kokoskin1996). Asymptomatic sporadic human infections were reported in Canada since 1945, particularly in aboriginal populations from Quebec to Saskatchewan and the eastern coast of Greenland (Babbott et al., Reference Babbott, Frye and Gordon1961; MacLean et al., Reference MacLean, Arthur, Ward, Gyorkos, Curtis and Kokoskin1996). In 1993, an outbreak of a common-source infection with this fluke, with acute clinical symptoms of fatigue, upper abdominal tenderness and pain, low-grade fever, headache, weight loss and anorexia, occurred among 19 Korean immigrants in a river north of Montreal, Canada; the patients consumed wild-caught fish (Catostomus commersoni) that had been undercooked (MacLean et al., Reference MacLean, Arthur, Ward, Gyorkos, Curtis and Kokoskin1996). Laboratory findings included high blood eosinophilia and raised liver enzymes (MacLean et al., Reference MacLean, Arthur, Ward, Gyorkos, Curtis and Kokoskin1996). However, serious liver diseases have not been reported in human infections.
Metorchis bilis (Braun, 1790) Odening, 1962 is a liver fluke of carnivorous mammals in Central and Eastern Europe and Western Siberia of Russia (Mordvinov et al., Reference Mordvinov, Yurlova, Ogorodova and Katokhin2012). The geographical range of M. bilis considerably overlaps with that of O. felineus (Mordvinov et al., Reference Mordvinov, Yurlova, Ogorodova and Katokhin2012). Human infections with M. bilis were first suggested by demonstrating serum antibodies to M. bilis in 51.3% of 37 patients (37.8% of them reacted against both O. felineus and M. bilis antigens) residing in the Novosibirsk area of Russia (Kuznetsova et al., Reference Kuznetsova, Naumov and Belov2000). Another serological study also demonstrated positive immunoreactivity of 29 (63.2%) of 46 small liver fluke egg-positive patients to both O. felineus and M. bilis antigens and of 3 (7.9%) patients only to M. bilis antigen (Fedorov et al., Reference Fedorov, Naumov, Kuznetsova and Belov2002). Now it seems that infections of both humans and piscivorous vertebrates by M. bilis are under-diagnosed (Sitko et al., Reference Sitko, Bizos, Sherrad-Smith, Stanton, Komorova and Heneberg2016).
Metorchis orientalis Tanabe, 1920 is a small liver fluke of piscivorous birds and mammals in Japan, China and South Korea (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). The first report of human infection was published in Guangdong Province, China (Lin et al., Reference Lin, Chen and Li2001); 4 (4.2%) of 95 residents examined in Ping Yuan County were egg positive and from 2 purged patients a total of 12 adult flukes were recovered (Lin et al., Reference Lin, Chen and Li2001). However, the epidemiological status and potential impact of human infection are unknown (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). The geographical range of this fluke considerably overlaps with that of C. sinensis (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019).
Amphimerus spp.
Amphimerus sp. (species undetermined) was found to be a human parasitic liver fluke in Ecuador. In 2009, 4 human fecal samples from indigenous Chachi communities along the Cayapas River on the northern coast of Ecuador were found to be positive for opisthorchiid eggs (Calvopiña et al., Reference Calvopiña, Cevallos, Kumazawa and Eisenberg2011). Next year, a follow-up survey was conducted in the same communities, and a high proportion, 71 (24%) of 297 fecal samples, of residents, were positive for the same eggs (Calvopiña et al., Reference Calvopiña, Cevallos, Kumazawa and Eisenberg2011). The biliary liquid was collected from 4 patients under duodenoscopy, and eggs identical to those found in the feces were found; they were treated with praziquantel followed by purging, and adult worms recovered were identified as a species of Amphimerus (Calvopiña et al., Reference Calvopiña, Cevallos, Kumazawa and Eisenberg2011). The patients had a history of consuming smoked or lightly cooked fish; the number of people living in endemic communities is about 24 000 (Calvopiña et al., Reference Calvopiña, Cevallos, Kumazawa and Eisenberg2011). A further study reported a very high prevalence of infection with this fluke in domestic cats and dogs living in Chachi communities, Ecuador (Calvopiña et al., Reference Calvopiña, Cevallos, Atherton, Saunders, Small, Kumazawa and Sugiyama2015). Several species of freshwater fish were confirmed molecularly to have metacercariae of Amphimerus sp. (Romero-Alvarez et al., Reference Romero-Alvarez, Valverde-Muñoz, Calvopina, Rojas, Cevallos, Kumazawa, Takagi and Sugiyama2020). Human Amphimerus sp. infection is in most cases asymptomatic; however, it occasionally can cause non-specific generalized symptoms (Cevallos et al., Reference Cevallos, Calvopiña, Nipáz, Vicente-Santiago, López-Alban, Fernández-Soto, Guevara and Muro2017). Liver cirrhosis and pancreatitis occurred in cats and a cormorant infected with this fluke (Cevallos et al., Reference Cevallos, Calvopiña, Nipáz, Vicente-Santiago, López-Alban, Fernández-Soto, Guevara and Muro2017).
Amphimerus noverca (Braun, 1902) Baker, 1911 is a common parasite in the bile duct or pancreatic duct of dogs, wolverines and pigs in India (Beaver et al., Reference Beaver, Jung and Cupp1984; Roy and Tandon, Reference Roy and Tandon1992). Human infection with A. noverca was mentioned two times (in 1876 and 1878) in India (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). No further reports on human infections are available.
Amphimerus pseudofelineus (Rodriguez, Gomez Lince et Montalvan, 1949) Artigas and Perez, 1964 was originally reported under the name Opisthorchis guayaquilensis from 18 of 245 humans (based on eggs in feces) and several dogs (based on eggs in feces and adult worms) in a rural area of Ecuador by Rodriguez and colleagues (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). Then, Thatcher (Reference Thatcher1970) transferred this fluke to the genus Amphimerus. This fluke is now known to be distributed in North and South America, probably taking aquatic snails shedding cercariae and fish harbouring encysted metacercariae (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997).
Pseudamphistomum truncatum and P. aethiopicum
Pseudamphistomum truncatum (Rudolphi, 1819) Lühe, 1908 is a species of liver or gall bladder fluke in mammals in Europe and North America (Yamaguti, Reference Yamaguti1971; Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). Its human infection was briefly mentioned in 1928 in Europe (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). Later, in the Aleksee District of Tartaria, Russia, 31 human cases were diagnosed among 61 patients who had hepatobiliary system damage and had undergone bile analysis (Khamidullin et al., Reference Khamidullin, Liubina, Khamidullin and Medinskiĭ1991). Human infections were also detected in five areas along the Volga, Kama and Belaya Rivers in Russia (Khamidullin et al., Reference Khamidullin, Fomina, Sultanaeva and Khamidullin1995). The eggs and metacercariae of P. truncatum can be morphologically differentiated from those of O. felineus (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). The pathogenicity in humans is unknown but in otters and mink, P. truncatum can cause cholecystitis (Simpson et al., Reference Simpson, Gibbons, Khalil and Williams2005; Sherrad-Smith et al., Reference Sherrad-Smith, Cable and Chadwick2009).
Pseudamphistomum aethiopicum Pierantoni, 1942 was reported, on one occasion, to have caused a human infection with a small tumour in the intestinal wall (Yamaguti, Reference Yamaguti1971).
Dicrocoelium dendriticum and D. hospes
Within the genus Dicrocoelium, 5 species are currently recognized to be valid; D. dendriticum (Rudolphi, 1819) Looss, 1899, D. chinensis Tang and Tang, 1978, D. hospes Looss, 1907, D. orientale Sudarikov and Ryjikov, 1951 and D. petrowi Kassimov, 1952 (Manga-González and Ferreras, Reference Manga-González, Ferreras, Toledo and Fried2014).
Dicrocoelium dendriticum and Dicrocoelium hospes are known to cause human infections in rare instances. D. dendriticum (the lancet fluke) is a small liver fluke infecting domestic animals in the Northern coasts of Africa, Asia and the Americas (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997; Traversa et al., Reference Traversa, Lorusso, Otranto and Liu2013; Manga-González and Ferreras, Reference Manga-González, Ferreras, Toledo and Fried2014). Using mitochondrial nd1 and cox1 sequences, D. dendriticum could be distinguished from D. chinensis and D. hospes (Hayashi et al., Reference Hayashi, Tang, Ohari, Ohtori, Mohanta, Matsuo, Sato and Itagaki2017; Khan et al., Reference Khan, Afshan, Nazar, Firasat, Chaudhry and Sargison2021). In human dicrocoeliasis, there may be true (genuine) and false (spurious) infections. The geographical localities where human infections were reported are very wide, and the incidence is undoubtedly underestimated (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997).
The first intermediate host includes more than 90 species of land snails, with Cionella (= Cochlicopa) lubrica as the most important species (Traversa et al., Reference Traversa, Lorusso, Otranto and Liu2013). The cercariae are shed from the snails in clusters of thousands forming a so-called ‘slime ball’ (Traversa et al., Reference Traversa, Lorusso, Otranto and Liu2013). These cercariae are ingested by ants (Formica fusca, F. pratensis and F. rufibarbis) (Traversa et al., Reference Traversa, Lorusso, Otranto and Liu2013). Humans are infected accidentally by swallowing an infected ant together with food (Traversa et al., Reference Traversa, Lorusso, Otranto and Liu2013).
In human infections, the pathogenicity depends on the number of flukes infected and the duration of infection, and in many instances, there may be no notable symptoms. However, a prolonged period of constipation or diarrhoea, nausea, vomiting, abdominal discomfort and epigastric pain may occur (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). The diagnosis is based on the recovery of eggs in feces. However, an egg-positive result does not necessarily indicate a ‘true infection’, and a ‘spurious infection’ should be ruled out.
Serodiagnosis, including immunofluorescence, immunoblot, haemagglutination, complement fixation and ELISA have been introduced (Traversa et al., Reference Traversa, Lorusso, Otranto and Liu2013; Dar et al., Reference Dar, Shabir, Dar and Ganai2020). For treatment, albendazole (Magi et al., Reference Magi, Frati, Bernini, Sansoni and Zanelli2009), praziquantel (Drabick et al., Reference Drabick, Egan, Brown, Vick, Sandman and Neafie1988; Khandelwal et al., Reference Khandelwal, Shaw and Jain2008), triclabendazole (Khandelwal et al., Reference Khandelwal, Shaw and Jain2008; Ashrafi, Reference Ashrafi2010) and myrrh (herbal drug in Egypt; Mirazid®) (Abdul-Ghani et al., Reference Abdul-Ghani, Loutfy and Hassan2009) showed favourable results (Table 2).
D. hospes is a small liver fluke (more slender and smaller than D. dendriticum) infecting the bile duct and gall bladder of domestic animals in sub-Saharan Africa (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). This liver fluke can also cause genuine and spurious infections in humans (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). Two human patients in Sierra Leone (spurious infection was not ruled out) showed hepatitis-like symptoms; one patient had jaundice, and both had elevated bilirubin and transaminase levels (King, Reference King1971). In animals, D. hospes does not seem to cause much damage even in heavy infections (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). Diagnosis can be made by the recovery of eggs in feces; however, the eggs are morphologically indistinguishable from those of D. dendriticum and Eurytrema pancreaticum (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). Anthelmintic drugs used against D. dendriticum may have similar effects on D. hospes.
Fasciola hepatica and F. gigantica
Fasciola hepatica Linnaeus, 1758 (the sheep liver fluke) infects the bile duct of domestic animals, including cattle and sheep, and occasionally humans (Beaver et al., Reference Beaver, Jung and Cupp1984; Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005; Liu and Zhu, Reference Liu, Zhu and Liu2013). The parasite life cycle, biology, pathogenesis, pathology, epidemiology and clinical symptoms are similar between F. hepatica and F. gigantica (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). Hybrid forms between F. hepatica and F. gigantica have been found in Asia (Tang et al., Reference Tang, Zhou, Liu, Cheng, Zhang and Xu2021). Humans are accidental hosts (Liu and Zhu, Reference Liu, Zhu and Liu2013). They are infected through eating aquatic vegetables or raw liver of infected livestock animals (Liu and Zhu, Reference Liu, Zhu and Liu2013). The history of human infection with Fasciola spp. seems to be very long, since archaeological studies in ancient mummies in Egypt revealed remains of parasites (possibly part of F. hepatica worm) in liver tissue (David, Reference David1997). Sporadic cases of human Fasciola spp. infections (genuine or spurious infection unclear) were reported in Egypt in the 1950s (Kuntz et al., Reference Kuntz, Lawless, Langbehn and Malakatis1958), and an epidemic occurred in France in 1956 (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005). An extensive review of human fascioliasis was performed by Chen and Mott (Reference Chen and Mott1990) in which 2595 cases from over 40 countries in Europe, Asia, Western Pacific, the Americas and Africa from 1970 to 1990 were analysed. Now human fascioliasis (F. hepatica and F. gigantica) is recognized by the WHO as an important FBT disease with an estimated 2.4 million people infected annually and 180 million at risk in over 61 countries (Haseeb et al., Reference Haseeb, El Shazly, Arafa and Morsy2002). Large-scale epidemics occurred in France, Egypt and Iran (Parkinson et al., Reference Parkinson, Dalton, O'Neill, Palmer, Soulsby, Torgerson and Brown2011).
In the genus Fasciola, three species are currently recognized; F. hepatica, F. gigantica Cobbold, 1855, and F. nyanzae Leiper, 1910 (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009; Rajapakse et al., Reference Rajapakse, Pham, Karunathilake, Lawton and Le2020). Among them, the most common species worldwide are F. hepatica, F. gigantica and their hybrid forms (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009, Reference Mas-Coma, Valero, Bargues, Toledo and Fried2019). In Europe, the Americas and Oceania, only F. hepatica is found, whereas both species and their hybrids are commonly found in Asia and Africa (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005, Reference Mas-Coma, Valero, Bargues, Toledo and Fried2019). For phylogenetic analyses of Fasciola spp. worms, sequences of nuclear rDNA, including ITS1-5.8S-ITS2 and 28S rDNA, and mitochondrial genes, including nd1 and cox1, have been found to be useful and popularly used to differentiate F. hepatica, F. gigantica and the intermediate forms (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009, Reference Mas-Coma, Bargues and Valero2018, Reference Mas-Coma, Valero, Bargues, Toledo and Fried2019). Molecular analyses of five Korean Fasciola worms from cattle using ITS-2 and 28S rDNA revealed that one possessed the F. hepatica-type sequence, two F. gigantica-type sequences, and two possessed sequences of both types (Agatsuma et al., Reference Agatsuma, Arakawa, Iwagami, Honzako, Cahyangsih, Kang and Hong2000). An adult specimen recovered from a Korean male patient revealed F. hepatica ITS-1 genotype (Kang et al., Reference Kang, Jung, Lee, Hwang, Lim, Cho, Hwang and Chai2014). In China, using ITS-2 sequences to perform RFLP analysis, the Fasciola worms from Sichuan Province represented F. hepatica, those from Guangxi Province represented F. gigantica, and the ones (from sheep) from Heilongjiang Province may represent an ‘intermediate genotype’ (Huang et al., Reference Huang, He, Wang and Zhu2004). In Japan, both ITS-1 and ITS-2 were useful to discriminate Fsp1 (F. hepatica), Fsp2 (F. gigantica) and Fsp1/2 (intermediate form), but nd1 and cox1 could not discriminate the intermediate form properly (Itagaki et al., Reference Itagaki, Kikawa, Sakaguchi, Shimo, Terasaki, Shibahara and Fukuda2005). Also in Europe, ITS-2 showed low variability for both fasciolid species and appeared to furnish valuable information (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009). It is also notable that in Asian countries there are aspermic Fasciola flukes that may reproduce parthenogenetically (Itagaki et al., Reference Itagaki, Kikawa, Sakaguchi, Shimo, Terasaki, Shibahara and Fukuda2005).
A wide variety of freshwater snails that belong to the Lymnaeidae play the role of the first intermediate host (Bargues and Mas-Coma, Reference Bargues and Mas-Coma2005). In Asia, Europe, Africa and the Western Pacific, Galba truncatula (syn. Lymnaea truncatula) and Austropeplea ollula (syn. Austropeplea viridis) snails are most frequently involved (Bargues and Mas-Coma, Reference Bargues and Mas-Coma2005; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). Encysted metacercariae of F. hepatica are found on leaves of aquatic vegetables (watercress, alfalfa, water lettuce etc.) or green vegetation, bark or other smooth surfaces above or below the waterline (Beaver et al., Reference Beaver, Jung and Cupp1984; Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997; Liu and Zhu, Reference Liu, Zhu and Liu2013). People in Latin America, France and Algeria frequently acquire this disease by eating raw watercress (Nasturtium officinale) (Beaver et al., Reference Beaver, Jung and Cupp1984). In Asia, water bamboo, water caltrop and morning glory were suspected to be important sources of human infections. Recently in South Korea, the water parsley (water dropwort) and its juice have been suspected as the potential source of sporadic human infections (unpublished data). Humans can also be infected by eating raw livers of infected animals containing immature or young worms, and these worms attach to the pharyngeal mucosa and cause pain, bleeding, oedema and dyspnoea, and this condition was once called ‘halzoun’ (Beaver et al., Reference Beaver, Jung and Cupp1984).
F. hepatica is distributed almost worldwide, especially where extensive sheep and cattle raising is popularly done (Liu and Zhu, Reference Liu, Zhu and Liu2013). Human fascioliasis is one of the major public health problems in northern Africa, western Europe, Andean countries, the Caribbean area and the Caspian areas (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). In South Korea, Japan, China, Thailand and Vietnam, sporadic cases have been reported (Chen and Mott, Reference Chen and Mott1990; Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). It is of particular note that the highest prevalence (up to 72% by coprological and 100% by serological tests) and intensity ever have been found in some communities in the Northern Bolivian Altiplano (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997).
The pathological changes in the liver and bile duct of infected humans or animals depend primarily on the number of flukes infected (Chen and Mott, Reference Chen and Mott1990), and the pathology in human infections is similar to that reported in animals (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). However, in about 50% of cases, human fascioliasis is asymptomatic probably due to infection with a low number of flukes (Liu and Zhu, Reference Liu, Zhu and Liu2013). In rare instances (<10% of all patients), ectopic fascioliasis can occur (Beaver et al., Reference Beaver, Jung and Cupp1984; Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). The pathogenicity of F. hepatica and F. gigantica in humans is not recognizably different, although in sheep F. gigantica was found to be more pathogenic (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997).
After excystation in the duodenum or jejunum of their definitive host, the metacercariae of F. hepatica migrate through the intestinal wall and peritoneal cavity, and then penetrate into the liver parenchyma through Glisson's capsule and finally reside in the bile duct and gall bladder or go astray to other ectopic locations (Beaver et al., Reference Beaver, Jung and Cupp1984; Liu and Zhu, Reference Liu, Zhu and Liu2013). They can cause mechanical damage with focal haemorrhage, inflammatory reactions and necrotic lesions (Beaver et al., Reference Beaver, Jung and Cupp1984). Adult worms may live between 9 and 13.5 years in humans (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005). Stone formation in the bile duct or gall bladder is frequent, but liver cirrhosis is less common (Mas-Coma et al., Reference Mas-Coma, Valero, Bargues, Toledo and Fried2019). The pathogenesis of ectopic fascioliasis is not fully understood. The ectopic locations include the skin, subcutaneous tissue, skeletal muscle, blood vessel, lungs, orbit, ventricles of the brain, stomach, appendix, pancreas, intestinal wall, heart, spleen, epididymis and lymph nodes (Beaver et al., Reference Beaver, Jung and Cupp1984; Chen and Mott, Reference Chen and Mott1990).
The diagnosis of human fascioliasis can be done directly using parasitological techniques and indirectly by immunological tests and radiologic images, including ultrasound, CT, MRI and radioisotope scanning (Mas-Coma et al., Reference Mas-Coma, Valero, Bargues, Toledo and Fried2019).
Triclabendazole is currently the drug of choice for human fascioliasis (Fairweather, Reference Fairweather2009; Mas-Coma et al., Reference Mas-Coma, Valero, Bargues, Toledo and Fried2019) (Table 2). A problem related to triclabendazole is the appearance of drug resistance particularly in livestock animals; it was first described in Australia and then in many European and South American countries (Mas-Coma et al., Reference Mas-Coma, Valero, Bargues, Toledo and Fried2019). Praziquantel was also used for human fascioliasis but treatment failure was experienced even at high doses (Mas-Coma et al., Reference Mas-Coma, Valero, Bargues, Toledo and Fried2019). Albendazole, nitazoxanide, Mirazid® and artesunate are alternative drugs for potential use in human and animal fascioliasis (Mas-Coma et al., Reference Mas-Coma, Valero, Bargues, Toledo and Fried2019; Chai et al., Reference Chai, Jung and Hong2021a). Infection may be prevented by strict avoidance of consuming watercress and other metacercaria-carrying aquatic plants in endemic areas (Mas-Coma et al., Reference Mas-Coma, Valero, Bargues, Toledo and Fried2019).
Fasciola gigantica Cobbold, 1855 (the giant cattle liver fluke) is a large liver fluke species infecting the bile duct of domestic animals, including cattle and sheep, and occasionally humans (Beaver et al., Reference Beaver, Jung and Cupp1984; Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997; Liu and Zhu, Reference Liu, Zhu and Liu2013). In Asia and Africa, F. hepatica, F. gigantica and their hybrid forms are found, but in Europe, the Americas and Oceania, only F. hepatica is found (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005, Reference Mas-Coma, Valero, Bargues, Toledo and Fried2019). The parasite's life cycle, biology, pathogenesis, pathology, epidemiology and clinical symptoms are similar to those of F. hepatica (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). The most important snail host for F. gigantica is Radix auricularia (Liu and Zhu, Reference Liu, Zhu and Liu2013). The absence of F. gigantica in the New World may be explained by the absence of these snail species (Radix spp.) (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009). The metacercariae are encysted on the leaves of aquatic plants (Garcia, Reference Garcia2016).
Lung flukes
Paragonimiasis is a zoonotic disease caused by lung flukes of the genus Paragonimus (Chai and Jung, Reference Chai, Jung and Robertson2018). As of 1999, more than 50 nominal species had been described in this genus (Blair et al., Reference Blair, Xu and Agatsuma1999a; Narain et al., Reference Narain, Agatsuma, Blair and Liu2010; Doanh et al., Reference Doanh, Horii and Nawa2013a). However, 16–17 of them were synonymized with the others, and the remaining 36 species were regarded as valid or potentially valid (Blair et al., Reference Blair, Xu and Agatsuma1999a). Thereafter, five new species were described from Asia and Africa; P. vietnamensis (Doanh et al., Reference Doanh, Shinohara, Horii, Habe, Nawa and Le2007), P. pseudoheterotremus (Waikagul, Reference Waikagul2007), P. sheni (Shan et al., Reference Shan, Lin, Li, Hu, Shen and Lou2009), P. gondwanensis (Bayssade-Dufour et al., Reference Bayssade-Dufour, Chermette, Šundić and Radujković2014) and P. kerberti (Bayssade-Dufour et al., Reference Bayssade-Dufour, Chermette, Šundić and Radujković2015). Among the 41 nominal species (or subspecies), at least 9 are known to cause human infections, including P. westermani, P. africanus, P. gondawanensis, P. heterotremus, P. kellicotti, P. mexicanus, P. skrjabini, P. skrjabini miyazakii and P. uterobilateralis (Table 3) (Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b; Bayssade-Dufour et al., Reference Bayssade-Dufour, Chermette, Šundić and Radujković2014; Chai and Jung, Reference Chai, Jung and Robertson2018; Blair, Reference Blair, Toledo and Fried2019). The lung flukes can cause pulmonary as well as extrapulmonary infections in humans. The global number of people infected with Paragonimus spp. was estimated at about 23 million in 48 countries (mostly in China) with 292 million people at risk worldwide (Blair, Reference Blair, Toledo and Fried2019).
Table 3. Lung flukes infecting humans with biological, clinical characteristics and geographical distribution

a The geographical distributions of lung flukes are mostly referred from Chai and Jung (Reference Chai, Jung, Toledo and Fried2019).
Species involved
Paragonimus westermani
Paragonimus westermani (Kerbert, 1878) Braun, 1899 (the Oriental lung fluke) was originally reported from the lungs of a Bengal tiger (India) that died in the Zoological Garden in Amsterdam (Beaver et al., Reference Beaver, Jung and Cupp1984). Since then, this species has been reported mainly in Asian countries (Blair et al., Reference Blair, Xu and Agatsuma1999a; Narain et al., Reference Narain, Agatsuma, Blair and Liu2010). Human infections were first found in a Portuguese residing in Taiwan through recovery of adult worm (s) after autopsy in 1880 and also in Japanese patients through detecting eggs from bloody sputum in 1880 and then adult worms in 1883 in Japan (Beaver et al., Reference Beaver, Jung and Cupp1984). Now human infections with this lung fluke continue to occur in Asian countries, including China, Taiwan, Japan, South Korea, Far Southeast Russia, the Philippines and recently India (Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b; Singh et al., Reference Singh, Sugiyama, Devi and Singh2015; Blair, Reference Blair, Toledo and Fried2019). While human infections may be decreasing in Japan and South Korea, new endemic foci have been detected in several other countries (Yoshida et al., Reference Yoshida, Doanh and Maruyama2019). Morphologically P. westermani differs from other Paragonimus species in the patterns of lobation of the ovary and testes (Miyazaki, Reference Miyazaki1991). The ovary of P. westermani has 6 simple lobes, whereas that of P. mexicanus or P. ohirai displays many delicate branches (Miyazaki, Reference Miyazaki1991).
Paragonimus siamensis was described as a new species from experimental cats infected with metacercariae from the freshwater crab Parathelphusa germaini in Thailand (Miyazaki and Wykoff, Reference Miyazaki and Wykoff1965). Later, this lung fluke was found in animals in Sri Lanka (Kannagara and Karunaratne, Reference Kannagara and Karunaratne1969), India (Devi et al., Reference Devi, Narain, Mahanta, Nirmolia, Blair, Saikia and Agatsuma2013) and the Philippines (Yoshida et al., Reference Yoshida, Doanh and Maruyama2019). An adult specimen recovered from a New Guinea native man in 1926 was preserved in School of Public Health and Tropical Medicine, Australia, and it was restudied and assigned as P. siamensis (Wang et al., Reference Wang, Blair, Min, Li and Wang2011). Filopaludina martensi martensi snails are the first intermediate host (Yaemput et al., Reference Yaemput, Dekumyoy and Visiassuk1994), and freshwater crabs, including Ceylonthelphusa rugosa, play the role of the second intermediate hosts (Blair et al., Reference Blair, Xu and Agatsuma1999a). Recent molecular studies reported that P. siamensis is nested within the P. westermani complex (Devi et al., Reference Devi, Narain, Mahanta, Nirmolia, Blair, Saikia and Agatsuma2013; Blair, Reference Blair, Toledo and Fried2019).
Phylogenetic studies revealed that P. westermani is a species complex comprising of two groups; the East Asia group (Japan, South Korea, China and Taiwan) and the Southeast Asia group (Malaysia and the Philippines) based on morphological and molecular data (Blair et al., Reference Blair, Agatsuma, Watanobe, Okamoto and Ito1997; Doanh et al., Reference Doanh, Shinohara, Horii, Habe and Nawa2009). However, recent discoveries of P. westermani in Thailand (Sugiyama et al., Reference Sugiyama, Morishima, Binchai, Rangsiruji and Ketudat2007), India (Tandon et al., Reference Tandon, Prasad, Chatterjee and Bhutia2007) and Sri Lanka (Iwagami et al., Reference Iwagami, Rajapakse, Paranagama, Okada, Kano and Agatsuma2008) provided evidence that P. westermani complex was constructed with more than two groups (Blair et al., Reference Blair, Agatsuma, Wang, Murrell and Fried2007).
The first intermediate host is variable species of freshwater snails (Table 3). The second intermediate host is crustaceans, including freshwater crayfish Cambaroides spp. (C. similis, C. dauricus and C. schrenki), Eriocheir spp. crabs (E. japonicus and E. sinensis) and a variety of other crab species belonging to the family Potamidae or Parathelphusidae (Blair et al., Reference Blair, Xu and Agatsuma1999a). In China, Taiwan and South Korea, freshwater shrimps (Macrobrachium nipponensis and other species) were also reported as the second intermediate hosts (Blair et al., Reference Blair, Xu and Agatsuma1999a). There are several kinds of paratenic hosts, for example, wild boars, bears, wild pigs and rats; in these hosts worms do not mature to be adults but remain at a juvenile stage; they can be an important source of human infection (Miyazaki and Habe, Reference Miyazaki and Habe1976; Shibahara et al., Reference Shibahara, Nishida, Torii, Gyoten, Tsuboi and Sakai1992). The egg-laying capacity of Paragonimus worms was reported to be 11 000– 104 000 eggs per day per worm (EPD/worm) in experimental dogs (Yokogawa, Reference Yokogawa1965).
The principal mode of human P. westermani infection is consumption of raw or improperly cooked (pickled) freshwater crabs or crayfish (Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b). In Asian countries, famous dishes causing human paragonimiasis have been known, for example, ‘drunken crab’ in China, ‘Kejang (= sauced crab)’ in South Korea, ‘Oboro-kiro (= crab juice soup)’ in Japan, ‘Goong ten (= raw crayfish salad)’ in Thailand, and ‘Kinuolao (= raw crab)’ in the Philippines (Kim, Reference Kim1984a; Nakamura-Uchiyama et al., Reference Nakamura-Uchiyama, Mukae and Nawa2002). It is of note that freshwater crab or crayfish juice was used in South Korea and Japan for traditional treatment of febrile diseases, such as measles, asthma and urticaria (Kim, Reference Kim1984a; Nakamura-Uchiyama et al., Reference Nakamura-Uchiyama, Mukae and Nawa2002). This kind of practice was formerly an important mode of contracting paragonimiasis, especially in children. Another important mode of infection in Japan is ingestion of raw or undercooked boar meat containing P. westermani metacercariae or juvenile worms (Miyazaki and Habe, Reference Miyazaki and Habe1976; Shibahara et al., Reference Shibahara, Nishida, Torii, Gyoten, Tsuboi and Sakai1992; Nakamura-Uchiyama et al., Reference Nakamura-Uchiyama, Mukae and Nawa2002). In paratenic hosts, worms do not mature and stay in muscles and tissues; when they were eaten by humans, they could develop into adult worms (Nakamura-Uchiyama et al., Reference Nakamura-Uchiyama, Mukae and Nawa2002; Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b).
The geographical distribution of P. westermani is wide, including East Asia, Southeast Asia, South Asia (including India) and even the Western Pacific (Blair, Reference Blair, Toledo and Fried2019). Human cases of P. westermani were reported also in the USA (Fried and Abruzzi, Reference Fried and Abruzzi2010; Boland et al., Reference Boland, Vaszar, Jones, Mathison, Rovzar, Colby, Leslie and Tazelaar2011); however, the existence of the life cycle of P. westermani in North America needs to be verified.
When the definitive host, including humans, consumes crab or crayfish meat containing metacercariae, they excyst in the duodenum and penetrate into the intestinal wall; during this process they mature into juvenile flukes (Chai and Jung, Reference Chai, Jung and Robertson2018). The juvenile flukes enter the inner wall (abdominal muscle) of the abdominal cavity and reappear in the abdominal cavity; they then penetrate the diaphragm and enter the pleural cavity in about 14 days after infection (Yokogawa, Reference Yokogawa1965). In the pleural cavity, two worms mate and then move to the lung parenchyma where a fibrous cyst called the ‘worm cyst’ or ‘worm capsule’ develops around them (Narain et al., Reference Narain, Agatsuma, Blair and Liu2010; Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b). The two worms exchange sperm and produce eggs within the worm cyst, which accumulate for some time in acute stages but in chronic stages when tissue necrosis occurs around the cyst, the eggs escape from the cyst into small bronchioles (Yokogawa, Reference Yokogawa1965; Blair et al., Reference Blair, Xu and Agatsuma1999a). There are triploid and tetraploid forms of P. westermani which do not produce sperm and reproduce parthenogenetically (Miyazaki, Reference Miyazaki1991; Terasaki et al., Reference Terasaki, Habe, Ho, Jian, Agatsuma, Shibahara, Sugiyama and Kawashima1995; Agatsuma et al., Reference Agatsuma, Iwagami, Sato, Iwashita, Hong, Kang, Ho, Su, Kawashima and Abe2003). The patients may undergo variable clinical types, including pulmonary, thoracic, abdominal, cerebral, spinal and cutaneous paragonimiasis (Nakamura-Uchiyama et al., Reference Nakamura-Uchiyama, Mukae and Nawa2002; Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b).
In pulmonary infections, P. westermani worms lie in worm cysts of host origin, 1–2 cm in diameter, within the lung parenchyma (Blair et al., Reference Blair, Xu and Agatsuma1999a). The lung lesions can be classified into infiltrative, nodular and cavitating shadow types, or combination of these types (Nakamura-Uchiyama et al., Reference Nakamura-Uchiyama, Mukae and Nawa2002). In early stages of infection, there is no exit from the worm cyst, and eggs as well as excretions, metabolic products and tissue debris may gather progressively within the cyst, which becomes distended, and the cyst wall becomes thick and fibrotic (Yokogawa, Reference Yokogawa1965). In chronic stages, eggs can be demonstrated in the blood-tinged portion of the sputum (Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b). The earliest possible clinical manifestations by day 15 after infection include abdominal pain, fever, chill, fatigue and diarrhoea (Choi, Reference Choi1990; Nakamura-Uchiyama et al., Reference Nakamura-Uchiyama, Mukae and Nawa2002; Procop, Reference Procop2009). Eosinophilia of up to 25% can occur at 2 months after infection (Choi, Reference Choi1990; Procop, Reference Procop2009). In chronic stages, the most common and important manifestations include chronic cough, rusty-coloured sputum (= haemoptysis), chest pain, dyspnoea and crepitation (Im et al., Reference Im, Kong, Shin, Yang, Song, Han, Kim, Cho and Ham1993; Nakamura-Uchiyama et al., Reference Nakamura-Uchiyama, Mukae and Nawa2002; Procop, Reference Procop2009). In chest radiographs, cavitating lesions called ‘ring shadows’ or ‘cysts’ are commonly seen (Im et al., Reference Im, Kong, Shin, Yang, Song, Han, Kim, Cho and Ham1993). Pleural effusion is frequently seen in Korean and Japanese patients (Im et al., Reference Im, Kong, Shin, Yang, Song, Han, Kim, Cho and Ham1993; Nakamura-Uchiyama et al., Reference Nakamura-Uchiyama, Mukae and Nawa2002).
The extrapulmonary migration of Paragonimus worms has been suggested to occur due to several reasons. The most important reason is the complex migration route of the worms (Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b). Another reason was suggested to be seeking sexual partners to exchange sperm (Miyazaki, Reference Miyazaki1991). The brain is the most commonly involved ectopic site, although the mechanisms and route of worm migration to the brain are not well understood (Choi, Reference Choi1990).
Cerebral paragonimiasis occurs quite commonly in P. westermani infection, about 1% of all paragonimiasis patients (Oh, Reference Oh1969). Five major symptoms include the Jacksonian type seizure, headache, visual disturbance, motor and sensory disturbances, and 5 major signs are optic atrophy, mental deterioration, hemiplegia, hemi-hypalgesia and homonymous hemianopsia (Oh, Reference Oh1969). Intracranial haemorrhage can also occur though rare in the incidence (Choo et al., Reference Choo, Suh, Lee, Lee, Song, Shin and Lee2003; Koh et al., Reference Koh, Kim, Wang, Chai, Chong, Park, Cheon and Phi2012). In skull radiography, cerebral calcification is the most commonly encountered finding, and temporal, occipital and parietal lobes of the brain are the predilection sites (Oh, Reference Oh1968b). Compared with cerebral infections, spinal paragonimiasis is relatively rare (Oh, Reference Oh1968a). The predilection site is extradural areas of the thoracic level (Choi, Reference Choi1990).
Abdominal paragonimiasis is probably more common than cerebral and spinal infections (Meyers and Neafie, Reference Meyers, Neafie, Binford and Conner1976). The affected organs include the abdominal wall (muscle), peritoneal cavity, liver, spleen, pancreas, heart, greater omentum, appendix, ovary, uterus, scrotum, inguinal regions, thigh and urinary tract (Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b). Cutaneous and subcutaneous paragonimiasis are very rare compared to pleuropulmonary and other ectopic types (Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b). P. westermani can cause abscesses and ulcers in the skin or subcutaneous tissue, but P. skrjabini can cause migrating subcutaneous nodules (Meyers and Neafie, Reference Meyers, Neafie, Binford and Conner1976).
Conventional methods to diagnose human Paragonimus infections are microscopic examinations of the sputum or fecal samples for detecting eggs, chest radiography to observe the lung lesions (differential diagnosis needed with tuberculosis and lung cancers), and serological tests, including intradermal test, indirect haemagglutination test, ELISA, and others to detect antibodies (Lee et al., Reference Lee, Kim, Back, Chai, Kim and Hwang2003; Sugiyama et al., Reference Sugiyama, Singh, Rangsiruji and Liu2013; Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b). Sputum eggs can usually be detected in chronic cases when the worm cyst is ruptured and connected to bronchioles. In children, aged-people or handicapped individuals, sputum is frequently swallowed, and in such cases, eggs could be detected in feces (Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b). Among the serological tests, ELISA (such as microplate ELISA and multiple-dot ELISA) is so far the most reliable tool because this assay method shows high sensitivity and high specificity (Lee et al., Reference Lee, Kim, Back, Chai, Kim and Hwang2003; Yoshida et al., Reference Yoshida, Doanh and Maruyama2019). Molecular methods, including PCR and DNA sequencing, have become useful for specific identification of Paragonimus eggs and worms (Sugiyama et al., Reference Sugiyama, Singh, Rangsiruji and Liu2013). Other methods, including PCR-RFLP, multiplex PCR, random amplified polymorphic DNA (RAPD), and DNA hybridization, have also been applied for specific identification of Paragonimus (Narain et al., Reference Narain, Agatsuma, Blair and Liu2010; Sugiyama et al., Reference Sugiyama, Singh, Rangsiruji and Liu2013).
Praziquantel is the drug of choice for treating paragonimiasis (P. westermani and other Paragonimus spp.), including cerebral infections (Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b) (Table 2). Rarely, allergic reactions may occur following praziquantel treatment; however, such patients can be successfully treated by desensitization to praziquantel (Kyung et al., Reference Kyung, Cho, Kim, Park, Jeong, Lee, Sung and Lee2011). Triclabendazole is a new promising drug for the treatment of pulmonary paragonimiasis (Keiser et al., Reference Keiser, Engels, Büscher and Utzinger2005). However, its efficacy on cerebrospinal paragonimiasis remains to be determined (Chai, Reference Chai, Garcia, Tanowitz and Del Brutto2013b). Prevention of contamination of the crab and crayfish with the cercariae of P. westermani depends on environmental control of surface waters through the elimination of freshwater snails. Long-term health education of young people to avoid eating raw or improperly cooked crayfish or crabs seems to be one of the best ways to prevent P. westermani infection in endemic areas.
Paragonimus africanus
Paragonimus africanus Voelker and Vogel, 1965 was originally described from the mongoose in Cameroon and is now known to be distributed throughout sub-Saharan Africa (Narain et al., Reference Narain, Agatsuma, Blair and Liu2010). Up to 2008, it was estimated that there had been 2295 confirmed cases of human paragonimiasis in Africa mostly due to P. africanus and P. uterobilateralis with the vast majority occurring in Nigeria and Cameroon (Aka et al., Reference Aka, Adoubryn, Rondelaud and Dreyfuss2008; Cumberlidge et al., Reference Cumberlidge, Rollinson, Vercruysse, Tchuem Tchuenté, Webster and Clark2018). Freshwater crabs Liberonautes spp. and Sudanonautes spp. carry the metacercariae (Aka et al., Reference Aka, Adoubryn, Rondelaud and Dreyfuss2008). Its morphological characters include the distinctly larger oral sucker than the ventral sucker, a delicately branched ovary, and highly branched testes which are significantly larger than the ovary (Narain et al., Reference Narain, Agatsuma, Blair and Liu2010). Uncooked crab meat is an important source of human infection (Aka et al., Reference Aka, Adoubryn, Rondelaud and Dreyfuss2008). Nucleotide sequences of ITS2 and cox1 have been used for specific diagnosis (as P. africanus) of fecal eggs from monkeys (Friant et al., Reference Friant, Brown, Saari, Segel, Slezak and Goldberg2015) and humans (Nkouawa et al., Reference Nkouawa, Okamoto, Mabou, Edinga, Yamasaki, Sako, Nakao, Nakaya, Blair, Agatsuma, Enyong, Shibahara, Moyou-Somo and Ito2009). Clinical manifestations are similar to P. westermani infection. Cerebral infection has been suspected but never proved (Aka et al., Reference Aka, Adoubryn, Rondelaud and Dreyfuss2008). Sputum and stool examinations to detect eggs are the main diagnostic procedures.
Paragonimus gondwanensis
Paragonimus gondwanensis Bayssade-Dufour et al., Reference Bayssade-Dufour, Chermette, Šundić and Radujković2014 was described as a new species from humans (only eggs) and carnivorous mammals (adult worms) in Cameroon (Bayssade-Dufour et al., Reference Bayssade-Dufour, Chermette, Šundić and Radujković2014). This species is morphologically distinct from all other Paragonimus species in having a very short excretory bladder, once used as a character for a different genus, Euparagonimus (Bayssade-Dufour et al., Reference Bayssade-Dufour, Chermette, Šundić and Radujković2014). However, molecular data are lacking, and the validity of this species remains to be determined (Rabone et al., Reference Rabone, Wiethase, Clark, Rollinson, Cumberlidge and Emery2021). The second intermediate host is freshwater crabs, Sudanonautes africanus (Bayssade-Dufour et al., Reference Bayssade-Dufour, Chermette, Šundić and Radujković2014).
Paragonimus heterotremus
Paragonimus heterotremus Chen and Hsia, 1964 was first discovered from rats in China and is now known to be distributed in China, Indochina peninsula, Sri Lanka and India (Sing et al., Reference Singh, Sugiyama, Umehara, Hiese and Khalo2009; Narain et al., Reference Narain, Agatsuma, Blair and Liu2010; Yoshida et al., Reference Yoshida, Doanh and Maruyama2019). In 2007, P. pseudoheterotremus was reported as a new species from a cat experimentally infected with the metacercariae in crabs from a mountainous area of Thailand (Waikagul, Reference Waikagul, Wongsaroj, Radomyos, Meesomboon, Praewanich and Jongsuksuntikul1997). Later, however, this species is considered a geographical variation of the P. heterotremus complex, based on molecular studies using ITS2 and cox1 sequences (Sanpool et al., Reference Sanpool, Intapan, Thanchomnang, Janwan, Nawa, Blair and Maleewong2013; Doanh et al., Reference Doanh, Thaenkham, An, Hien, Horii and Nawa2015; Tantrawatpan et al., Reference Tantrawatpan, Tapdara, Agatsuma, Sanpool, Intapan, Maleewong and Saijuntha2021). Human infection with P. heterotremus was first identified by the recovery of an adult worm from a 13-year-old boy in Nakorn-Nayok Province, Thailand in 1965 (Miyazaki and Harinasuta, Reference Miyazaki and Harinasuta1966). In the same year, eggs presumed to be of P. heterotremus were detected in the bloody sputum of a patient in Guangxi, China (Zhou et al., Reference Zhou, Yang, Tan, Zhang, Shi and Zhou2021). Since then, a lot of human cases have been reported (Miyazaki, Reference Miyazaki1991; Singh et al., Reference Singh, Sugiyama, Umehara, Hiese and Khalo2009; Doanh et al., Reference Doanh, Horii and Nawa2013a). Freshwater snails, Assiminea sp., Oncomelania hupensis and Neotricula aperta (syn. Tricula aperta), are known to serve as the first intermediate host, and freshwater crabs, including Larnaudia beusekomae, Siamthelphusa paviei and Potamiscus smithianus (Thailand), Potamon flexum and Sinolapotamon patellifer (China), take the role of the second intermediate hosts (Blair et al., Reference Blair, Xu and Agatsuma1999a). In adult specimens, the ventral sucker is characteristically small, about a half the diameter of its oral sucker (Narain et al., Reference Narain, Agatsuma, Blair and Liu2010). The ovary and testes are both delicately branched (Miyazaki, Reference Miyazaki1991). The pathology and clinical manifestations in P. heterotremus infection is similar to those seen in P. westermani infection. The diagnosis is based on the recovery of eggs in bloody sputum, serology, chest radiography and molecular genetic analysis.
Paragonimus kellicotti
Paragonimus kellicotti Ward, 1908 was originally discovered in a cat and a dog in the USA (Blair et al., Reference Blair, Xu and Agatsuma1999a; Procop, Reference Procop2009). The lobation of the ovary and testes is more complex than in P. westermani (Procop, Reference Procop2009). Now this species is known to occur in central and eastern parts of the USA and adjacent areas of Canada (Procop, Reference Procop2009). Beaver et al. (Reference Beaver, Jung and Cupp1984) considered the first human case to be a German labourer who worked in the USA in the 1890s and had eaten crayfish. Another case was a Canadian man aged 51 years who had never been outside of Quebec and complained of systemic and pulmonary symptoms (Béland et al., Reference Béland, Boone, Donevan and Mankiewicz1969; Coogle et al., Reference Coogle, Sosland and Bahr2021). Thereafter, from 1984 until 2017, a total of 20 human cases have been documented (Lane et al., Reference Lane, Barsanti, Santos, Young, Lubner and Weil2009, Reference Lane, Marcos, Onen, Demertzis, Hayes, Davilla, Nurutdinova, Bailey and Weil2012; Procop, Reference Procop2009; Fried and Abruzzi, Reference Fried and Abruzzi2010; Coogle et al., Reference Coogle, Sosland and Bahr2021). Molecular studies were done on ITS2, cox1 and other genetic loci (Blair et al., Reference Blair, Wu, Chang, Gong, Agatsuma, Zhang, Chen, Lin, Chen, Waikagul, Guevara, Feng and Davis1999b; Fischer et al., Reference Fischer, Curtis, Marcos and Weil2011; McNulty et al., Reference McNulty, Fischer, Townsend, Curtis, Weil and Mitreva2014). Based on ITS2 and cox1 sequences, P. kellicotti was genetically closest to P. macrorchis and P. mexicanus, and then to P. heterotremus, but distant from P. westermani and P. siamensis (Blair et al., Reference Blair, Wu, Chang, Gong, Agatsuma, Zhang, Chen, Lin, Chen, Waikagul, Guevara, Feng and Davis1999b). The draft genome of P. kellicotti has been established and analysed in comparison with those of P. westermani, P. skrjabini miyazakii and P. heterotremus (Rosa et al., Reference Rosa, Choi, McNulty, Jung, Martin, Agatsuma, Sugiyama, Le, Doanh, Maleewong, Blair, Brindley, Fischer and Mitreva2020). The second intermediate host is the crayfish of Cambarus bartoni, C. robustus and C. virilis, Orconectes propinquus, O. rusticus and Procambarus blandingi acutus, or crabs Geothelphusa dehaani (Blair et al., Reference Blair, Xu and Agatsuma1999a). In the USA, frozen or pickled crabs available at markets are suspected to be the source of P. kellicotti infection (Procop, Reference Procop2009). Pleuropulmonary infection is dominant in P. kellicotti infection, and the most frequent clinical manifestations among 21 North American patients were cough, pleural effusion, fever, fatigue or malaise, weight loss, chest pain, dyspnoea, haemoptysis and eosinophilia (Coogle et al., Reference Coogle, Sosland and Bahr2021). Their diagnosis was based on microscopic examinations of sputum, pleural fluid or bronchoalveolar lavage and serological tests using complement fixation test, immunoblot or western blot (Coogle et al., Reference Coogle, Sosland and Bahr2021).
Paragonimus mexicanus
Paragonimus mexicanus Miyazaki and Ishii, Reference Miyazaki and Ishii1968 (syn. Paragonimus peruvianus) was first described from opossums in Colima, Mexico (Miyazaki and Ishii, Reference Miyazaki and Ishii1968). Its characteristic morphology includes a somewhat larger oral sucker than the ventral sucker and a strongly lobed ovary and two testes (Miyazaki, Reference Miyazaki1991). Metacercariae have no cyst wall (Sugiyama et al., Reference Sugiyama, Singh, Rangsiruji and Liu2013). P. mexicanus is now known to be distributed in Central and South Americas (Blair et al., Reference Blair, Xu and Agatsuma1999a). A human case reported by Báez M. and Galán J. in 1961, a 35-year-old Mexican man from whom eggs were detected in lung tissue, was believed to have been due to P. mexicanus infection (Miyazaki and Ishii, Reference Miyazaki and Ishii1968). In Costa Rica, 2 human cases (total 4) were reported in 1968 and 1982 (Beaver et al., Reference Beaver, Jung and Cupp1984), and since then, 28 additional cases were described until 2013 (Hernández-Chea et al., Reference Hernández-Chea, Jiménez-Rocha, Castro, Blair and Dolz2017). In Ecuador, as early as in 1922, a paragonimiasis patient was found from a coastal region of Chone-Manabi (Calvopiña et al., Reference Calvopiña, Romero, Castañeda, Hashiguchi and Sugiyama2014); this seems to be the first human case infected with P. mexicanus. Thereafter, until 2007, the total number of human infections in Ecuador was 3822 patients (Calvopiña et al., Reference Calvopiña, Romero, Castañeda, Hashiguchi and Sugiyama2014). Using a combination of ITS2, 28S and cox1 sequences, the phylogenetic position of P. mexicanus was analysed, and it was closest to P. heteretremus and then P. macrorchis; however, far from P. westermani and P. siamensis (Devi et al., Reference Devi, Narain, Mahanta, Nirmolia, Blair, Saikia and Agatsuma2013). However, in Mexico, Ecuador (López-Caballero et al., Reference López-Caballero, Oceguera-Figueroa and León-Règagnon2013) and Guatemala (Landaverde-González et al., Reference Landaverde-González, Osgood, Quinoñez, Monzón, Rodas and Monroy2022), several new genetic groups distinct from P. mexicanus (origin; Colima, Mexico) were found based on cox1 sequences. One from Ecuador could be assigned as P. ecuadoriensis Voelker and Arzube, 1979 (once synonymized with P. mexicanus by Vieira et al. in 1992), and two possible new species (or subspecies) groups included one from Chiapas, Mexico and San José, Guatemala and the other from Veracruz, Mexico (Iwagami et al., Reference Iwagami, Monroy, Rosas, Pinto, Guevara, Vieira, Agatsuma and Agatsuma2003; López-Caballero et al., Reference López-Caballero, Oceguera-Figueroa and León-Règagnon2013). Freshwater crabs, Hypolobocera aequatorialis, H. chilensis and H. guayaquilensis, Pseudothelphusa dilatata, P. nayaritae, P. propinqua and P. terrestris carry the metacercariae (Blair et al., Reference Blair, Xu and Agatsuma1999a; Calvopiña et al., Reference Calvopiña, Romero-Alvarez, Rendon, Takagi and Sugiyama2018). Raw crabs with vegetables and lemon juice is the main infection source for Peruvians (Nakamura-Uchiyama et al., Reference Nakamura-Uchiyama, Mukae and Nawa2002), and ceviche that contains uncooked crustacean meat is an important source for Mexicans (Procop, Reference Procop2009). Clinically P. mexicanus infection is mostly of the pulmonary type (99.7% of 3822 cases in Ecuador) (Calvopiña et al., Reference Calvopiña, Romero, Castañeda, Hashiguchi and Sugiyama2014). However, cerebral infection with intracerebral haemorrhage can occur (Brenes Madrigal et al., Reference Brenes Madrigal, Rodriguez-Ortiz, Vargas Solano, Ocamp Obando and Ruiz Sotela1982).
Paragonimus skrjabini
Paragonimus skrjabini Chen, 1959 (syn. P. szechuanensis, P. hueitungensis and P. veocularis) was first described from the lungs of viverrid cats in Guangzhou, China (Blair et al., Reference Blair, Xu and Agatsuma1999a). Phylogenetic studies revealed that there exists a P. skrjabini complex, which includes P. skrjabini, P. skrjabini miyazakii and P. proliferus (syn. P. hocuoensis) (Doanh et al., Reference Doanh, Hien, Nonaka, Horii and Nawa2013b; Yang et al., Reference Yang, Li and Zhou2021; Shu et al., Reference Shu, Li, Tian, Meng, He, Wang and Wang2021a). P. skrjabini is now known to occur in China, Thailand, Vietnam and northeast India (Singh et al., Reference Singh, Singh and Sugiyama2006; Doanh et al., Reference Doanh, Hien, Nonaka, Horii and Nawa2013b; Blair, Reference Blair, Toledo and Fried2019). Human infections were reported for the first time in Sichuan and then in Hunan (Blair et al., Reference Blair, Xu and Agatsuma1999a). Numerous provinces in China have been found to be endemic for P. skrjabini infection (Zhou et al., Reference Zhou, Yang, Tan, Zhang, Shi and Zhou2021). The first intermediate host is freshwater snails, including Assiminea lutea, Tricula spp. and Neotricula spp., and the second host is freshwater crabs, Aprapotamon grahami, Isolapotamon spp., Sinopotamon spp., Tenuilapotamon spp., Stigmatomma denticulatum and Haberma nanum (Blair et al., Reference Blair, Xu and Agatsuma1999a; Shu et al., Reference Shu, Yang, Wang, Li, Tian, Bai, Meng, He and Wang2021b). Morphologically this species is characterized by profusely branched ovary and testes and an elongated body (Blair et al., Reference Blair, Chang, Chen, Cui, Wu, Agatsuma, Iwagami, Corlis, Fu and Zhan2005; Narain et al., Reference Narain, Agatsuma, Blair and Liu2010). P. skrjabini more frequently cause cutaneous or cerebral infections than pulmonary lesions, and the skin lesions usually contain juvenile flukes; thus, humans are considered an abnormal definitive host (Nakamura-Uchiyama et al., Reference Nakamura-Uchiyama, Mukae and Nawa2002). Diagnosis is most commonly done by serological tests, including intradermal test and ELISA (Yu et al., Reference Yu, Zhang, Chen, Zheng, Ai, Ye and Wang2017).
Paragonimus skrjabini miyazakii
Paragonimus skrjabini miyazakii (Kamo, Nishida, Hatsushika and Tomimura, 1961) Blair et al., Reference Blair, Chang, Chen, Cui, Wu, Agatsuma, Iwagami, Corlis, Fu and Zhan2005 (syn. P. miyazakii) was first described from dogs experimentally fed the metacercariae from a freshwater crab in Japan (Kamo et al., Reference Kamo, Nishida, Hatsushika and Tomimura1961). Human infections were first identified in the Kanton District of Honshu, Japan (Yokogawa et al., Reference Yokogawa, Araki, Saito, Momose, Kimura, Suzuki, Chiba, Kutsumi and Minai1974). This lung fluke has been found in Shikoku, Kyushu, and the southern half of Honshu, but never from outside of Japan (Miyazaki, Reference Miyazaki1991). In the University of Miyazaki, Japan, approximately 800 cases (17–49 cases annually) of human paragonimiasis (predominantly by P. westermani and less frequently by P. skrjabini miyazakii) were diagnosed between 1986 and 2018 (Yoshida et al., Reference Yoshida, Doanh and Maruyama2019). Yatera et al. (Reference Yatera, Hanaka, Hanaka, Yamasaki, Nishida, Kawanami, Kawanami, Ishimoto, Kanazawa and Mukae2015) reviewed 46 P. skrjabini miyazakii patients reported from 1974 to 2009 having pulmonary involvement. Phylogenetic studies revealed that P. skrjabini miyazakii is clearly included among the P. skrjabini complex (Blair et al., Reference Blair, Chang, Chen, Cui, Wu, Agatsuma, Iwagami, Corlis, Fu and Zhan2005; Doanh et al., Reference Doanh, Hien, Nonaka, Horii and Nawa2013b; Yang et al., Reference Yang, Li and Zhou2021; Shu et al., Reference Shu, Li, Tian, Meng, He, Wang and Wang2021a; Reference Shu, Yang, Wang, Li, Tian, Bai, Meng, He and Wang2021b). Freshwater crabs, Geothelphusa dehaani carry the metacercariae (Blair et al., Reference Blair, Xu and Agatsuma1999a). The adult flukes are somewhat elongated with severely branched ovary and testes (Miyazaki, Reference Miyazaki1991; Blair et al., Reference Blair, Chang, Chen, Cui, Wu, Agatsuma, Iwagami, Corlis, Fu and Zhan2005). Whereas the main clinical features of P. skrjabini miyazakii infection are pleural manifestations with pneumothorax and pleural effusion (in most cases worms cannot mature to become adults in humans), those of P. westermani infection are pulmonary involvement (Yatera et al., Reference Yatera, Hanaka, Hanaka, Yamasaki, Nishida, Kawanami, Kawanami, Ishimoto, Kanazawa and Mukae2015).
Paragonimus uterobilateralis
Paragonimus uterobilateralis Voelker and Vogel, 1965 was originally described from the mongoose in Cameroon (Narain et al., Reference Narain, Agatsuma, Blair and Liu2010), and is now known to be distributed in mid-western sub-Saharan Africa (Blair et al., Reference Blair, Xu and Agatsuma1999a). Human infection was first found in Nigeria (Blair et al., Reference Blair, Xu and Agatsuma1999a). Up to 2008, there had been 2295 confirmed cases of human paragonimiasis in Africa, which was mostly due to P. africanus and P. uterobilateralis; the vast majority of these cases occurred in Nigeria and Cameroon (Aka et al., Reference Aka, Adoubryn, Rondelaud and Dreyfuss2008; Cumberlidge et al., Reference Cumberlidge, Rollinson, Vercruysse, Tchuem Tchuenté, Webster and Clark2018). Freshwater crabs, Liberonautes spp. and Sudanonautes spp., are the second intermediate hosts (Blair et al., Reference Blair, Xu and Agatsuma1999a). This lung fluke is morphologically similar to P. africanus but differs in having similar-sized oral and ventral suckers (Miyazaki, Reference Miyazaki1991). It has a delicately branched ovary; however, it has moderately branched testes larger than the ovary (Blair et al., Reference Blair, Xu and Agatsuma1999a). Uncooked crab meat is an important source of human infection (Aka et al., Reference Aka, Adoubryn, Rondelaud and Dreyfuss2008). Clinical manifestations are similar to P. westermani infection. Cerebral infection has been suspected but never proved (Aka et al., Reference Aka, Adoubryn, Rondelaud and Dreyfuss2008).
Intestinal flukes
Intestinal flukes are taxonomically diverse, including three large groups, namely, heterophyids (family Heterophyidae), echinostomes (family Echinostomatidae), and other groups, including amphistomes, brachylaimids, cyathocotylids, diplostomes, fasciolids, gymnophallids, isoparorchiids, lecithodendriid-like flukes, microphallids, nanophyetids, plagiorchiids and strigeids (Yamaguti, Reference Yamaguti1971; Chai, Reference Chai2019; Chai and Jung, Reference Chai and Jung2020). At least 75 different species have been described which infect an estimated 40–50 million people (Chai et al., Reference Chai, Shin, Lee and Rim2009; Chai and Jung, Reference Chai and Jung2020). Among them, heterophyids include 28 species (14 species are with more than 10 human cases; Table 4), echinostomes include 24 species (16 species; Table 5), and other groups are comprised of 23 species (10 species; Table 6). In this section, 40 species having more than 10 human cases are briefly introduced in the text, and the other 35 miscellaneous species are briefly mentioned in Supplementary Table. Molecular techniques using ITS and cox1 genes have been applied to differentiate eggs, larvae, as well as adults of various heterophyid species (Duflot et al., Reference Duflot, Setbon, Midelet, Brauge and Gay2021). Intestinal flukes are easily treated with 10 mg kg−1 single dose of praziquantel or 2 g single dose of niclosamide (Table 2).
Table 4. Heterophyid intestinal flukes infecting humans with their life cycle and geographical distributiona

a The geographical distributions of heterophyids are referred from Chai (Reference Chai2019) and Chai and Jung (Reference Chai, Jung, Toledo and Fried2019).
Table 5. Echinostomes infecting humans with their life cycle and geographical distribution

a The geographical distributions of these echinostomes are referred from Chai (Reference Chai2019) and Chai and Jung (Reference Chai, Jung, Toledo and Fried2019).
Table 6. Other intestinal fluke species infecting humans with their life cycle and geographical distribution
a The geographical distributions of these intestinal flukes are referred from Chai (Reference Chai2019) and Chai and Jung (Reference Chai, Jung, Toledo and Fried2019).
Heterophyid species
Metagonimus spp.
Metagonimus yokogawai (Katsurada, 1912) Katsurada, 1912 was originally described from an experimentally infected dog in Taiwan and then reported mostly from Asian countries (Yu and Chai, Reference Yu, Chai and Liu2013). Sequences of nuclear 28S rDNA and mitochondrial cox1 genes were used to discriminate this species from the related ones, M. miyatai and M. takahashii (Chai, Reference Chai2019). The main clinical symptoms of M. yokogawai infection are mild-to-severe gastrointestinal trouble, including abdominal pain, diarrhoea and indigestion (Chai and Jung, Reference Chai and Jung2020). The histopathology of the small intestine is characterized by villous atrophy and crypt hyperplasia accompanied by inflammatory reactions (Chai, Reference Chai2019). The first case of human infection was discovered in Japan by Yokogawa S. in 1913, and thereafter numerous succeeding reports were published (Ito, Reference Ito1964). The sweetfish Plecoglossus altivelis, dace Tribolodon hokonensis or T. taczanowskii, and perch Lateolabrax japonicus are the major fish intermediate host (Yu and Chai, Reference Yu, Chai and Liu2013). The principal mode of human infection is ingestion of raw or improperly cooked fish (Chai et al., Reference Chai, Shin, Lee and Rim2009; Chai and Jung, Reference Chai and Jung2017). M. yokogawai is distributed mainly in the Far East and East Asia (Chai, Reference Chai2019). In South Korea, endemic areas are scattered along almost all large and small rivers and streams in eastern and southern coastal areas (Chai and Lee, Reference Chai and Lee2002; Chai et al., Reference Chai, Shin, Lee and Rim2009). In Japan, the prevalence reported in humans has been generally lower than that in South Korea (Chai and Jung, Reference Chai and Jung2020). In China, human infections were reported in Guangdong, Anhui, Hubei and Zhejiang Province (Yu and Mott, Reference Yu and Mott1994). The diagnosis is based on the recovery of eggs in fecal examinations. In Russia, Metagonimus infection has been reported under the name M. yokogawai in the Amur and Ussuri valleys of the Khabarovsk Territory, and the prevalence was 20–70% among the ethnic minority group (Yu and Mott, Reference Yu and Mott1994). Recently, however, the parasite in this area (obtained from experimental rats) was reported as a new species (Metagonimus suifunensis) based on molecular analyses (Shumenko et al., Reference Shumenko, Tatanova and Besprozvannykh2017).
Metagonimus miyatai Saito, Chai, Kim, Lee et Rim, Reference Saito, Chai, Kim, Lee and Rim1997 was first found in Japan in 1941 but reported a long time later as a distinct species (Saito et al., Reference Saito, Chai, Kim, Lee and Rim1997). Human infections seem to have existed in Japan but there had been no confirmed reports. In South Korea, human infections were first recognized along the Geum River in 1980 by detecting eggs in feces (Chai, Reference Chai2019). Adult flukes were recovered from 32 people living along the Namhan River in Umsong and Yongwol County (Chai et al., Reference Chai, Huh, Yu, Kook, Jung, Park, Sohn, Hong and Lee1993). Freshwater fish, including Zacco platypus, Z. temminckii, P. altivelis, Tribolodon hakonensis and T. taczanowskii, are the second intermediate hosts (Yu and Chai, Reference Yu, Chai and Liu2013). In Japan, fish from small rivers in the Shizuoka Prefecture were found to have M. miyatai infection (Kino et al., Reference Kino, Suzuki, Oishi, Suzuki, Yamagiwa and Ishiguro2006).
Metagonimus takahashii (Takahashi, 1929) Suzuki, 1930 was originally described in Japan and now known to exist also in South Korea (Yu and Chai, Reference Yu, Chai and Liu2013). The crussian carp C. carassius, carp C. carpio, dace T. taczanowskii and perch L. japonicus carry the metacercariae (Yu and Chai, Reference Yu, Chai and Liu2013). In Japan, this fluke was reported in Okayama, Hiroshima and around the Lake Biwa (Ito, Reference Ito1964; Urabe, Reference Urabe2003). In South Korea, human infections were first identified from riparian people along the Hongcheon River, Gangwon-do (Province) (Ahn and Ryang, Reference Ahn and Ryang1988). Subsequently, an endemic focus was discovered in 1993 from Umsong County along the upper reaches of the Namhan River (Chai et al., Reference Chai, Huh, Yu, Kook, Jung, Park, Sohn, Hong and Lee1993).
Heterophyes heterophyes and H. nocens
Heterophyes heterophyes (v. Siebold, 1852) Stiles and Hassall, 1900 was first described based on specimens obtained at autopsy of an Egyptian, and the Nile Delta of Egypt and Sudan is now known to be important endemic areas (Yu and Mott, Reference Yu and Mott1994; Chai, Reference Chai, Murrell and Fried2007). The distribution of H. heterophyes is also known in Europe, the Middle East and North Africa (Yu and Mott, Reference Yu and Mott1994; Chai, Reference Chai, Murrell and Fried2007). Sequences of nuclear ITS2 and 28S rDNA and mitochondrial cox1 have been used to differentiate Heterophyes species (Chai, Reference Chai2019). Brackish water fish, including the mullet Mugil cephalus and tilapia Tilapia nilotica, are the second intermediate hosts (Chai, Reference Chai, Murrell and Fried2007). Humans are infected by eating raw or inadequately cooked brackish water fish.
Heterophyes nocens Onji and Nishio, 1916 was first described from experimental dogs and cats fed mullets in Japan (Chai, Reference Chai, Murrell and Fried2007). This fluke has been reported also in South Korea and Japan (Chai et al., Reference Chai, Shin, Lee and Rim2009; Chai and Jung, Reference Chai and Jung2017). Brackish water fish, including the mullet and goby Acanthogobius flavimanus, are the second intermediate hosts (Guk et al., Reference Guk, Shin, Kim, Sohn, Hong, Yoon, Lee, Rim and Chai2007). Humans are infected by consuming raw or inadequately cooked brackish water fish. In South Korea, residents of southwestern coastal areas and islands showed 10–70% egg-positive rates (Chai et al., Reference Chai, Park, Han, Shin, Kim, Guk, Hong, Lee and Rim2004, Reference Chai, Shin, Lee and Rim2009). In Japan, Kochi, Chiba, Yamaguchi, Chugoku, Hiroshima and Shizuoka Prefectures were the places where human infections were recorded (Kino et al., Reference Kino, Oishi, Ohno and Mitsuru2002). Diagnosis is based on the recovery of eggs in fecal examinations, but eggs should be differentiated from those of other heterophyids and small liver flukes.
Haplorchis spp.
Haplorchis taichui (Nishigori, 1924) Chen, 1936 was originally described from birds and mammals in Taiwan (Chai, Reference Chai, Murrell and Fried2007; Chai and Jung, Reference Chai and Jung2017). The first report of human infections was published from the Philippines (Africa et al., Reference Africa, de Leon and Garcia1940). Now this fluke is known to be distributed widely in Asia and the Middle East (Chai et al., Reference Chai, Han, Guk, Shin, Sohn, Yong, Eom, Lee, Jeong, Ryang, Hoang, Phommasack, Insisiengmay, Lee and Rim2007, Reference Chai, Shin, Lee and Rim2009, Reference Chai, De and Sohn2012a; Sohn et al., Reference Sohn, Yong, Eom, Min, Lee, Jung, Banouvong, Insisiengmay, Phammasack, Rim and Chai2014). PCR targeting ITS1, ITS2 and cox1 has been proved to be useful for genetic studies of Haplorchis species and opisthorchiids (Chai, Reference Chai2019). In Laos, hyperendemic areas with average individual worm loads of 12 079 (Champasak Province) and 21 565 specimens (Saravane Province) were reported (Chai et al., Reference Chai, Yong, Eom, Min, Jeon, Kim, Jung, Sisabath, Insisiengmay, Phommasack and Rim2013a). Freshwater fish, Cyprinus spp., Gambusia affinis, Hampala dispar and Puntius spp., harbour the metacercariae (Chai et al., Reference Chai, Shin, Lee and Rim2009).
Haplorchis pumilio (Looss, 1896) Looss, 1899 was first recorded from birds and mammals in Egypt (Chai et al., Reference Chai, Shin, Lee and Rim2009). Later, in Taiwan, a successful experimental infection of a human volunteer was reported in 1924 (Chai et al., Reference Chai, Shin, Lee and Rim2009). Numerous human infections were found thereafter, and now this fluke is known to be distributed widely from Africa to Asia, Oceania and the Americas (Chai et al., Reference Chai, Shin, Lee and Rim2009, Reference Chai, De and Sohn2012a, Reference Chai, Sohn, Na, Yong, Eom, Yoon, Hoang, Jeoung and Socheat2014, Reference Chai, Sohn, Jung, Yong, Eom, Min, Insisiengmay, Insisiengmay, Phommasack and Rim2015; Chung et al., Reference Chung, Lee, Kim, Sohn, Kwak and Seo2011). The second intermediate host is freshwater fish of the Cyprinidae, Siluridae and Cobitidae (Chai et al., Reference Chai, Shin, Lee and Rim2009).
Haplorchis yokogawai (Katsuta, 1932) Chen, 1936 was first reported from dogs and cats experimentally fed mullets in Taiwan (Chai et al., Reference Chai, Shin, Lee and Rim2009). An experimental human infection was successful in Taiwan but natural human infections were reported for the first time in the Philippines (Africa et al., Reference Africa, de Leon and Garcia1940). This fluke is now known to be distributed in Asia, Australia and Egypt (Chai et al., Reference Chai, Han, Guk, Shin, Sohn, Yong, Eom, Lee, Jeong, Ryang, Hoang, Phommasack, Insisiengmay, Lee and Rim2007, Reference Chai, Shin, Lee and Rim2009, Reference Chai, Sohn, Na, Yong, Eom, Yoon, Hoang, Jeoung and Socheat2014). Freshwater fish Cyclocheilichthys armatus, Hampala dispar, Misgurnus sp., Mugil spp. and Puntius spp. harbour the metacercariae (Chai et al., Reference Chai, Shin, Lee and Rim2009).
Centrocestus formosanus
Centrocestus formosanus (Nishigori, 1924) Price, 1932 (syn. Centrocestus caninus) was first found in a dog experimentally infected with metacercariae from freshwater fish in Taiwan (Chai et al., Reference Chai, Shin, Lee and Rim2009; Chai and Jung, Reference Chai and Jung2017). A successful experimental infection of a human volunteer was reported at the same time (Chai et al., Reference Chai, Shin, Lee and Rim2009; Chai and Jung, Reference Chai and Jung2017). Two human infections reported in Thailand under the name C. caninus (Waikagul et al., Reference Waikagul, Wongsaroj, Radomyos, Meesomboon, Praewanich and Jongsuksuntikul1997) are now considered as C. formosanus infection. In Laos, 7 human infections were detected (Chai et al., Reference Chai, Garcia, Tanowitz and Del Brutto2013b). Now this fluke is distributed almost all over the world, including Asia, Europe and North and South America (Chai et al., Reference Chai, Shin, Lee and Rim2009, Reference Chai, De and Sohn2012a, Reference Chai, Sohn, Yong, Eom, Min, Lee, Lim, Insisiengmay, Phommasack and Rim2013b, Reference Chai, Sohn, Na, Yong, Eom, Yoon, Hoang, Jeoung and Socheat2014; Wanlop et al., Reference Wanlop, Wongsawad, Prattapong, Wongsawad, Chontananarth and Chai2017). Human infections were reported from China, Taiwan, the Philippines, Thailand, Laos and Vietnam (Yu and Mott, Reference Yu and Mott1994; Chai et al., Reference Chai, Shin, Lee and Rim2009; Chai, Reference Chai2019). The freshwater fish, including Cyclocheilichthys repasson and Puntius brevis, are the second host (Chai et al., Reference Chai, Shin, Lee and Rim2009).
Heterophyopsis continua
Heterophyopsis continua (Onji and Nishio, 1916) Price, 1940 was originally described from experimental cats infected with metacercariae in the mullet in Japan. Without proper literature background, the presence of human infections was mentioned in Japan (Yamaguti, Reference Yamaguti1958). In South Korea, adult flukes were recovered from more than 10 human cases (Chai, Reference Chai2019). The second intermediate host includes the perch L. japonicus, goby A. flavimanus, shad Clupanodon punctatus, conger eel Conger myriaster, and sweetfish P. altivelis (Chai et al., Reference Chai, Shin, Lee and Rim2009). This fluke is distributed in Asia and the Middle East (Chai et al., Reference Chai, De and Sohn2012a; Chai, Reference Chai2019).
Pygidiopsis summa and P. genata
Pygidiopsis summa Onji and Nishio, 1916 was originally described from dogs experimentally infected with metacercariae from brackish water fish in Japan and thereafter this fluke was reported also from South Korea (Chai et al., Reference Chai, Shin, Lee and Rim2009; Chai and Jung, Reference Chai and Jung2017). In Japan, human infections were suggested by the recovery of eggs in feces in 1929, and adult flukes were subsequently isolated in 1965 (Chai et al., Reference Chai, Shin, Lee and Rim2009). In South Korea, human infections (8 cases) were first discovered from a salt-farm village in a western costal area of Jeollabuk-do (Province) (Seo et al., Reference Seo, Hong and Chai1981). Now this fluke is known to be distributed on many western and southern coastal islands of South Korea (Chai et al., Reference Chai, Park, Han, Shin, Kim, Guk, Hong, Lee and Rim2004). Its presence was also reported in Vietnam (Vo et al., Reference Vo, Murrell, Dalsgaard, Bristow, Nguyen, Bui and Vo2008). Sequences of 28S rDNA and cox1 were used to assess the phylogenetic relationships of P. summa with C. sinensis, M. yokogawai and M. takahashii (Chai, Reference Chai2019).
Pygidiopsis genata Looss, 1907 was originally described from a pelican in Egypt, and then also found in pelicans in Romania, experimental dogs and cats in the Philippines, a Persian wolf (in Berlin Museum), and dogs and cats in Israel (Palestine) (Witenberg, Reference Witenberg1929; Africa et al., Reference Africa, de Leon and Garcia1940). This parasite is distributed in Egypt, the Middle East and the Philippines (Chai and Jung, Reference Chai and Jung2020). Human infections were first found in Egypt (Youssef et al., Reference Youssef, Mansour, Awadalla, Hammouda, Khalifa and Boulos1987a). Brackish water fish, including tilapia, mullet and Barbus canis, are the second intermediate hosts (Witenberg, Reference Witenberg1929; Youssef et al., Reference Youssef, Mansour, Hammouda, Awadalla, Khalifa and Boulos1987b).
Stellantchasmus falcatus
Stellantchasmus falcatus Onji and Nishio, 1916 was first described from cats experimentally fed the mullet harbouring the metacercariae in Japan (Chai et al., Reference Chai, Shin, Lee and Rim2009). Human infections were first reported in Japan and thereafter in many Asian-Pacific countries, including South Korea, the Philippines, Hawaii, Thailand and Vietnam (Chai et al., Reference Chai, Shin, Lee and Rim2009, Reference Chai, De and Sohn2012a, Reference Chai, Sohn, Na, Jeoung, Sinuon and Socheat2016).
Stictodora fuscata
Stictodora fuscata (Onji and Nishio, 1916) Yamaguti, Reference Yamaguti1958 was first discovered from experimental cats fed infected mullets in Japan (Chai and Jung, Reference Chai and Jung2017). Human infection was first found in South Korea in a young man who regularly consumed mullets and gobies raw (Chai et al., Reference Chai, Hong, Lee and Seo1988). Subsequently, 13 additional cases were detected in a southwestern coastal area (Chai and Lee, Reference Chai and Lee2002). Gobies A. flavimanus and topmouth gudgeons Pseudorasbora parva are the fish hosts (Yamaguti, Reference Yamaguti1958; Chai et al., Reference Chai, Shin, Lee and Rim2009).
Miscellaneous heterophyid species
Fourteen species of miscellaneous heterophyid flukes having less than 10 human infection cases are briefly introduced in Supplementary Table. See Chai and Jung (Reference Chai and Jung2020) for details.
Echinostome species
Echinostoma spp.
Echinostoma revolutum (Froelich, 1802) Dietz, 1909 is the oldest echinostome species ever recorded in the literature (Chai, Reference Chai, Fried and Toledo2009; Chai et al., Reference Chai, Cho, Chang, Jung and Sohn2020). It was originally described in 1798 from a wild duck Anas boschas fereae naturally infected in Germany (Kanev, Reference Kanev1994). This species is now known to be distributed widely in Asia, Europe, Africa, Oceania and North and South America (Chai et al., Reference Chai, Shin, Lee and Rim2009). The first report of human infection was published from Taiwan in 1929, where its prevalence among people was 2.8–6.5% (Yu and Mott, Reference Yu and Mott1994). Subsequently, human infections were reported in China, Indonesia, Thailand and Russia (Chai et al., Reference Chai, Shin, Lee and Rim2009). The flukes can cause gastrointestinal trouble, mucosal ulceration and bleeding (Chai, Reference Chai, Fried and Toledo2009). Tadpoles, gastropod snails (Physa occidentalis, Lymnaea sp. and Filopaludina sp.) and clams (Corbicula producta) can be the second intermediate hosts (Beaver et al., Reference Beaver, Jung and Cupp1984; Yu and Mott, Reference Yu and Mott1994; Chai et al., Reference Chai, Sohn, Na and Nguyen2011; Chantima et al., Reference Chantima, Chai and Wongsawad2013).
Echinostoma cinetorchis Ando and Ozaki, 1923 was first discovered from naturally infected rats in Japan (Chai et al., Reference Chai, Shin, Lee and Rim2009). Adult flukes of this echinostome species are morphologically characterized by having a reduced number (none or 1, rarely 2) and abnormal location of testes (Chai, Reference Chai, Fried and Toledo2009). Human infections were first documented in Japan, and subsequently also detected in South Korea and China (Chai et al., Reference Chai, Shin, Lee and Rim2009).
Echinostoma ilocanum (Garrison, 1908) Odhner, 1911 was first described from 5 prisoners in Manila, the Philippines (Chai et al., Reference Chai, Shin, Lee and Rim2009). Subsequently, numerous human infection cases were discovered in Asian countries (Sohn et al., Reference Sohn, Kim, Yong, Eom, Jeong, Kim, Kang, Kim, Park, Ji, Sinuon, Socheat and Chai2011; Chai et al., Reference Chai, Sohn, Cho, Eom, Yong, Min, Hoang, Phommasack, Insisiengmay and Rim2018). For example, in northern Luzon, the Philippines, the prevalence among the people was high, in the range from 7% to 17% (Chai et al., Reference Chai, Shin, Lee and Rim2009). In Oddar Meanchey Province, Cambodia, the general population and the student group revealed 1.8% and 0.7% egg-positive rates, respectively (Sohn et al., Reference Sohn, Kim, Yong, Eom, Jeong, Kim, Kang, Kim, Park, Ji, Sinuon, Socheat and Chai2011). Large snails, Pila conica (the Philippines) and Viviparus javanicus (Java) are the second intermediate hosts (Beaver et al., Reference Beaver, Jung and Cupp1984; Yu and Mott, Reference Yu and Mott1994).
Echinostoma lindoense Sandground and Bonne, 1940 was described from human population in the Lake Lindoe region of Central Celebes, Indonesia (Chai, Reference Chai2019). A high prevalence (24–96%) of human infection was reported in Indonesia (Chai et al., Reference Chai, Shin, Lee and Rim2009). It is now known to be distributed in South Asian countries (Chai, Reference Chai, Fried and Toledo2009).
Echinostoma mekongi Cho, Jung, Chang, Sohn, Sinuon and Chai, 2020 was described as a new species based on adult flukes recovered from 6 humans residing along the Mekong River in Kratie and Takeo Province, Cambodia (Cho et al., Reference Cho, Jung, Chang, Sohn, Sinuon and Chai2020). Further human cases (more than 10) were found in Kandal Province, Cambodia (unpublished data). The freshwater snails, Filopaludina martensi cambodjiensis, from Pursat Province, Cambodia were found to carry the metacercariae (Chai et al., Reference Chai, Sohn, Cho, Jung, Chang, Lee, Khieu and Huy2021b). This echinostome seems to exist in neighbouring countries, such as Laos, Thailand and Vietnam.
Isthmiophora hortensis
Isthmiophora hortensis (Asada, 1926) Kostadinova and Gibson, 2002 (syn. Echinostoma hortense) was first found from rats in Japan, and then also from rats in South Korea and China (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). Human infections were first documented in Japan in 1976 and then also found in South Korea and China (Chai and Lee, Reference Chai and Lee2002; Chai et al., Reference Chai, Shin, Lee and Rim2009). In South Korea, clinical cases with significant abdominal symptoms were diagnosed by extracting living worms by gastroduodenal endoscopy (Chai et al., Reference Chai, Shin, Lee and Rim2009). Loaches, Misgurnus anguillicaudatus and M. mizolepis, and other freshwater fish, including Odontobutis obscura interrupta and Moroco oxycephalus, are the second intermediate hosts (Chai et al., Reference Chai, Shin, Lee and Rim2009).
Echinochasmus spp.
Echinochasmus japonicus Tanabe, 1926 was originally reported in Japan from dogs, cats, rats, mice and birds experimentally fed the metacercariae encysted in freshwater fish (Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). This fluke is now known to occur mainly in Far Eastern countries (Chai and Lee, Reference Chai and Lee2002; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). In Japan, an experimental human infection was reported to be successful, and in China and South Korea natural human infections were discovered (Chai et al., Reference Chai, Shin, Lee and Rim2009). Freshwater fish, including P. parva, Hypomesus olidus and Gnathopogon strigatus, are the second intermediate hosts (Chai et al., Reference Chai, Hong, Son, Lee and Seo1985; Choi et al., Reference Choi, Kim, Chung, Jang, Eom, Chung, Sohn, Chai and Hong2006).
Echinochasmus perfoliatus (Ratz, 1908) Gedoelst, 1911 was originally reported from dogs in Romania, and then found again in dogs and cats in Hungary (Chai et al., Reference Chai, Shin, Lee and Rim2009). Now its distribution is known to be wide from Asia to Europe (Chai et al., Reference Chai, Shin, Lee and Rim2009). Human infections were reported in Japan (Chai et al., Reference Chai, Shin, Lee and Rim2009). In Guangdong, Fujian, Anhui and Hubei Provinces of China, 1.8% prevalence was reported among people (Yu and Mott, Reference Yu and Mott1994). Freshwater fish, including Carassius sp., Zacco platypus, Z. temminckii, and P. parva, are the second intermediate hosts (Rim, Reference Rim, Steele, Hillyer and Hopla1982c; Yu and Mott, Reference Yu and Mott1994).
Echinochasmus liliputanus (Looss, 1896) Odhner, 1910 was found from dogs, cats, and birds in Egypt, Syria and Israel (Palestine) (Yamaguti, Reference Yamaguti1958). The first human infections, with a high prevalence of 13.4% among 2426 people, were reported in Anhui Province, China in 1991 (Yu and Mott, Reference Yu and Mott1994). Since that time, more than 2500 human cases had been reported in Anhui Province, China (Xiao et al., Reference Xiao, Wang, Zheng, Shen and Tian2005). P. parva and goldfish are the second intermediate hosts (Xiao et al., Reference Xiao, Wang, Zheng, Shen and Tian2005). The mode of human infections is consumption of raw or improperly cooked fish or drinking untreated water containing the cercariae (Chai et al., Reference Chai, Shin, Lee and Rim2009).
Echinochasmus fujianensis Cheng, Lin, Chen, et al., 1992 was originally reported from humans, dogs, cats, pigs and rats in Fujian Province, China (Yu and Mott, Reference Yu and Mott1994). The prevalence among residents (mostly children) in Fujian Province ranged 1.6–7.8% (Yu and Mott, Reference Yu and Mott1994). P. parva and Cyprinus carpio are the second hosts (Yu and Mott, Reference Yu and Mott1994).
Echinochasmus caninus (Verma, 1935) Chai et al., 2019 (syn. Episthochasmus caninum, Episthmium caninum) was first described from dogs in India. Its metacercariae were found in fish Macropodus opercularis, and adult flukes were detected in dogs in Hainan Island, China (Chai et al., Reference Chai, Chang, Jung, Shin, Soh, Eom, Yong, Min, Phommasack, Insisiengmay and Rim2019b). Human infections were reported in northeastern Thailand and Lao PDR (Chai et al., Reference Chai, Chang, Jung, Shin, Soh, Eom, Yong, Min, Phommasack, Insisiengmay and Rim2019b).
Artyfechinostomum spp.
Artyfechinostomum malayanum (Leiper, 1911) Mendheim, 1943 (syn. Echinostoma malayanum) was originally reported from a human in Malaysia (Beaver et al., Reference Beaver, Jung and Cupp1984), and subsequently found in other Asian countries (Chai et al., Reference Chai, Shin, Lee and Rim2009, Reference Chai, Sohn, Yong, Eom, Min, Hoang, Phammasack, Insisiengmay and Rim2012b; Sohn et al., Reference Sohn, Yong, Eom, Sinuon, Jeoung and Chai2017). Large snails, i.e. I. exustus, A. convexiusculus and Pila scutate, play the role of the second intermediate hosts (Yu and Mott, Reference Yu and Mott1994; Belizario et al., Reference Belizario, Geronilla, Anastacio, de Leon, Suba-an, Sebastian and Bangs2007).
Artyfechinostomum sufrartyfex Lane, 1915 (syn. Artyfechinostomum mehrai) was originally discovered from an Assamese girl in India (Yu and Mott, Reference Yu and Mott1994) and later in Vietnam (Tran et al., Reference Tran, Nguyen, Nguyen, Luc, Mafie, Rupa and Sato2016). Freshwater snails, fish or frogs harbour the metacercariae (Raghunathan and Srinivasan, Reference Raghunathan and Srinivasan1962). Further human cases were reported in India (Chai and Jung, Reference Chai and Jung2020).
Artyfechinostomum oraoni Bandyopadhyay, Manna et Nandy, 1989 was originally reported from 20 human infection cases in a tribal community near Calcutta, India (Bandyopadhyay et al., Reference Bandyopadhyay, Manna and Nandy1989, Reference Bandyopadhyay, Maji, Manna, Bera, Addy and Nandy1995). The life cycle is to be determined. This fluke may provoke fatal diarrhoea in pigs (Bandyopadhyay et al., Reference Bandyopadhyay, Manna and Nandy1989).
Hypoderaeum conoideum
Hypoderaeum conoideum (Bloch, 1872) Dietz, 1909 was originally reported from various species of birds in Europe (Yamaguti, Reference Yamaguti1958). This echinostome is now known to be distributed in Europe, Asia and Siberia (Yamaguti, Reference Yamaguti1958; Rim, Reference Rim, Steele, Hillyer and Hopla1982c; Beaver et al., Reference Beaver, Jung and Cupp1984). The first human infections were reported in northeastern Thailand; the prevalence among 254 residents was 55% (Yokogawa et al., Reference Yokogawa, Harinasuta and Charoenlarp1965). Large snails and tadpoles play the role of the second intermediate hosts (Chai et al., Reference Chai, Shin, Lee and Rim2009).
Acanthoparyphium tyosenense
Acanthoparyphium tyosenense Yamaguti, 1939 was originally reported from the duck Melanitta fusca stejnegeri and M. nigra americana caught in South Korea (Chai, Reference Chai, Fried and Toledo2009; Chai and Jung, Reference Chai, Jung, Toledo and Fried2019). Its geographical distribution is confined to South Korea and Japan (Kim et al., Reference Kim, Yu, Chung and Chung2004). Humans infected with this echinostome were first discovered in coastal villages of Jeollabuk-do (Province), South Korea (Chai et al., Reference Chai, Han, Park, Guk and Lee2001). Brackish water bivalves, including Mactra veneriformis and Solen spp., and brackish water gastropod Neverita bicolor, were found to harbour the metacercariae (Chai et al., Reference Chai, Han, Park, Guk and Lee2001; Kim et al., Reference Kim, Yu, Chung and Chung2004).
Miscellaneous echinostome species
Eight species of miscellaneous echinostome flukes having less than 10 human infection cases are briefly introduced in Supplementary Table. See Chai and Jung (Reference Chai and Jung2020) for details.
Other intestinal fluke species
Brachylaima cribbi
Brachylaima cribbi Butcher and Grove, Reference Butcher and Grove2001 was first described by Butcher and Grove (Reference Butcher and Grove2001) in Australia. Human infections (about 15 cases) were reported in Australia (Butcher et al., Reference Butcher, Parasuramar, Thompson and Grove1998, Reference Butcher, Palethope and Grove2003). Helicid land snails, such as, Cernuella virgata, serve as the source of human infection (Butcher and Grove, Reference Butcher and Grove2005). Clinical symptoms include diarrhoea, abdominal pain, low-grade fever and fatigue (Butcher et al., Reference Butcher, Palethope and Grove2003).
Caprimolgorchis molenkampi
Caprimolgorchis molenkampi (Lie, 1951) Baugh, 1957 (syn. Prosthodendrium molenkampi) was originally reported from 2 human autopsies in Indonesia (Manning and Lertprasert, Reference Manning and Lertprasert1973). Later, this fluke was found again in 14 human autopsies in northeastern Thailand (Manning et al., Reference Manning, Diggs, Viyanant, Lertprasert and Watanasirmkit1970; Manning and Lertprasert, Reference Manning and Lertprasert1973). Currently, this fluke shows a considerable prevalence in northeast Thailand and Laos (Chai et al., Reference Chai, Shin, Lee and Rim2009). Naiads and adults of dragon- and damselflies serve as the second intermediate hosts (Manning and Lertprasert, Reference Manning and Lertprasert1973).
Fasciolopsis buski
Fasciolopsis buski (Lankester, 1857) Odhner, 1902 was originally described in 1843 based on the worms in the duodenum of an Indian sailor (Beaver et al., Reference Beaver, Jung and Cupp1984; Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005; Tandon et al., Reference Tandon, Roy, Prasad and Liu2013). Now this fluke is known to be a common intestinal parasite of humans and pigs in Asia (Chai et al., Reference Chai, Shin, Lee and Rim2009; Tandon et al., Reference Tandon, Roy, Prasad and Liu2013). The estimated number of Asian populations infected with F. buski is about 10 million (Tandon et al., Reference Tandon, Roy, Prasad and Liu2013). The prevalence ranged from 0.04% in Cambodia to 8.6–50% in Bangladesh, 25–61% in Taiwan and up to 85% in some areas of China (Tandon et al., Reference Tandon, Roy, Prasad and Liu2013). Metacercariae can attach to the surface of aquatic plants, such as, water chestnut, water caltrop, water hyacinth, roots of the lotus, water bamboo and other aquatic vegetations; they may also float on the water (Beaver et al., Reference Beaver, Jung and Cupp1984; Yu and Mott, Reference Yu and Mott1994; Tandon et al., Reference Tandon, Roy, Prasad and Liu2013). The main mode of human infection is the consumption of raw or improperly cooked aquatic plants, or peeling off the hull or skin of the plants by mouth before eating the raw nut (Yu and Mott, Reference Yu and Mott1994).
Gastrodiscoides hominis
Gastrodiscoides hominis (Lewis and McConnell, 1876) Leiper, 1913 was first found from an Indian patient (Beaver et al., Reference Beaver, Jung and Cupp1984). This fluke is now known to be a common parasite of humans and pigs in various countries of Asia and Africa (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005). Adult worms attach to the caecum and ascending colon of humans and may produce mucous diarrhoea (Beaver et al., Reference Beaver, Jung and Cupp1984). The cercariae encyst on aquatic plants, or in tadpoles, frogs and crayfish (Yu and Mott, Reference Yu and Mott1994).
Gymnophallloides seoi
Gymnophalloides seoi Lee, Chai et Hong, 1993 was originally reported from a woman suffering from acute pancreatitis and gastrointestinal discomfort in South Korea (Lee et al., Reference Lee, Chai and Hong1993; Chai et al., Reference Chai, Choi, Yu and Lee2003). Subsequently, a southwestern coastal island was found to be a highly endemic area with 49% prevalence and heavy worm loads (Chai et al., Reference Chai, Choi, Yu and Lee2003). This fluke is now known to be distributed in 25 seashore villages of western and southern coastal islands and 3 coastal inlands of South Korea (Chai et al., Reference Chai, Shin, Lee and Rim2009). Oysters, Crassostrea gigas were verified to be the second intermediate hosts (Chai et al., Reference Chai, Choi, Yu and Lee2003). Consumption of raw oysters is the main mode of human infection.
Microphallus brevicaeca
Microphallus brevicaeca (Africa and Garcia, 1935) Baer, 1943 (syn. Spelotrema brevicaeca) was first described from birds and subsequently also found from 12 human autopsies in the Philippines (Africa et al., Reference Africa, de Leon and Garcia1940). Eggs were shown to cause acute cardiac dilatation and egg granuloma in the heart, brain and spinal cord, especially in immunocompromised patients (Africa et al., Reference Africa, de Leon and Garcia1940). Brackish water crabs Carcinus maenas and shrimps Macrobrachium sp. serve as the second intermediate hosts (Beaver et al., Reference Beaver, Jung and Cupp1984).
Nanophyetus salmincola and N. schikhobalowi
Nanophyetus salmincola (Chapin, 1926) Chapin in Hall, 1927 (syn. Troglotrema salmincola) was first found in dogs suffering from a fatal disease after the ingestion of uncooked salmon in the Pacific Coast of North America (Witenberg, Reference Witenberg1932). Since 1974, at least 20 human infections were found in the USA (Witenberg, Reference Witenberg1932; Eastburn et al., Reference Eastburn, Tritsche and Terhune1987). N. salmincola has been proven to be the vector of a rickettsia Neorickettsia helmintheca (Chai and Jung, Reference Chai and Jung2020). This rickettsial infection can cause a serious and often fatal systemic disease known as salmon poisoning in animals such as dogs and foxes (Chai and Jung, Reference Chai and Jung2020). However, salmon poisoning has never been reported in humans (Chai and Jung, Reference Chai and Jung2020).
Nanophyetus schikhobalowi Skrjabin and Podjapolskaja, Reference Skrjabin and Podjapolskaja1931 was discovered from native people in far eastern Siberia (Skrjabin and Podjapolskaja, Reference Skrjabin and Podjapolskaja1931). It was described as a new species mainly because of their smaller egg size compared to N. salmincola (Skrjabin and Podjapolskaja, Reference Skrjabin and Podjapolskaja1931). The far eastern part of Russia including Amur and Ussuri valleys of Khabarovsk Territory and north Sakhalin is an important endemic area, with an average prevalence of 5% (Yu and Mott, Reference Yu and Mott1994).
Neodiplostomum seoulense
Neodiplostomum seoulense (Seo, Rim et Lee, 1964) Hong and Shoop, 1995 was first found in house rats in South Korea (Seo, Reference Seo1990). Its geographical distribution is confined to mountainous areas of South Korea (Seo, Reference Seo1990) and a northeastern part of China (Chai et al., Reference Chai, Shin, Lee and Rim2009). Adult flukes were recovered from a young man who suffered from acute abdominal pain and fever and had a history of consuming improperly cooked snakes (Seo, Reference Seo1990). Experimental mice and rats showed severe mucosal damage in the intestinal tract with frequent host death and fecundity reduction (Lee et al., Reference Lee, Yoo, Hong, Chai, Seo and Chi1985; Chai et al., Reference Chai, Shin, Lee and Rim2009; Shin et al., Reference Shin, Im, Park, Cho, Kim and Chai2016). Military soldiers who had eaten raw snakes during their survival training were found to be infected with this fluke (Hong et al., Reference Hong, Cho, Hong, Chai, Lee and Seo1984; Chai et al., Reference Chai, Shin, Lee and Rim2009). Tadpoles and frogs of Rana sp. are the second intermediate hosts, and the snake Rhabdophis tigrina is a paratenic host (Seo, Reference Seo1990).
Phaneropsolus bonnei
Phaneropsolus bonnei Lie, 1951 was originally reported based on adult flukes recovered from a human autopsy in Indonesia, and subsequently this fluke was found in monkeys in Malaysia and India (Manning et al., Reference Manning, Diggs, Viyanant, Lertprasert and Watanasirmkit1970). Human infections with this fluke were first reported in 15 human autopsies in northeastern Thailand (Manning et al., Reference Manning, Diggs, Viyanant, Lertprasert and Watanasirmkit1970). Thailand and Laos are currently important countries where this fluke infection is considerably high among the people (Chai et al., Reference Chai, Shin, Lee and Rim2009). Insects, particularly naiads and adults of dragon- and damselflies, serve as the second intermediate hosts (Manning and Lertprasert, Reference Manning and Lertprasert1973).
Other miscellaneous species
Thirteen species of miscellaneous intestinal flukes having less than 10 human cases are presented in Supplementary Table. See Chai and Jung (Reference Chai and Jung2020) for details.
Issues and perspectives
FBT are taxonomically diverse, and a lot of species have been involved in causing zoonotic human and animal infections. Recently, FBT infections have been included among the neglected tropical diseases by the World Health Organization. Because of the increased risk of human exposure to these parasites, the public health significance of each FBT species is expected to increase. The life cycles of many kinds of FBT have been elucidated, and a variety of human infection sources (including types of foods) have been identified. However, problems still remain including difficulties in the diagnosis of infection (fecal examination alone frequently do not provide exact/specific diagnosis) as well as poor understanding of the epidemiological situation, including the geographical distribution, prevalence, intensity of infection and other characteristics. In addition, the clinicopathological significance of each FBT infection requires further investigation.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022000725.
Acknowledgements
The authors would like to thank the members of the MediCheck Research Institute, Korea Association of Health Promotion, Seoul, South Korea for their help in gathering information and references used in this article.
Author contributions
J.-Y. C. and B.-K. J. designed this review and wrote the article.
Financial support
This review received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors declare that they have no conflicts of interest related to this review article.