An integrated multidisciplinary approach to diagnosing and managing complex disorders such as dementia is generally recommended 1–3 because no single medical specialty has the expertise to deal with the complex range of mental, physical and social problems that accompany dementia. Reference Collighan, Macdonald, Herzberg and Lindesay4,Reference Verhey, Jolles, Ponds, Rozendaal, Plugge, de Vet, Vreeling and van der Lugt5 However, to date no randomised clinical trial has investigated the value of such an approach to dementia care. Reference Wolfs, Dirksen, Severens and Verhey6 Recently, an out-patient diagnostic facility, the Diagnostic Observation Centre for Psychogeriatric Patients (DOC–PG), was established in Maastricht, The Netherlands. This facility combines the hospital-based approach of a memory clinic with the care-oriented approach of a regional community mental health team and aims to provide general practitioners with detailed diagnostic and therapeutic advice for patients with cognitive disorders.
The Maastricht Evaluation of a Diagnostic Intervention for Cognitively Impaired Elderly (MEDICIE) study is a randomised controlled trial comparing the efficacy and efficiency of DOC–PG and usual care. We predicted that the DOC–PG intervention would have beneficial effects on health-related quality of life (HRQoL) compared with usual care, based on the assumption that both diagnosis of the cognitive disorder according to specialist guidelines and appropriate assessment of the patient's social circumstances are prerequisites for the best possible care for the patient and the patient's family. The trial registration number is NCT00402311.
Method
We used a cluster randomised study design. The sample size was determined using a power calculation that ensures the detection of at least 80% of the differences in the mean score on the visual analogue scale (VAS) of the EuroQd measure EQ–5D 7 at 5% significance. Assuming an intraclass correlation coefficient of 0.10, a total of 108 patients per group were required.
Study participants
The MEDICIE study was approved by the medical ethics committee of Maastricht University Hospital. Patients were recruited from July 2002 to August 2004 from 60 general practices in the Maastricht region, 7 practices in the Sittard region and 3 practices in the east Heerlen region (all three regions are in the province of Limburg, in the south of The Netherlands). General practitioners in these practices were asked to refer all patients with possible dementia or a cognitive disorder. The inclusion criteria were age 55 years or older; a suspected diagnosis of dementia or a cognitive disorder; no referral to other local or regional services in the past 2 years and availability of a proxy (visiting the patient at least once a week). Exclusion criteria were the presence of an acute disorder requiring prompt therapeutic intervention, and living in a nursing home.
Randomisation
Randomisation took place at the practice level to prevent contamination at a patient and general practitioner level. In order to control for effects related to differences in general practices, all practices were asked to supply information about the practitioner's experience, demographic characteristics of the practice population, and the practitioner's affinity with geriatric problems. On the basis of these data two groups of practices were formed, and the patients from these practices were randomly assigned (by means of a computer program) to either the intervention group or the control group (usual care). The general practitioners were initially masked to this procedure and the random allocation sequence was concealed for most of the participants.
Interventions
DOC–PG
The DOC–PG has expertise in the fields of old age psychiatry, geriatric medicine, neuropsychology, physiotherapy, occupational therapy, geriatric nursing and mental health nursing, and hence enables multidisciplinary assessment of patients, covering aspects such as somatic screening, psychogeriatric assessment, and evaluation of the required levels of care for patients and their carers. General practitioners can refer patients to the DOC–PG if a cognitive disorder is suspected. During a 2-week diagnostic screening procedure, patients are visited once at home and are asked to visit the university hospital departments of geriatric medicine and geriatric psychiatry. A computed tomographic scan and various blood tests are performed. The results are then discussed at a weekly interdisciplinary meeting in which a definite diagnosis is made and a treatment plan is formulated. The patient's general practitioner is sent a summary of the assessments, the multi-axis diagnosis and recommendations for treatment and management; thereafter the general practitioner is responsible for the patient even though further investigations might have been recommended.
Usual care
In the control group general practitioners provided care as usual. This means that the patients were not referred to the DOC–PG and that either the diagnosis was made by the general practitioner or the patient was referred to one of the separate regional services, such as the Maastricht Memory Clinic, geriatric medicine or the department of mental health for the elderly at the mental health community service. Reference Verhey, Jolles, Ponds, Rozendaal, Plugge, de Vet, Vreeling and van der Lugt5
Outcome measures
Interviewers, who for practical reasons could not be kept totally unaware of the treatment assignment, assessed participants at baseline (within 2 weeks of the DOC–PG or usual care intervention) and at 6 months and 12 months after the baseline measurement. All outcome measures (except the Mini-Mental Scale Examination (MMSE)) were collected through personal interviews with the patient's proxy (i.e. we measured the proxy's perception of the patient's health). The HRQoL of the patient and the carer was the primary outcome of this study. Because we expected that the patients would show a complex range of mental, physical and social problems, we chose to use the EQ–5D to measure HRQoL. This instrument has been validated in a number of European countries including The Netherlands Reference Brooks8 and provides a simple descriptive profile and a single index value for health status. It is widely used in cost–utility analyses. Reference Dolan9,Reference Kind, Brooks, Rabin and Charro10 The EQ–5D consists of a scale, VAS, ranging from 0 (worst imaginable health state) to 100 (best imaginable health state). Change in VAS scores over the course of 1 year was the primary outcome variable in this study. A difference of 10% or more between the intervention group and the control group on the VAS was a priori considered to be a clinically relevant difference, as described in the original protocol that preceded the start of the study. The number of patients experiencing this clinically relevant difference were compared between both groups.
As the secondary outcome instrument we used the 36-item Short Form Health Survey (SF-36), Reference Ware and Sherbourne11 a generic questionnaire used to measure nine relevant aspects of the health-related functioning of patients. Higher scores reflect better functioning. Reference Ware and Sherbourne11–Reference VanderZee, Sanderman, Heyink and de Haes13 Additional secondary outcome measures were scores on the MMSE, Reference Folstein, Folstein and McHugh14 the Global Deterioration Scale (GDS), Reference Reisberg, Ferris, de Leon and Crook15 the Neuropsychiatric Inventory (NPI), Reference Cummings16,Reference Cummings and McPherson17 the Instrumental Activities of Daily Living scale (IADL), Reference Lawton and Brody18 and the Cornell Scale for Depression in Dementia (CSDD). Reference Alexopoulos, Abrams, Young and Shamoian19,Reference Kurlowicz, Evans, Strumpf and Maislin20 The MMSE assesses the severity of cognitive decline, the GDS evaluates seven stages of global functioning in patients with a primary degenerative dementia such as Alzheimer's disease and the NPI appraises patients' behavioural and psychological problems. The IADL scale measures seven areas of more complex activities required for optimal independent functioning, with scores reflecting whether patients are completely independent, are in need of assistance, or are completely dependent on others for the performance of specific activities. Reference Lawton and Brody18 The CSDD is a 19-item depression scale that was developed specifically to measure the severity of depressive symptoms in older adults with dementia. Higher scores on all instruments, except for the MMSE, are indicative of more severe problems.
Statistical analyses
Missing data
Missing items were imputed using a regression model, and missing data or data missing covariates were imputed using Rubin's multiple imputation procedure. Reference Rubin21 This method generates ten different data-sets for imputed data. All analyses were performed with each of these ten data-sets and the results were pooled. Complete missing data were imputed if participants had completed the instrument on two occasions but not if they had completed only the baseline measurement. These patients were considered as having withdrawn from the study. With a logistic regression analysis the probability of withdrawing from the study was assessed and, with these probabilities, P weights were calculated as 1/(1–predicted probability). This allowed for differential weighting of people in data analysis. Reference Little and Rubin22 The data of patients who had died after the baseline measurement and before the follow-up investigations were not analysed.
Data analysis
Weighted regression models, clustered on general practice level, were used to examine the influence of group (intervention or usual care) on outcome on each of the instruments. The cluster option was used to account for the correlated data within general practices. The dependent variables in the models were the scores and the change over time of the participants' scores on the instruments, with baseline characteristics (group, gender, age, diagnosis and MMSE score) as independent variables. The software SPSS version 12.0.1 for Windows was used to calculate the P-weights, to examine group differences and to impute the missing items by means of a regression model (missing value analysis). Rubin's multiple imputation procedure and our main regression analyses were performed using Stata version 8.2 for Windows. The background characteristics of the participants (both the patients and their proxies) were summarised using descriptive statistics. Response distributions of the instruments are provided.
Results
Of the general practices included in this study, 33 were randomised to the DOC–PG intervention and 37 to usual care. Between July 2002 and August 2004, a total of 414 patients were referred for further treatment. Of these patients, 351 were eligible for this study and 230 (65%) agreed to participate. Non-participants were comparable to the participants with respect to age (77.8 years, s.d.=6.4 and 77.8 years, s.d.=6.7 respectively) and gender (females constituted 59.5% and 66.2% of the groups respectively). The main reason for not participating was that participation would be too much of a burden for either the patient or the proxy. We followed up 94.3% of the patients. Eight patients (5.8%) in the intervention group and five patients (5.4%) in the control group withdrew from the study because ‘the burden is too high’ (intervention group n=2, control group n=2), ‘the proxy has health problems’ (intervention group n=4, control group n=1) and ‘participation in the study has no beneficial effects for the patient’ (intervention group n=2, control group n=2). The flow of participants through the trial is shown in Fig. 1. The demographic and clinical characteristics of the participants were similar at baseline in both groups (Table 1).

Fig. 1 Flow of patients through trial. DOC–PG, Diagnostic Observation Centre for Psychogeriatric Patients
Table 1 Baseline characteristics of the sample
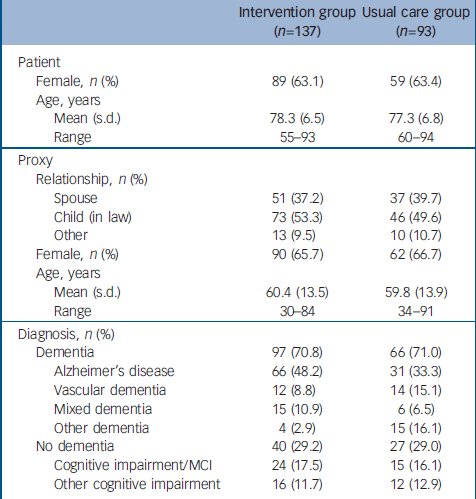
| Intervention group (n=137) | Usual care group (n=93) | |
|---|---|---|
| Patient | ||
| Female, n (%) | 89 (63.1) | 59 (63.4) |
| Age, years | ||
| Mean (s.d.) | 78.3 (6.5) | 77.3 (6.8) |
| Range | 55–93 | 60–94 |
| Proxy | ||
| Relationship, n (%) | ||
| Spouse | 51 (37.2) | 37 (39.7) |
| Child (in law) | 73 (53.3) | 46 (49.6) |
| Other | 13 (9.5) | 10 (10.7) |
| Female, n (%) | 90 (65.7) | 62 (66.7) |
| Age, years | ||
| Mean (s.d.) | 60.4 (13.5) | 59.8 (13.9) |
| Range | 30–84 | 34–91 |
| Diagnosis, n (%) | ||
| Dementia | 97 (70.8) | 66 (71.0) |
| Alzheimer's disease | 66 (48.2) | 31 (33.3) |
| Vascular dementia | 12 (8.8) | 14 (15.1) |
| Mixed dementia | 15 (10.9) | 6 (6.5) |
| Other dementia | 4 (2.9) | 15 (16.1) |
| No dementia | 40 (29.2) | 27 (29.0) |
| Cognitive impairment/MCI | 24 (17.5) | 15 (16.1) |
| Other cognitive impairment | 16 (11.7) | 12 (12.9) |
MCI, mild cognitive impairment
Outcomes
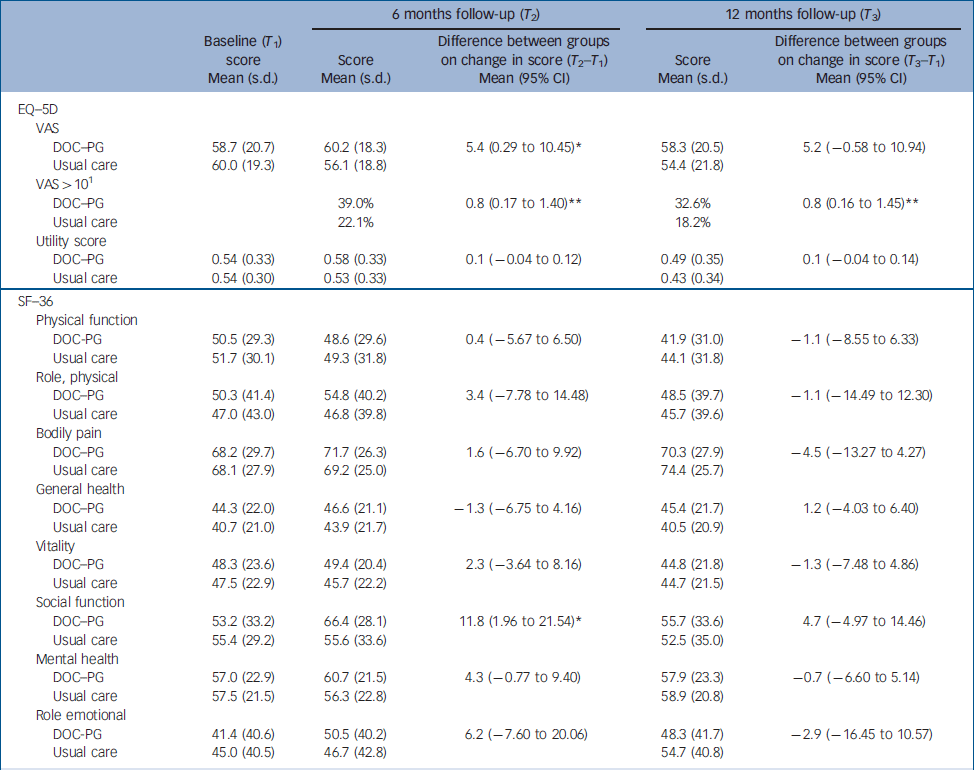
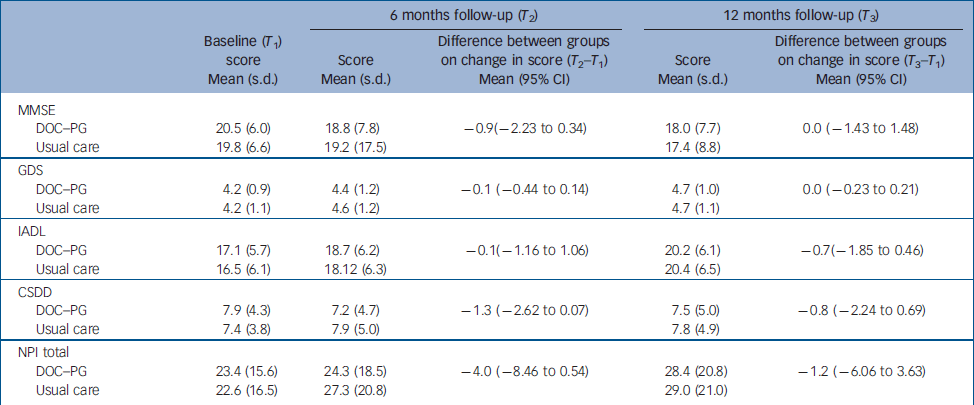
The mean score on the social functioning component of the SF–36 was significantly higher (P=0.03) in the intervention group than in the usual care group at 6 months (Table 2); no other difference in mean scores was found between the groups. The mean difference scores for the EQ–5D over time were significantly different between the two groups (P=0.04). Health-related quality of life measured with the VAS improved slightly in the intervention group (1.5 points) but decreased in the usual care group (4 points). We found a mean group difference of 9.6% in VAS after 12 months, which was close to our initial expectations. The proportion of patients who improved more than 10% (of the group difference) on the VAS was significantly greater (P=0.01) in the intervention group (39.0%) than in the control group (22.1%). With an improvement of 0.03 on the population utility score of the EQ–5D being considered a clinically relevant improvement, Reference Marra, Woolcott, Kopec, Shojania, Offer, Brazier, Esdaile and Anis23 significantly (P=0.04) more patients in the intervention group than in the usual care group showed a clinically relevant improvement after 6 months (42.1% v. 37.7%). Furthermore, the groups differed significantly (P=0.02) on the change score in the social functioning component of the SF–36, with patients in the intervention group showing a larger improvement than patients in the usual care group. After 12 months, more patients in the intervention group than in the usual care group showed a clinically relevant improvement in HRQoL measured as an improvement of more than 10% of the group difference on the VAS (32.6% v. 18.6%, P=0.01) and on the utility score of the EQ–5D (40.6% v. 24.7%, P<0.0001). The groups did not differ in terms of clinical outcome measures (Table 3).
Table 2 Health-related quality of life outcomes at follow-up and results of regression analyses (group differences)
| 6 months follow-up (T 2) | 12 months follow-up (T 3) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline (T 1) score Mean (s.d.) | Score Mean (s.d.) | Difference between groups on change in score (T 2–T 1) Mean (95% CI) | Score Mean (s.d.) | Difference between groups on change in score (T 3–T 1) Mean (95% CI) | |||
| EQ–5D | |||||||
| VAS | |||||||
| DOC–PG | 58.7 (20.7) | 60.2 (18.3) | 5.4 (0.29 to 10.45) * | 58.3 (20.5) | 5.2 (–0.58 to 10.94) | ||
| Usual care | 60.0 (19.3) | 56.1 (18.8) | 54.4 (21.8) | ||||
| VAS>10 1 | |||||||
| DOC–PG | 39.0% | 0.8 (0.17 to 1.40) ** | 32.6% | 0.8 (0.16 to 1.45) ** | |||
| Usual care | 22.1% | 18.2% | |||||
| Utility score | |||||||
| DOC–PG | 0.54 (0.33) | 0.58 (0.33) | 0.1 (–0.04 to 0.12) | 0.49 (0.35) | 0.1 (–0.04 to 0.14) | ||
| Usual care | 0.54 (0.30) | 0.53 (0.33) | 0.43 (0.34) | ||||
| SF–36 | |||||||
| Physical function | |||||||
| DOC-PG | 50.5 (29.3) | 48.6 (29.6) | 0.4 (–5.67 to 6.50) | 41.9 (31.0) | –1.1 (–8.55 to 6.33) | ||
| Usual care | 51.7 (30.1) | 49.3 (31.8) | 44.1 (31.8) | ||||
| Role, physical | |||||||
| DOC–PG | 50.3 (41.4) | 54.8 (40.2) | 3.4 (–7.78 to 14.48) | 48.5 (39.7) | –1.1 (–14.49 to 12.30) | ||
| Usual care | 47.0 (43.0) | 46.8 (39.8) | 45.7 (39.6) | ||||
| Bodily pain | |||||||
| DOC–PG | 68.2 (29.7) | 71.7 (26.3) | 1.6 (–6.70 to 9.92) | 70.3 (27.9) | –4.5 (–13.27 to 4.27) | ||
| Usual care | 68.1 (27.9) | 69.2 (25.0) | 74.4 (25.7) | ||||
| General health | |||||||
| DOC–PG | 44.3 (22.0) | 46.6 (21.1) | –1.3 (–6.75 to 4.16) | 45.4 (21.7) | 1.2 (–4.03 to 6.40) | ||
| Usual care | 40.7 (21.0) | 43.9 (21.7) | 40.5 (20.9) | ||||
| Vitality | |||||||
| DOC–PG | 48.3 (23.6) | 49.4 (20.4) | 2.3 (–3.64 to 8.16) | 44.8 (21.8) | –1.3 (–7.48 to 4.86) | ||
| Usual care | 47.5 (22.9) | 45.7 (22.2) | 44.7 (21.5) | ||||
| Social function | |||||||
| DOC–PG | 53.2 (33.2) | 66.4 (28.1) | 11.8 (1.96 to 21.54) * | 55.7 (33.6) | 4.7 (–4.97 to 14.46) | ||
| Usual care | 55.4 (29.2) | 55.6 (33.6) | 52.5 (35.0) | ||||
| Mental health | |||||||
| DOC–PG | 57.0 (22.9) | 60.7 (21.5) | 4.3 (–0.77 to 9.40) | 57.9 (23.3) | –0.7 (–6.60 to 5.14) | ||
| Usual care | 57.5 (21.5) | 56.3 (22.8) | 58.9 (20.8) | ||||
| Role emotional | |||||||
| DOC-PG | 41.4 (40.6) | 50.5 (40.2) | 6.2 (–7.60 to 20.06) | 48.3 (41.7) | –2.9 (–16.45 to 10.57) | ||
| Usual care | 45.0 (40.5) | 46.7 (42.8) | 54.7 (40.8) | ||||
DOC–PG, Diagnostic Observation Centre for Psychogeriatric Patients; SF–36, 36-item Short Form Health Survey; VAS, Visual Analogue Scale
Table 3 Clinical outcomes at follow-up and results of regression analyses (group differences).
| 6 months follow-up (T 2) | 12 months follow-up (T 3) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline (T 1) score Mean (s.d.) | Score Mean (s.d.) | Difference between groups on change in score (T 2–T 1) Mean (95% CI) | Score Mean (s.d.) | Difference between groups on change in score (T 3–T 1) Mean (95% CI) | |||
| MMSE | |||||||
| DOC–PG | 20.5 (6.0) | 18.8 (7.8) | –0.9(–2.23 to 0.34) | 18.0 (7.7) | 0.0 (–1.43 to 1.48) | ||
| Usual care | 19.8 (6.6) | 19.2 (17.5) | 17.4 (8.8) | ||||
| GDS | |||||||
| DOC–PG | 4.2 (0.9) | 4.4 (1.2) | –0.1 (–0.44 to 0.14) | 4.7 (1.0) | 0.0 (–0.23 to 0.21) | ||
| Usual care | 4.2 (1.1) | 4.6 (1.2) | 4.7 (1.1) | ||||
| IADL | |||||||
| DOC–PG | 17.1 (5.7) | 18.7 (6.2) | –0.1(–1.16 to 1.06) | 20.2 (6.1) | –0.7(–1.85 to 0.46) | ||
| Usual care | 16.5 (6.1) | 18.12 (6.3) | 20.4 (6.5) | ||||
| CSDD | |||||||
| DOC–PG | 7.9 (4.3) | 7.2 (4.7) | –1.3 (–2.62 to 0.07) | 7.5 (5.0) | –0.8 (–2.24 to 0.69) | ||
| Usual care | 7.4 (3.8) | 7.9 (5.0) | 7.8 (4.9) | ||||
| NPI total | |||||||
| DOC–PG | 23.4 (15.6) | 24.3 (18.5) | –4.0 (–8.46 to 0.54) | 28.4 (20.8) | –1.2 (–6.06 to 3.63) | ||
| Usual care | 22.6 (16.5) | 27.3 (20.8) | 29.0 (21.0) | ||||
CSDD, Cornell Scale for Depression in Dementia; DOC–PG, Diagnostic Observation Centre for Psychogeriatric Patients; GDS, Global Deterioration Scale; IADL, Instrumental Activities of Daily Living; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory
1. Values are the proportion of patients who improved by 10% or more of the group difference on the VAS compared with baseline (0, <10, 1, ≥10)
* P<0.05
** P<0.01
We investigated whether these differences in HRQoL between the groups were related to the use of cholinesterase inhibitors, in a post hoc analysis. In general, few patients received cholinesterase inhibitors (mean 14.6%), but significantly more patients in the intervention group than in the usual care group were treated with these drugs (18.3% v. 9.1%, P=0.01). However, the use of cholinesterase inhibitors had no influence on the proportion of patients who showed a clinically relevant improvement in HRQoL after 6 months (P=0.15) and after 12 months (P=0.53).
Discussion
In this study we found a modest but significant improvement in the proxy perception of HRQoL 6 months after the baseline measurement, confirming our initial hypothesis. Furthermore, more patients in the intervention group experienced a clinically relevant improvement of 10% or more of the group difference on the VAS and 0.03 or more on the utility score of the EQ–5D, after both 6 months and 12 months. These differences were not attributable to the use of cholinesterase inhibitors. We found no difference in cognitive functioning, behavioural and psychological problems, ability to perform activities of daily living, or emotional functioning. In the absence of any significant effect on the secondary clinical outcome measures, it is difficult to establish what might have caused this favourable outcome in the intervention group. Because the DOC–PG provides general practitioners with different types of advice – such as adaptation of medication, improvement of sensory function by ear syringing or testing eyesight, further referral to other hospital departments and to paramedical disciplines, and advice to initiate extra care, e.g. nursing home placement, respite care or services like ‘meals on wheels’ Reference Wolfs, Verhey, Kessels, Winkens, Severens and Dirksen24 – the improved outcome probably reflects the sum of the different advice and recommendations given.
The similarity of outcomes other than HRQoL in the two groups might be because the intervention provided access to two healthcare facilities that were available to the usual care group. Whereas medical centres tend to focus on medical diagnostics and pharmacotherapy, community mental health services focus on the provision of appropriate levels of care and support for patients and their carers. It is thus not surprising that the two approaches had comparable effects on psychological and behavioural problems, emotional functioning and ability to manage daily life. In this context, usual care in our region is very good and is provided by an active university medical centre and a community mental health service that have collaborated in the past on several projects. Thus, the contrast between DOC–PG and usual care might have been smaller than would be the case in other regions.
To our knowledge, this is the first randomised controlled trial of a multidisciplinary diagnostic approach to dementia. Our results suggest that an integrated approach to dementia as recommended by international dementia guidelines improves patient outcomes. In the absence of a cure for dementia, the finding that (the proxy perception of) HRQoL can be improved with an integrated treatment plan formulated on the basis of a multidisciplinary diagnostic evaluation is important. It should, however, be noted that the results of this study cannot be generalised to nursing-home care.
The study had potential weaknesses. First, the design of the study was not optimal because it was not feasible to mask the interviewers assessing the patients and their carers to the treatment assigned. However, all instruments were standardised and the participants received neutral instructions for every instrument. Another potential problem is linked to our inability to keep the random allocation sequence completely concealed, because the person responsible for the allocation of patients also recruited a small number of patients (5%). However, the people who recruited the majority of the patients were unaware of patient allocation. The masking of the referring general practitioners could not be maintained until the end of the study. In order to investigate the potential effects of this on the study results, we compared post hoc the characteristics of patients in the two groups who were recruited in the first year and in the second year. We did not find any difference within the intervention group with respect to age, gender, diagnosis, MMSE score and GDS score; however, there were non-significant differences in diagnosis and GDS score in the control group – in the second year of the inclusion period more people with a cognitive disorder other than dementia and with a lower GDS score were included. The general practitioners probably wanted to refer patients to DOC–PG but this was only possible after recruitment was completed. The inclusion of slightly healthier patients (with consequently higher quality of life and lower costs) in the latter half of the inclusion period probably resulted in a less favourable outcome for the DOC–PG intervention. Another potential limitation is the use of proxies to complete the questionnaires. We chose to use proxies because of the longitudinal nature of the study, the complex health problems of the study population and the anticipated progressive global deterioration of intellect and personality of the study population. In the later stages of dementia, proxy measures are generally considered necessary because patients are no longer able to evaluate their own health. Reference Selai, Rabin, Busschbach and Charro25,Reference Jonsson, Andreasen, Kilander, Soininen, Waldemar, Nygaard, Winblad, Jonhagen, Hallikainen and Wimo26 The proxy scores on the various instruments might have been biased because of a perceived caregiver burden, Reference Logsdon, Gibbons, McCurry and Teri27 but this bias would apply to both groups. Furthermore, it should be emphasised that we measured the proxy's perception of the HRQoL of the patient rather than a direct estimate of HRQoL. Another problem is the presence of missing data, which could have affected the statistical analyses. However, very few data (5%) were missing, and multiple imputation procedures provide a useful strategy for dealing with data-sets with missing values. Instead of filling in a single value for each missing value, the missing value is replaced by a set of ten plausible values that represent the uncertainty with respect to choosing the right value to impute. This results in statistically valid inferences that properly reflect the uncertainty brought on by missing values. Reference Rubin21,Reference Rubin and Schenker28
We chose the VAS of the EQ–5D as main outcome because it has good clinimetric properties, is reliable Reference Parkin, Rice, Jacoby and Doughty29 and is easy to administer. Unfortunately, the VAS is more subjective than the descriptive component of the EQ–5D and this could be considered a limitation. A person's state of mind, goals and expectations can influence VAS scores; Reference Dolan30,Reference Ubel, Loewenstein and Jepson31 however, we expected that these effects would be present in both groups. Moreover, the VAS enables a personal valuation of the patient's HRQoL, which is an important outcome in the absence of a cure.
There is a growing interest in studies on effectiveness and efficacy of multidisciplinary healthcare models. We are currently conducting an economic evaluation comparing the costs of DOC–PG and of usual care. Although a multidisciplinary model is more effective than a monodisciplinary model, it is also more complex, requiring a higher level of organisation. It is therefore a challenge for clinicians to combine their professional expertise and share responsibility for a patient given their different – and sometimes opposing – approaches and views on patient care and management. For instance, the role of memory clinics is debated. Although some claim that these clinics merely prescribe and monitor drug treatment, Reference Pelosi, McNulty and Jackson32 such clinics are becoming increasingly integrated in the standard care for dementia in The Netherlands. Reference Verhey, Scheltens and Olde Rikkert33 We recommend that all services involved with dementia care integrate (such as in the DOC–PG) rather than polarise, because greater integration will lead to greater continuity of care for patients with dementia. The value of DOC–PG has already been recognised by general practitioners, as evidenced by the high referral rate by these doctors and by the high compliance with DOC–PG recommendations. Reference Wolfs, Verhey, Kessels, Winkens, Severens and Dirksen24
Acknowledgements
We thank the patients, their carers and the general practitioners who participated in this study. Additionally, we thank Daniëlle Willems, Audrey Fiddelers, Heidi Lansdaal and Ilvy Mayen for collecting and entering the data. We also thank everyone working at the DOC–PG, the Maastricht Memory Clinic and the community mental health team for their help in recruiting patients.
The study was funded by the Dutch Research Institute for Care – Medical Sciences (ZorgOnderzoek Nederland-Medische wetenschappen), grant 945-02-055.







eLetters
No eLetters have been published for this article.