Although obesity is not a disease, it has harmful effects on human health. It is very important to prevent and cure the symptoms of obesity, as obesity increases the risk of various diseases, including non-insulin-dependent diabetes mellitus and arteriosclerosis. Obesity is the result of an incorrect energy balance leading to increased stores of energy as fat. Most people desire to avoid obesity, and hope to maintain slimmer body profiles. As a result, people are increasingly trying to reduce body weight and adipose tissue mass. Thus, ideal diets for prevention and amelioration of obesity are needed.
Conjugated linoleic acid (CLA) is a generic term for positional and geometrical isomers of LA, and is present in food items produced from ruminant animals(Reference Kepler, Hirons and McNell1, Reference Kepler and Tove2). The double-bond structure in CLA is different from that in LA, although CLA and LA have the same carbon chain length and number of double bonds(Reference Eulitz, Yurawecz and Sehat3). The double bonds of CLA form a conjugated diene structure, and numerous CLA isomers have been discovered. CLA has been found to have several functions, including anti-obesity, anti-carcinogenic and anti-atherogenic effects(Reference Nakanishi, Oikawa and Koutoku4–Reference Pariza, Park and Cook6). Because CLA induces liver enlargement with high lipid contents in mice(Reference Nakanishi, Oikawa and Koutoku4, Reference Tsuboyama-Kasaoka, Takahashi and Tanemura7), research into preventing fatty liver symptoms, as well as its causative mechanisms, is needed.
Long-chain n-6 PUFA are essential fatty acids. PUFA are metabolized by elongases and desaturases. In n-6 PUFA, linoleic acid (LA) is metabolized to γ-linolenic acid (GLA), dihomo-γ-linolenic acid and arachidonic acid (ARA). Dihomo-γ-linolenic acid and ARA are metabolized to PGE1 and PGE2, respectively. PG are known to have roles in ovulation, nidation, platelet aggregation, vascularization, protection of gastric mucosa and kidney function(Reference Gurr, Harwood and Frayn8). In addition, increased PGE2 levels protect against alcoholic liver injury(Reference Lukivskaya, Maskevich and Buko9), while PGE1 or PGE2 injections in the rat liver prevents toxicity induced by carbon tetrachloride(Reference Masaki, Ohta and Shirataki10).
Long-term administration of CLA lowers PGE2 levels in the serum and liver(Reference Nakanishi, Oikawa and Koutoku4, Reference Sugano, Tsujita and Yamasaki11). Nakanishi et al. (Reference Nakanishi, Oikawa and Koutoku4) reported that the combination of CLA and GLA prevented fatty liver induced by CLA and suggested that GLA inhibits CLA-induced fatty liver, as GLA prevents the CLA-mediated decreases in PGE2 production in the liver. The combination of CLA and GLA, as well as CLA alone, had anti-obesity effects on visceral fat(Reference Nakanishi, Oikawa and Koutoku4) and adipocytes of subcutaneous tissue in the skin(Reference Oikawa, Nakanishi and Nakamura12). However, GLA was converted to PGE1 through dihomo-γ-linolenic acid metabolism. Thus, the results by Nakanishi et al. (Reference Nakanishi, Oikawa and Koutoku4) could not be attributed to PGE2 alone. On the other hand, ARA is a direct precursor of PGE2, rather than GLA.
Therefore, the purpose of the present study was to investigate whether the combination of CLA and ARA attenuates fatty liver induced by CLA in mice.
Materials and methods
Animals and treatments
Male mice (8 weeks old; Sea:ddY strain; purchased from CLEA Japan Inc., Tokyo, Japan) were kept at 25°C on a 12 h light–dark cycle (08.00–20.00 hours), housed individually, and had free access to a commercial diet (MF, Oriental Yeast Co. Ltd, Tokyo, Japan) and water. Food intake was monitored individually using clean animal feeding equipment (Type M, Rodent Café; Oriental Yeast Co. Ltd). The chemical composition (weight %) of the commercial diet was as follows: moisture, 7·7; crude protein, 23·6; crude fat, 5·3; crude ash, 6·1; crude fibre, 2·9; nitrogen-free extract, 54·4. Fatty acid composition (%) of the diet was 16 : 0, 14·6; 18 : 0, 2·6; 18 : 1n-9, 24·6; 18 : 2n-6, 46·6; 18 : 3n-3, 3·8; 20 : 4n-6, 0·2; others, 7·6, respectively. Mice were divided into five groups of seven mice each according to body weight v. average weight of each group.
CLA diet in the three CLA groups and LA diets in the LA group were prepared by adding 3 % CLA oil (TAG-type) and 3 % LA oil (high LA safflower oil) to standard diets, respectively. Both oils were provided by Nisshin Oillio Group Ltd (Tokyo, Japan). The CLA oil contained 83·0 % CLA (40·5 % 9cis, 11trans-CLA; 39·6 % 10trans, 12cis-CLA). The LA group was given high LA safflower oil, which contained 73·0 % LA. SUNTGA40S (TAG-type), provided by Suntory Ltd (Osaka, Japan), including 45·3 % TAG-type ARA, was given to the ARA groups. The fatty acid composition of the oils is shown in Table 1. Mice were given access ad libitum to commercial diet alone (control), or LA, CLA, CLA+1 % ARA or CLA+2 % ARA diets for 4 weeks. Diets were replaced with fresh diet every 3 d, and remaining diet was weighed to determine food intake. Mice were killed by cervical dislocation and decapitation at the end of the feeding period.
Table 1 Compositions (% of total fatty acids) of experimental oils
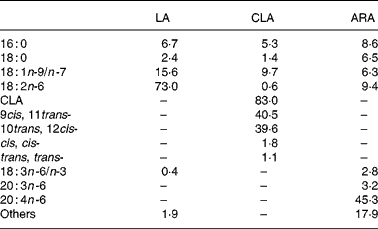
LA, linoleic acid; CLA, conjugated linoleic acid; ARA, arachidonic acid.
Experimental procedures followed the guidelines for Animal Experiments of the Faculty of Agriculture and of the Graduate Course of Kyushu University, as well as the relevant Laws (no. 105) and Notifications (no. 6) of the Japanese Government.
Sample collection
Blood was collected from the carotid artery into tubes containing heparin when the mice were decapitated. Blood was centrifuged for 10 min at 690 g, and the collected plasma was stored at − 30°C until assay for lipids and hepatitis toxicity. Liver, as well as perirenal and epididymal adipose tissues, were then removed and weighed. Liver was stored at − 80°C until lipid analysis.
Plasma assay
TAG, cholesterol and NEFA contents were analysed using commercial kits (Triglyceride E-test, Cholesterol E-test and NEFA C-test, respectively; Wako Pure Chemical Industries Ltd, Osaka, Japan). Glutamic oxaloacetic transaminase and glutamic pyruvic transaminase concentrations were analysed using a commercial kit (transaminase CII-test; Wako Pure Chemical Industries Ltd).
Liver analysis
TAG and cholesterol contents
Total lipids in the liver were extracted using the modified method of Bligh & Dyer(Reference Bligh and Dyer13). An acetic acid (0·1 mol/l)–chloroform–methanol (1:2·5:1·25, by vol.) solution (2 ml) was added to 0·4 g liver sample, followed by homogenization. The mixture was left for 10 min at room temperature. Chloroform (2·5 ml) and distilled water (2·7 ml) were added, and the mixture was stirred. The solution was centrifuged for 10 min at 890 g for separation into two distinct phases, and the lower phase was collected. Chloroform (4 ml) was again added into the upper phase followed by mixing, centrifugation and collection of the lower phase. Both fractions were filtered and mixed with 6 ml distilled water–methanol–chloroform (1:1:0·1, by vol.) solution. Samples were centrifuged at 890 g for 10 min. The lower phase was dried using a centrifuge evaporator. The dried extract was diluted with 1 ml isopropanol, and was then assayed using the TAG and cholesterol determination kits mentioned earlier.
PGE1 and PGE2 concentration
PGE1 and PGE2 were extracted from the liver using the methods recommended by GE Health Care Ltd (Little Chalfont, UK). The PGE1 assay used the PGE1 enzyme immunoassay kit (Assay Designs Inc., Ann Arbor, MI, USA) and the PGE2 assay used the PGE2 enzyme immunoassay kit (GE Health Care Ltd).
Statistical methods
Data analysed by one-way ANOVA. When significant effects were found, the five dietary groups were compared by Tukey–Kramer's test. Statistical significance was set at P < 0·05. The results are shown as means and their standard errors.
Results
Table 2 shows the effects of LA, CLA and combinations of CLA and ARA on food intake, body weight gain and physiological parameters in mice. Significant (F(4,30) 4·48, P = 0·006) dietary effects on food intake were detected. Food intake in the CLA group and CLA+1 % ARA group were significantly lower than in the control group. However, body weight gain and food efficiency were not significantly different (F(4,30) 1·09, P = 0·38 and F(4,30) 0·80, P = 0·54) among groups. Livers in the CLA group were significantly heavier than in the control and LA groups, whereas those in the CLA+1 % ARA and CLA+2 % ARA groups were markedly lighter than in the CLA alone group (F(4,30) 9·39, P < 0·0001). Dietary CLA alone significantly reduced visceral fats (perirenal fat and epididymal fat) as compared to the control and LA groups (F(4,30) 26·86, P < 0·0001 and F(4,30) 8·16, P = 0·0001). All combinations of CLA and ARA reduced perirenal fat weight similarly to CLA alone. Conversely, epididymal fat weight tended to be higher depending on ARA levels when compared to CLA alone.
Table 2 The effect of linoleic acid (LA), conjugated linoleic acid (CLA) or combinations of CLA and arachidonic acid (ARA) on food intake, body weight gain, tissue weight and plasma*
(Mean values with their standard errors for seven mice)

GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures, see Materials and methods.
Plasma glutamic oxaloacetic transaminase and glutamic pyruvic transaminase concentrations were not influenced by dietary treatment (F(4,30) 0·34, P = 0·85 and F(4,30) 1·38, P = 0·26). Plasma TAG concentrations (F(4,30) 7·41, P = 0·0003) in the CLA+1 % ARA and CLA+2 % ARA groups were significantly lower than those in the CLA group, and were not significantly different from the control and LA groups. Cholesterol (F(4,30) 2·01, P = 0·12) and NEFA (F(4,30) 1·87, P = 0·14) concentrations did not significantly differ among the groups.
TAG (F(4,30) 22·9, P < 0·0001) and cholesterol (F(4,30) 15·8; P < 0·0001) contents in the liver were greatest in the CLA diet group (Fig. 1). TAG and cholesterol contents when CLA was combined with 1 or 2 % ARA were 0·37 or 0·31 times and 0·41 or 0·47 times lower than those with CLA alone. Fig. 2 indicates PGE2 contents in the liver. In contrast to lipid contents in the liver, PGE2 contents (F(4,30) 4·54, P = 0·006) were significantly higher in the CLA+1 or 2 % ARA groups than in the CLA group. However, the liver PGE1 contents (ng/g liver weight) were not significantly (F(4,30) 1·32, P = 0·285) different among the treatment groups (control, 1·87 (sem 0·47); LA, 1·57 (sem 0·38); CLA, 1·13 (sem 0·28); CLA+1 % ARA, 1·62 (sem 0·32); CLA+2 % ARA, 2·26 (sem 0·32)).
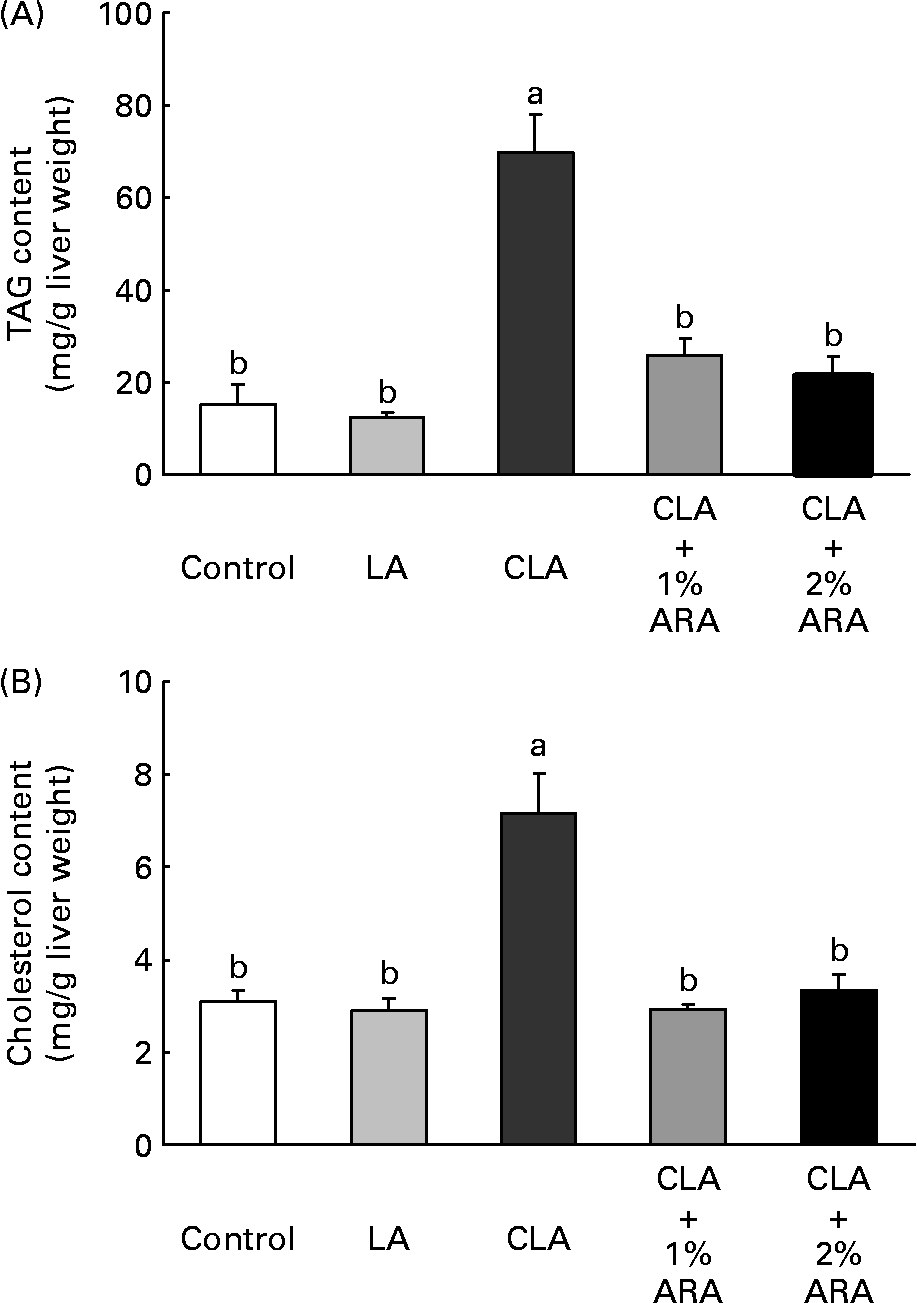
Fig. 1 TAG (A) and cholesterol (B) contents of the liver in mice given control, linoleic acid (LA), conjugated linoleic acid (CLA) or CLA+arachidonic acid (ARA) diets. Values are means with their standard errors depicted by vertical bars (n 7). a,b Mean values with unlike letters were significantly different (P < 0·05).
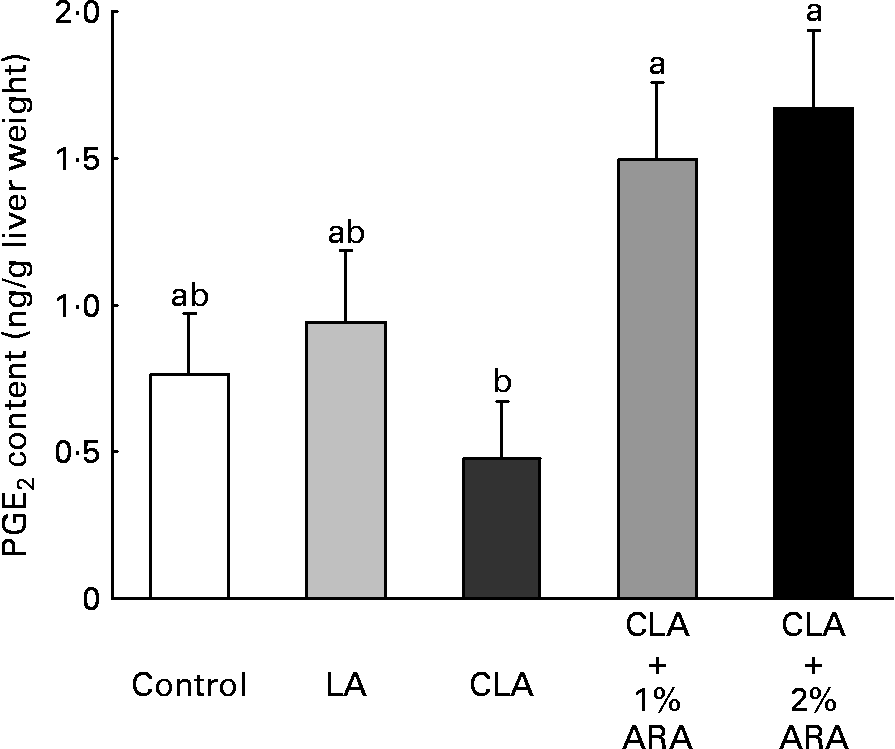
Fig. 2 PGE2 contents in livers of mice given control, linoleic acid (LA), conjugated linoleic acid (CLA) or CLA+arachidonic acid (ARA) diets. Values are means with their standard errors depicted by vertical bars (n 7). a,b Mean values with unlike letters were significantly different (P < 0·05).
Discussion
Food intake in the CLA and CLA+1 % ARA groups was lower as compared to the control group, although the CLA+2 % ARA group did not significantly differ from the control and LA groups (Table 2). The present results may be the result of both inhibition of neuropeptide Y production by CLA and high-energy intake, similarly to LA(Reference Cao, Wang and Xiang14). Perirenal and epididymal fat weights in the CLA group were the lowest among the five groups; however, body weight did not vary among the groups. The regulation of body weight by CLA may be explained by increased liver weight (Table 2), and increased amounts of protein and keeping of water in the body by CLA-feeding(Reference Park, Albright and Liu15, Reference Park, Storkson and Albright16). CLA alone or CLA mixed with ARA markedly suppressed the weight of white adipose tissue surrounding the kidney (Table 2).
The employed CLA contained 40·5 % 9cis, 11trans and 39·6 % 10trans, 12cis isomers (Table 1). Brown et al. (Reference Brown, Halvorsen and Lea-Currie17) reported that the 10trans, 12cis-CLA isomer inhibited accumulation of fat in the body, as the isomer played a role in the inhibition of hepatic stearoyl-CoA desaturase activity(Reference Park, Storkson and Ntambi18) and affected lipoprotein lipase activity(Reference Lin, Kreeft and Schuurbiers19). Moreover, the CLA isomer suppressed the gene expression of sterol regulatory element-binding protein-1, PPARγ, acetyl-CoA carboxylase and fatty acid synthase in adipocytes(Reference House, Cassady and Eisen20). On the other hand, the combination of CLA+ARA did not fully preserve the anti-obesity effects of CLA, as epididymal fat weight was higher in both groups than in the CLA alone group (Table 2). Nakanishi et al. (Reference Nakanishi, Oikawa and Koutoku4) reported that perirenal adipose tissue weight with supplementation of 250 μl CLA+250 μl GLA was maintained at similar levels obtained as with CLA alone, but supplementation with 250 μl CLA+250 μl GLA tended to increase epididymal fat mass. For anti-obesity effects, CLA+1 % ARA was the best combination, as both visceral fat weights were significantly lower than with LA intake.
Co-administration of ARA successfully inhibited fatty liver induced by CLA in the present study. The value for liver weight per body weight (g/kg) was calculated as follows: control, 44·4 (sem 1·6); LA, 44·3 (sem 1·1); CLA, 71·2 (sem 4·5); CLA+1 % ARA, 55·7 (sem 2·2); and CLA+2 % ARA, 52·2 (sem 2·1). ARA reduced the liver weight gain induced by CLA by 21·8 and 26·7 % at 1 and 2 %, respectively. Nakanishi et al. (Reference Nakanishi, Oikawa and Koutoku4) orally administered 250 μl CLA alone or 250 μl CLA+250 μl GLA daily to mice. The combination of CLA+GLA reduced liver weight (30·2 %) with reductions in TAG content. In contrast, the present study revealed that CLA increased both TAG and cholesterol contents. However, the contribution of TAG was high, as the liver contains high levels of TAG when compared with cholesterol (Fig. 1). Additionally, fatty liver induced by CLA is not apparently associated with liver damage. Glutamic oxaloacetic transaminase and glutamic pyruvic transaminase activities were not significantly different among any of the groups (Table 2). While CLA potentially attenuates liver toxicity in both fatty (Zucker) and conventional (Sprague–Dawley) rats after partial hepatectomy(Reference Nagao, Inoue and Wang21, Reference Hirao, Yamasaki and Chujo22), ARA tended to prevent elevation of plasma glutamic oxaloacetic transaminase levels in rats given the 1·5–5·0 % SUNTGA40S oil used in the present study(Reference Lina, Wolterbeek and Suwa23). Furthermore, ARA itself is not toxic to the liver, as liver weight did not change with 0·5–5·0 % SUNTGA40S administration.
Nakanishi et al. (Reference Nakanishi, Oikawa and Koutoku4) suggested that GLA attenuated fatty liver induced by CLA, as GLA increases PGE2 in the liver. However, GLA is metabolized to both PGE1 and PGE2, and these eicosanoids have protective effects on the liver(Reference Masaki, Ohta and Shirataki10, Reference Mater, Thelen and Jump24, Reference Sugawara, Kubota and Ogura25). These PG inhibit hepatic toxicities induced by carbon tetrachloride or tert-butyl hydroperoxide, and PGE2 suppresses gene expressions and activity of enzymes and proteins associated with lipogenesis, but not lipolysis. It is possible that both PGE1 and PGE2 prevented CLA-induced fatty liver, as PGE1 in the liver was not analysed in the previous report(Reference Nakanishi, Oikawa and Koutoku4). The lipid contents in the liver were markedly lower in the CLA+1or 2 % ARA groups than in the CLA alone group (Fig. 1). The hepatic lipid reductions were influenced by PGE2 produced from the administered ARA, as PGE2 content in the liver was significantly increased in the CLA+ARA groups compared to the CLA alone group. However, PGE1 was not affected by ARA intake.
The present results suggest that even if a large amount of ARA is given, dihomo-γ-linolenic acid would be metabolized to ARA but not to PGE1. At 3 %, CLA reduced ARA contents in the total liver, as well as in liver phospholipids such as phosphatidylcholine, phosphatidylethanolamine and phosphatidylserine(Reference Kelley, Bartolini and Newman26, Reference Stangl27). Hence, liver PGE2 levels were decreased by CLA. However, it is possible that CLA+ARA groups restored normal ARA levels in phospholipids, as PGE2 production was not inhibited.
Treatment with ARA or PGE2 clearly prevented gene expression of S14, a lipogenesis-related nuclear protein, and fatty acid synthase in rat hepatocytes. While, gene expressions of acyl-CoA oxidase and cytochrome P450 were not changed(Reference Mater, Thelen and Jump24). S14 and fatty acid synthase are regulated by sterol regulatory element-binding protein, and induce lipid production in the liver(Reference Kinlaw, Quinn and Wells28). Long-term 1·5 % CLA supplementation significantly elevates mRNA sterol regulatory element-binding protein-1 levels in the mouse liver, and enhanced fatty acid synthase gene expression(Reference Takahashi, Kushiro and Shinohara29). It was assumed that the CLA+ARA groups showed suppressed fatty liver because dietary ARA influenced the gene expression or activity of these lipogenic enzymes or proteins.
CLA induces hyperinsulinaemia and insulin resistance in mice(Reference Clément, Poirier and Niot30, Reference Wendel, Purushotham and Liu31). On the other hand, it was described that ARA administration reduced plasma high glucose and insulin resistance induced by high-fat diet in rats(Reference Wu, Wang and Duan32). Additionally, Papadimitriou et al. (Reference Papadimitriou, King and Jones33) showed that ARA and PGE2 prevented the cultured pancreatic β-cells from apoptosis. ARA treatment may suppress insulin disorders induced by CLA. Therefore, hepatic lipogenic and lipolytic enzyme activities or gene expressions were changed by both dietary CLA and ARA, and effects of the mixture oil on insulin secretion or activity should be examined in the future.
Ide(Reference Ide34), Yanagita et al. (Reference Yanagita, Wang and Nagao35) and Vemuri et al. (Reference Vemuri, Kelley and Mackey36) reported that a combination of CLA with n-3 fatty acids attenuated CLA-induced fatty liver in mice. The data reported by Ide were particularly similar to those of the present study. Liver weight was significantly lower in mice given CLA and fish oil than those given CLA alone, and fish oil intake dose-dependently decreased liver TAG and cholesterol contents. In addition, the anti-obesity effects of CLA were maintained in the CLA+fish oil groups in the visceral fat of mice, but the influence became weak depending on high fish oil concentration. Ide(Reference Ide34) reported that the attenuation of fatty liver was due to decreases in gene expression of hepatic lipogenic enzymes by n-3 fatty acids. Vemuri et al. (Reference Vemuri, Kelley and Mackey36) suggested EPA and DHA prevented CLA-induced fatty liver in mice. On the other hand, insulin resistance induced by CLA could recover in only the DHA group, but not EPA group.
In conclusion, fatty liver induced by CLA was attenuated by co-administration with ARA, furthermore, a combination of these fatty acids maintained the anti-obese effect of CLA.
Acknowledgements
This study was supported by a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists (no. 09551). None of the authors has conflicts of interest with respect to this work. Contributions to this work were as follows: D. O. designed and conducted the experiment and wrote the manuscript. S. T., Y. A. and Y. M. cared for the animals, helped in sampling and discussion writing. M. F. helped to design the experiment and participated in critical revision of the manuscript.






