Impact statement
While there has been much focus on the environmental occurrence and impacts of microplastic (MP; 1–5,000 μm), small MPs (sMP; <100 μm) and nanoplastics (NPs) are hypothesised to be an even greater risk to organisms due to their ability to transfer across biological membranes. High-quality, well-characterised and environmentally relevant test and reference materials are crucial for assessing sMP and NP fate and effects, but these are not currently widely available to the scientific community. This has led to recent efforts to develop methods for the production of more environmentally relevant test/reference materials in sufficient quantities for use in environmental fate and effects assessment, as well as analytical method validation. To the best of the author’s knowledge, the work presented here represents the first summary on the current status of the use and production of environmentally relevant sMP and NP reference materials. The work serves as a basis for describing the current state of the art, highlighting the challenges and limitations associated with the different methods reported alongside the advantages and benefits of each approach. It also provides the reader with a clear overview of the most promising methods and approaches for further development and optimisation. Finally, the work acts as a reference point for identifying and summarising the critical physicochemical properties that should be considered when producing sMP and NP test/reference materials. The impact of this work is considered to have regional and international relevance and reach through the future development and utilisation of more environmentally relevant test and reference materials for use in the fate and effects assessment of sMP and NP. In turn, this will help to improve the accuracy and robustness of risk assessments that form the basis for future mitigation actions and policy development towards plastic pollution, especially in the form of sMP and NP.
The urgent need for environmentally relevant small microplastic and nanoplastic test and reference materials
It is estimated that ~10% of all plastic produced enters the global oceans, accounting for 80–85% of the total marine litter load (Coyle et al., Reference Coyle, Hardiman and Driscoll2020). High-production polymers such as polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinylchloride, PE terephthalate (PET) and polyamide (PA), are used in many consumer products because of their durability and chemical resistance. Under most environment conditions, they are considered non-biodegradable over long timescales, implying that they will persist and accumulate in the environment (Tokiwa et al., Reference Tokiwa, Calabia, Ugwu and Aiba2009; Hakvåg et al., Reference Hakvåg, Brakstad, Kubowicz, Booth, Sarkar, Sharma and Shekhar2023). However, when exposed to a combination of UV irradiation and mechanical degradation/abrasion, most polymers will undergo physicochemical property changes that lead to fragmentation or ‘shedding’ (Geyer et al., Reference Geyer, Jambeck and Law2017). Such processes can occur during the use phase and once the materials reach the natural environment. The consequence is the fragmentation of the material into particles of millimetres, micrometres or even nanometres in size. Mechanic pressures and abrasion during all life stages, that is, production, consumer use and waste handling, may also cause the release of small- and medium-sized plastic particles from plastics of all types. Importantly, many plastic consumer products contain additive chemicals that are specifically included to protect the material from degradation (e.g., UV stabilisers).
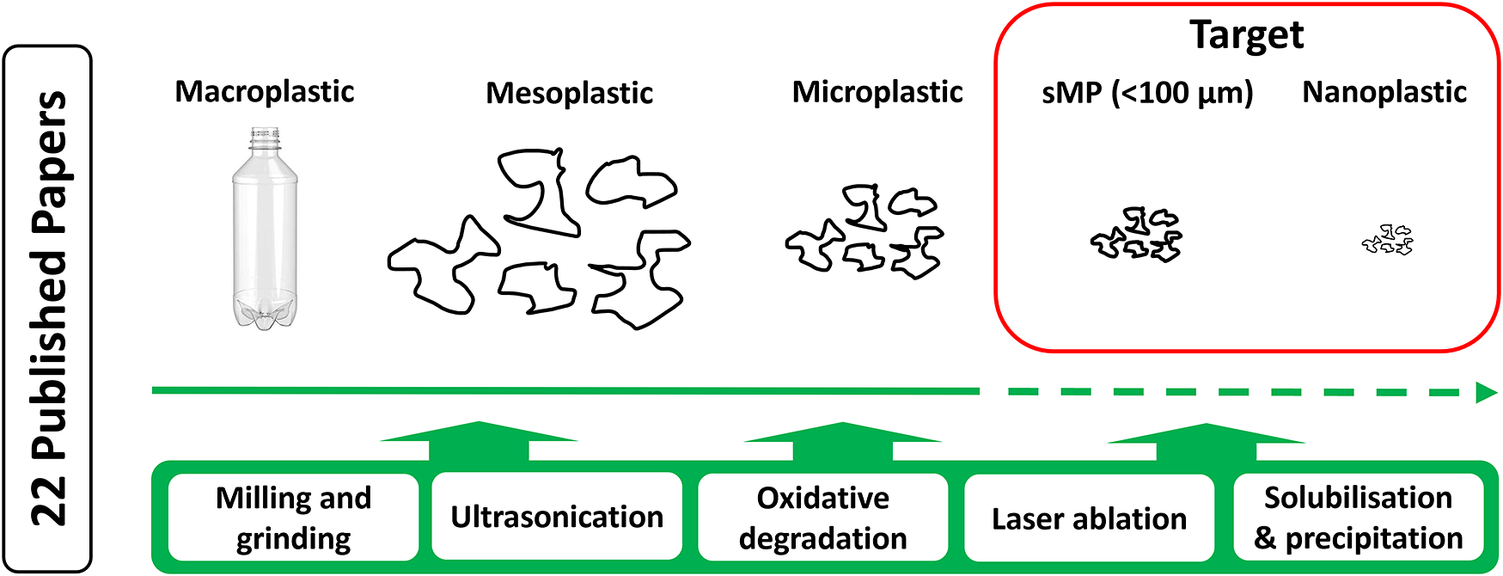
Many studies have investigated the occurrence and distribution of microplastic (MP; 1–5,000 μm) in the environment and food matrices, where robust, if not standardised, methods for their characterisation and quantification are widely available. The large body of available data suggests that larger MPs in the natural environment are irregular shaped, partially degraded (chemically and physically), a continuum of sizes, shapes and densities, represent a wide range of polymer types, and acts as a reservoir for thousands of plastic-associated chemicals. However, small MPs (sMPs; here defined as <100 μm) and particularly nanoplastics (NPs; <1 μm or <0.1 μm in all dimensions (da Costa et al., Reference da Costa, Santos, Duarte and Rocha-Santos2016; Gigault et al., Reference Gigault, El Hadri, Nguyen, Grassl, Rowenczyk, Tufenkji, Feng and Wiesner2021; Mitrano et al., Reference Mitrano, Wick and Nowack2021) in natural systems have been much less studied, primarily due to significant analytical challenges associated with their combined small size (Gigault et al., Reference Gigault, ter, Baudrimont, Pascal, Gauffre, Phi, El Hadri, Grassl and Reynaud2018, Reference Gigault, El Hadri, Nguyen, Grassl, Rowenczyk, Tufenkji, Feng and Wiesner2021). Although direct measurement data of true NP in the environment remain elusive, they are expected to be as equally diverse as MPs (Koelmans et al., Reference Koelmans, Besseling, Shim, Bergmann, Gutow and Klages2015; da Costa et al., Reference da Costa, Santos, Duarte and Rocha-Santos2016; Mattsson et al., Reference Mattsson, Jocic, Doverbratt, Hansson and Zeng2018). Sources of NP release into the environment have been assessed, including primary sources such as NP added to cosmetic products (Hernandez et al., Reference Hernandez, Yousefi and Tufenkji2017) and secondary NP released from surgical masks (Liang et al., Reference Liang, Wang, Liu, Ge, Song, Wang and Chai2022). The formation of sMP and NP during (simulated) environmental degradation of plastic or larger MP has been indicated through indirect and direct environmental observations (Lambert and Wagner, Reference Lambert and Wagner2016; Sait et al., Reference Sait, Sørensen, Kubowicz, Vike-Jonas, Gonzalez, Asimakopoulos and Booth2021; Tong et al., Reference Tong, Zhong, Duan, Yi, Cheng, Xu and Yang2022).
Recent research has suggested that NP may be more hazardous than MP, given the ability of smaller particles to cross biological membranes (Gong et al., Reference Gong, Li, Li, Guo, Xu, Gan, Yan and Wang2023). It has been demonstrated that the toxicological mechanisms and subsequent observed effects of different size plastic particles differ (Mitrano et al., Reference Mitrano, Wick and Nowack2021; Yin et al., Reference Yin, Wang, Zhao, Wang, Guo, Mu, Liu, Nie, Li, Li and Xing2021). To date, the vast majority of (s)MP and NP fate and effects studies have been performed with pristine (non-degraded), spherical, monodisperse and near-neutrally buoyant particles (Pradel et al., Reference Pradel, Catrouillet and Gigault2023), often without any assessment of the additive chemical composition (Delaeter et al., Reference Delaeter, Spilmont, Bouchet and Seuront2022). For example, studies demonstrating membrane transfer and subsequent toxicity have, in several cases utilised surface-modified particles meant for drug delivery (Shen et al., Reference Shen, Zhang, Zhu, Song, Zeng, Hu, Wen and Ren2019). Crucially, a few studies have shown significant differences in the toxicological effects observed after exposure to spherical and irregular sMP (Xia et al., Reference Xia, Sui, Du, Wang, Jing, Zhu, Zhao, Sun, Booth, Chen, Qu and Xing2022), indicating the importance of particle morphology. As a result, accurate assessment of the environmental fate, effects and risks of sMP and NP is challenging because of the lack of both field observations and laboratory investigations using environmentally relevant test materials (da Costa et al., Reference da Costa, Santos, Duarte and Rocha-Santos2016; Pradel et al., Reference Pradel, Catrouillet and Gigault2023).
It is evident that a lack of available sMP and NP test and reference materials, representative of those that may be expected to be released into or formed through plastic degradation in the environment, is limiting progress in understanding the fate, effects and ultimately, the environmental and human health risks of this ubiquitous form of pollution. The current situation is primarily explained by the significant challenges in developing effective and reproducible methods for producing well-characterised reference sMP and NP particles in sufficient qualities (Huber et al., Reference Huber, Ivleva, Booth, Beer, Bianchi, Drexel, Geiss, Mehn, Meier, Molska, Parot, Sørensen, Vella, Prina-Mello, Vogel and Caputo2023; Martínez-Francés et al., Reference Martínez-Francés, van Bavel, Hurley, Nizzetto, Pakhomova, Buenaventura, Singdahl-Larsen, Magni, Johansen and Lusher2023). This is further compounded by the development of suitable analytical tools representing a major bottleneck with respect to understanding the fate and effects of sMP and NP (Nguyen et al., Reference Nguyen, Claveau-Mallet, Hernandez, Xu, Farner and Tufenkji2019).
To address the challenges and current limitations associated with environmental fate and effects assessment, methods to produce significant yields of sMP and NP with a variety of shapes, sizes, polymer types and chemical compositions are needed. While having a repository of available reference materials is desirable for purposes such as analytical method validation and larger comparable studies, we propose that there is an equally large need for transferable methods that can be used to produce test materials from bulk plastics (e.g., specific consumer products) on a case-by-case basis. The aim of the current review is to provide an overview of the currently tested (reported in literature) methods for the production of representative sMP and NP materials and to suggest which may show the best promise for future development and optimisation. We focus mainly to top-down methods for producing particles of plausible relevance as fragments of ‘real’ leaked plastic in the environment.
Methods
Peer-reviewed literature was accessed using databases such as Science Direct, Google Scholar, Scopus, ACS Publications, IOP Sciences and the Norwegian University of Science and Technology Library in the period August 2021 to January 2023. Search strings were “degradation MP”, “degradation NP”, “milling MP”, “milling NP”, “MP reference material”, “NP reference material”. Further literature was also selected cascading from the primary results. Conference proceedings and non-accessible articles were omitted from the review. Papers on NP synthesis methods were omitted from the review. A total of 22 papers were selected for inclusion in the review (Table 1). For the purposes of this review, test materials are defined as those that can be made for specific testing applications but are not widely available, while reference materials are defined as those that are widely available for all to use in studies.
Table 1. Overview of top-down and re-precipitation sMP and NP test material production methods available in literature

* Yield is quoted as “Not reported” if produced amounts cannot be directly compared to the amount of starting material on a mass- or volume-basis. Polymer types studied were polystyrene (PS), polyethylene terephthalate (PET), polyethylene (PE), polypropylene (PP), polylactic acid (PLA), polyhydroxybutyrate (PHB), polybutylene adipate terephthalate (PBAT), polyether ether ketone (PEEK) and polycarbonate (PC)
Methods for production of MP and NP test and reference materials
From a physicochemical perspective, reference materials should be as similar as possible to the sMP and NP particles found in environmental matrices (Crawford and Quinn, Reference Crawford, Quinn, Crawford and Quinn2017). The production method must attempt to imitate the natural weathering processes that plastics undergo without physicochemically modifying the particle/material in a non-relevant way. It is the produced material from these processes that is referred to as secondary MPs. As most degradation mechanisms progress, the chemical properties of the particles change through the addition of various functional groups (e.g., hydroxyl, carbonyl and carboxyl) (Cai et al., Reference Cai, Wang, Peng, Wu and Tan2018). This is hugely important as such chemical changes can strongly impact particle environmental behaviour and fate (especially for smaller particles), as well as possibly increasing their chemical reactivity. Therefore, an essential factor in producing representative sMP and NP test and reference material is therefore to emulate the addition of functional groups and environmentally relevant chemical changes. Given the potentially significant role that plastic-associated chemicals play in impacting MP and NP toxicity, it is also the opinion of the authors that test and reference material production should also preserve chemical composition.
Methods for producing test MP and NP particles can be divided into whether they are produced through bottom-up synthesis procedures (Mitrano et al., Reference Mitrano, Beltzung, Frehland, Schmiedgruber, Cingolani and Schmidt2019; Sander et al., Reference Sander, H-PE and McNeill2019; Al-Sid-Cheikh et al., Reference Al-Sid-Cheikh, Rowland, Kaegi, Henry, M-A and Thompson2020) or via top-down degradation of larger particles, granules or even environmentally collected items (Kefer et al., Reference Kefer, Friedenauer and Langowski2022). While the former may allow relatively fast, controlled and reproducible production of larger quantities of particles, the latter is a desirable approach to mimic environmentally relevant particles (Pradel et al., Reference Pradel, Catrouillet and Gigault2023). Bottom-up approaches can be readily used to produce NP, typically resulting in the production of mainly spherical, homogenous and monodisperse particles, that is, not representative of environmental NPs. Furthermore, they typically do not allow the plastic-associated chemical composition of environmental sMP and NP to be replicated. A possible ‘intermediary case’ is the solvent-dissolution or melting of polymer particles in a solvent followed by extrusion or spray drying of particles in the desired size range (Bhagia et al., Reference Bhagia, Gallego, Hiremath, Harper, Lowden, Lowden, Pu, Vaidya, Ozcan and Ragauskas2021). Using this approach, it is possible to partially retain the chemical composition of the plastics, but the physical properties (shape, density, porosity and morphology) are not representative of the particles formed from the degradation of larger particles or plastic items during use or in the environment. In this review, 17 papers representing top-down production methods have been reviewed and 5 papers describing approaches for the re-dissolution of polymers are reviewed. Bottom-up synthesis methods for sMP and NPs were not included, although several are reported, as these are not considered appropriate for producing environmentally relevant particles and are thus out of the scope of the review.
Milling and grinding
It is almost impossible in the laboratory to mimic or reproduce all of the degradation processes simultaneously acting up an item of plastic debris in the natural environment. However, some individual degradation mechanisms can be reproduced in the laboratory quite accurately and effectively, with the potential for more than one to occur at the same time. A good starting point is to grind or mill pieces of plastic as finely as possible, and this process has generally been shown to produce polydisperse particles with a range of sizes, highly irregular shapes and surface morphologies representative of plastic particles found in the environment (Takacs, Reference Takacs2002; Seghers et al., Reference Seghers, Stefaniak, La Spina, Cella, Mehn, Gilliland, Held, Jacobsson and Emteborg2022). The two primary forms of milling available are rotary milling and ball milling, with the former acting more as a ‘cutting’ technique and the latter acting as a ‘smashing’ technique. Crucially, milling approaches allow the plastic-associated chemical composition of the start material to be preserved, as long as any subsequent fractionation or particle size range isolation step avoids the use of water or other solvents. For the production of MP >30 μm in size, these methods have proven to be relatively cheap and fast (Seghers et al., Reference Seghers, Stefaniak, La Spina, Cella, Mehn, Gilliland, Held, Jacobsson and Emteborg2022), although the production of large quantities of material can involve some investment of time. It is also important to note that different polymer types respond differently to the process, which impacts the final yields and particle size/morphology.
A critical issue with common grinding and milling techniques, however, is that they are not able to grind the plastics finely enough to produce reasonable yields of sMP and NP (Eitzen et al., Reference Eitzen, Paul, Braun, Altmann, Jekel and Ruhl2019; Ciobotaru et al., Reference Ciobotaru, Marcu, Maria, Ivanov, Savin, Moncea, Tociu and Deák2020). While particles in this size range are often reported as being produced, they typically represent yields of <<1%, if they are calculated and reported at all. It was found that approximately half of the reviewed papers applied some form of grinding or milling process to produce sMP and NPs, while most others utilised previously ground or milled particles as a starting material (Table 1). For thermoplastic materials, which may soften in ambient temperatures, a common approach has been to cool the material (cryomilling), making it more brittle and facilitating the fragmentation process into smaller particles sizes. Cryomilling is conducted at low temperatures (e.g., using liquid nitrogen), which increases the yield of fine structured particles and allows for rapid grain refinement (Suryanarayana, Reference Suryanarayana2001). Cryogenic conditions can be applied to both ball and rotary milling, either by pre-cooling the sample to be milled or by pre-cooling the sample holder and maintaining a low temperature throughout the milling process (Eitzen et al., Reference Eitzen, Paul, Braun, Altmann, Jekel and Ruhl2019). While cryomilling has been shown to be effective for producing larger MP reference materials (>30 μm) in reasonable yields (Kühn et al., Reference Kühn, van Oyen, Booth, Meijboom and van Franeker2018), the technique is still unable to produce larger quantities of sMP and NP particles (Eitzen et al., Reference Eitzen, Paul, Braun, Altmann, Jekel and Ruhl2019).
It has recently been demonstrated that conducting cryomilling and ball milling at ambient temperature in series can help to increase the production of smaller particles, especially NPs (Caldwell et al., Reference Caldwell, Lehner, Balog, Rhême, Gao, Septiadi, Weder, Petri-Fink and Rothen-Rutishauser2021). Although yields were not explicitly reported, the authors describe stock solutions of the final NP materials at concentrations ranging from 4.8 to 40.8 μg mL−1, which was sufficient for conducting toxicity tests in the study. A combination of dry and wet milling in water, solvent or surfactant has also been shown to improve the yield of lower size ranges (Bhagia et al., Reference Bhagia, Gallego, Hiremath, Harper, Lowden, Lowden, Pu, Vaidya, Ozcan and Ragauskas2021), and has been applied in most of the studies reviewed herein (Table 1). The approach has achieved the production of non-homogenous (in size and shape) particles for a range of polymers, including PS, PP, PE, PET, polybutylene adipate terephthalate and polyether ether ketone. Wet high-speed friction grinding has recently been investigated as an option for the production of MP and sMP, and a significant improvement in the yield of PE, PP and PLA particles in the lower size range was obtained by modifying the aqueous grinding media with 0.5% guar gum (Bhagia et al., Reference Bhagia, Gallego, Hiremath, Harper, Lowden, Lowden, Pu, Vaidya, Ozcan and Ragauskas2021). Minimal impact on the physicochemical properties was observed by this process.
Ultrasonication
Exposing plastic materials and particles to ultrasonication, where sound waves create mechanical forces that can help to break down plastic particles, has the potential to produce MP and NP test/reference materials. To date, only a few studies have been conducted using this approach, but they have demonstrated promising results, including the production of particles in the nanoscale (von der Esch et al., Reference von der Esch, Lanzinger, Kohles, Schwaferts, Weisser, Hofmann, Glas, Elsner and Ivleva2020). Ultrasonication is typically conducted by suspending the material to be fragmented in a solvent, where different chemical characteristics can further influence the fragmentation process. For example, using alkaline conditions has been proven to be effective (von der Esch et al., Reference von der Esch, Lanzinger, Kohles, Schwaferts, Weisser, Hofmann, Glas, Elsner and Ivleva2020). A significant advantage of ultrasonic-based methods is the wide availability of ultrasonication equipment in laboratories globally, increasing the possibility for many researchers to produce their own test materials. These methods are also cost and time efficient. However, there are still some limitations with the technique, including sonication being a time-demanding method and that it requires further development and harmonisation in order to achieve reproducible results when using different sonicators (von der Esch et al., Reference von der Esch, Lanzinger, Kohles, Schwaferts, Weisser, Hofmann, Glas, Elsner and Ivleva2020).
Oxidative degradation
An environmentally relevant method for degrading polymers and plastic materials is to expose them to UV radiation. Many studies have investigated the environmental UV degradation of plastics in the laboratory under simulated sunlight conditions, typically reporting significant fragmentation and chemical (polymer and additive) composition changes to the start material (Gigault et al., Reference Gigault, Pedrono, Maxit and Halle2016; Lambert and Wagner, Reference Lambert and Wagner2016; Sait et al., Reference Sait, Sørensen, Kubowicz, Vike-Jonas, Gonzalez, Asimakopoulos and Booth2021; Sørensen et al., Reference Sørensen, Groven, Hovsbakken, Del Puerto, Krause, Sarno and Booth2021). The irradiation causes photocatalysed oxidative degradation, resulting in broken polymer chains and a reduction in the molecular weight of the particles (Yousif and Haddad, Reference Yousif and Haddad2013). In such studies, the UV wavelengths are usually limited to those present in natural sunlight (e.g., UVA and UVB; >280 nm), but the time scales required to achieve significant degradation typically range from months to years (Sait et al., Reference Sait, Sørensen, Kubowicz, Vike-Jonas, Gonzalez, Asimakopoulos and Booth2021). The process can be sped up by conducting the UV exposure continuously and using high UV intensities but kept within natural ranges. However, shorter wavelengths are more effective at degrading plastic materials, and exposing them to light containing wavelengths that include the UVC region (200–280 nm) can significantly speed up the degradation process (Lee et al., Reference Lee, Busquets, Choi, Lee, Kim and Campos2020; Doğan, Reference Doğan2021), as can using intensities above natural levels. It should be noted that UVC exposure may lead to the formation of different degradation products and oxygenated radicals compared to UVB exposure (Doğan, Reference Doğan2021).
One way of achieving this enhanced degradation rate in the laboratory is to expose the starting polymer material in a UVC-Ozone (UV-O) chamber (Sarkar et al., Reference Sarkar, Rubin and Zucker2021), such as those commonly used for sterilisation purposes. The UV-O exposure facilitates the UV irradiation of the particles while simultaneously oxidising them via ozone, leading to radical reactions that break down the particles into smaller fragments (Sarkar et al., Reference Sarkar, Rubin and Zucker2021). The application of UV-induced fragmentation for the production of test materials has been investigated for polyhydroxybutyrate and PS, in two different studies, producing sMP and NP, respectively (González-Pleiter et al., Reference González-Pleiter, Tamayo-Belda, Pulido-Reyes, Amariei, Leganés, Rosal and Fernández-Piñas2019; Sarkar et al., Reference Sarkar, Rubin and Zucker2021). While the approach has been demonstrated to produce sMP and NP particles, yields and production volumes were not reported in either study. Advanced oxidation techniques such as UV-O typically require costly specialist equipment, and while degradation rates may be fast for UV-labile polymers, the method is not applicable to UV-stabile polymers. The applicability of such methods for the harmonised production of test and reference materials is therefore likely low.
Laser ablation
Magrì et al. (Reference Magrì, Sánchez-Moreno, Caputo, Gatto, Veronesi, Bardi, Catelani, Guarnieri, Athanassiou, Pompa and Fragouli2018) applied laser ablation (irradiation wavelength 248 nm) to create top-down NP fragments of PET at concentrations of up to 300 μg mL−1. Small NP size ranges were isolated by filtration (0.2 μm), and the mean diameter was found to be ~30 nm, one of the smallest sizes among the studies addressed herein. The benefits of this protocol would include not needing to add any particular solvent or stabiliser, whereas the downsides include the low yield and application of (costly) specialist instrumentation and time consumed. Upon characterisation, the produced particles were found to be nearly spherical or spherical, and oxidised at the surface compared to the original material. It is hypothesised by the authors that the oxidised surface is representative of (environmentally) degraded PET, but this is not proven. This technique potentially allows the production of NPs without impurities, chemical precursors and the byproducts associated with bottom-up processes.
Combined degradation protocols
As highlighted above, combining multiple degradation mechanisms has the potential to be more effective at degrading plastic materials and generating sMP and NP test materials in usable quantities (e.g., environmental fate and effects assessment). In an attempt to produce sMP particles in the range 1–10 μm, Sarkar et al. (Reference Sarkar, Rubin and Zucker2021) first used cryomilling to produce PS particles of <100 μm, followed by a sequential combination of thermal treatment (70°C) followed by UV(C)-ozone exposure (both dry) and probe sonication in ethanol–water suspension. The application of the post-cryomilling steps increased the number of particles in the 1–10 μm size range, although the yields were not determined. While not measured and reported directly by the authors, the size distributions presented indicated that an increase in the NP size fraction was likely also obtained using this method. The produced sMP particles were found to be comparable in size, shape, surface morphology and functionality to particles subjected to environmental weathering. However, the measured surface charge (zeta potential, ZP) was significantly different between the sMPs produced by this accelerated protocol and those produced from environmental weathering. Compared to the spherical model PS and environmentally weathered particles, the PS sMPs from the accelerated protocol represented an intermediary ZP. A major downside to the protocol may be the difficulty in scaling up the process to facilitate the bulk production of sMP (and potentially NP). Furthermore, the approach was optimised particularly for PS, known to be ‘easily’ UV-degradable due to its carbon–carbon backbone (Yousif and Haddad, Reference Yousif and Haddad2013; Gewert et al., Reference Gewert, Plassmann and MacLeod2015), and so its applicability for other polymer types is unknown.
Most recently, Schmitt et al. (Reference Schmitt, Altmann, Fengler and Gehde2023) used a combination of UV exposure and mechanical fragmentation using a custom made air-based fragmentation device to produce high yields of PE MP (~80%) using a thin film/foil as a starting material. The approach was able to produce PE flakes with irregular shapes, where a decrease in size occurred with longer UV exposure times (up to 2.5 months). Although a minimum average particle size of 110 μm was reported in the study, the relationship between particle size and UV exposure times, combined with the high yields, suggests the approach may have potential for further development and assessment for its applicability in producing sMP and NP materials. A common downside to both protocols presented above is the need for specialist instrumentation that is not readily available in most laboratories (e.g., UV-O chamber and custom-built fragmentors), making them difficult to reproduce in other laboratories without significant cost and commitment. On the positive side, both methods appear to be able to produce polymer fragments of environmental relevance, due to their partially degraded nature.
Solubilisation and precipitation methods
Several studies have reported the production of nanosized PET through the dissolution of larger PET particles in a strong solvent, followed by precipitation in (mainly) aqueous media (Rodríguez-Hernández et al., Reference Rodríguez-Hernández, Muñoz-Tabares, Aguilar-Guzmán and Vazquez-Duhalt2019; Johnson et al., Reference Johnson, Mecham, Krovi, Caffaro, Aravamudhan, Kovach, Fennell and Mortensen2021; Elhady et al., Reference Elhady, Elbarbary, Gad and Fathy2022). Another study applied a similar approach for PE, replacing direct precipitation by emulsification with a biosurfactant in seawater (Balakrishnan et al., Reference Balakrishnan, Déniel, Nicolai, Chassenieux and Lagarde2019). Although the methodologies are reasonably simple and transferable, they are highly similar to conventional bottom-up synthesis approaches that produce mainly spherical particles with a narrow size range, and which have limited environmental relevance. Additional downsides include the use of toxic solvents and the need to remove them before any particles can be utilised in toxicity studies. It also remains unclear and undocumented whether such approaches to test/reference material production sufficiently retain the additive chemical composition of the starting material. This is increasingly considered critical for achieving a more accurate and environmentally relevant assessment of sMP and NP.
Most recently, Peller et al. (Reference Peller, Mezyk, Shidler, Castleman, Kaiser, Faulkner, Pilgrim, Wilson, Martens and Horne2022) applied partial solubilisation with small volumes of long-chained solvents to successfully transform larger particles of several polymers (PE, PET and PS) into sMP and NP directly in aqueous media. The relatively simple approach involved the addition of plastic granules, particles or pieces to water, followed by the addition of a small volume (10–30 μL) of long-chain alkanes. Vigorous shaking created a cloudy suspension with average particle sizes of 1.3–4.4 μm for the different materials. Subsequent ultrasound treatment resulted in an average particle size of 338–724 nm. Differences were observed in the particle morphology, with some materials producing smoother and more spherical particles, and other materials producing more irregularly shaped and rougher-surfaced particles. Again, there was no attempt to assess whether the (additive) chemical composition of the resulting particles accurately reflected that of the start material. Given the challenges in producing bulk quantities of sMP and NP particles using mechanical (e.g., cryomilling) and UV-based degradation techniques, dissolution and partial dissolution approaches appear an attractive alternative. The protocols can be easily scaled and facilitate reasonably fast production of particles without the need for specialist instrumentation. However, more work is required to characterise the physicochemical properties of the resulting particles and compare them with those found in the natural environment. Furthermore, there is a need for assessing such techniques for their applicability to a much wider range of polymer types, again reflecting those found in the natural environment.
Discussion and considerations
Yields of sMP and NP
One of the key requirements of test and reference materials is being sufficiently available at a low or reasonable cost. Unfortunately, only a small number of papers report yields or produced mass in a way that allows calculation of yield relative to the starting amount of plastic. Where reported, yields are (on a mass basis) very low (<<1%). Dissolution–precipitation methods appear to be much quicker and easier to perform and may have the potential to be scaled up to provide higher sMP and NP yields. In contrast, entirely top-down methods may appear more desirable in terms of their potential for producing sMP and NP with a higher degree of environmental relevance, but are typically costly, time consuming and only able to produce small amounts of material. For a variety of reasons, very few studies reporting methods for the production of sMP and NP directly assess (or attempt to assess) the environmental relevance of the particles from a physical and chemical perspective (especially the latter). In line with previous assessments, we also see that most reported methods (10 papers) have focussed on production of PS (Figure 1), although this is not the most abundant polymer in the environment (Pradel et al., Reference Pradel, Catrouillet and Gigault2023). A similar number of studies have produced PET particles, whereas only a handful have produced PP and/or PE particles. This probably reflects not only the applicability of the methods to the various polymers, but also the interest in their production. We believe that it is important for methods to be able to produce comparable particles of several/most polymer types found in environmental plastic.

Figure 1. Overview of the number of peer-reviewed manuscripts reporting methods for the production of sMP and NP comprised of various polymer types (left) and the resulting particle sizes (right). Polymer types studied were polystyrene (PS), polyethylene terephthalate (PET), polyethylene (PE), polypropylene (PP), polylactic acid (PLA), polyhydroxybutyrate (PHB), polybutylene adipate terephthalate (PBAT), polyether ether ketone (PEEK) and polycarbonate (PC).
Size and shape of sMPs and NPs
Top-down methods generally produce irregularly shaped particles, whereas particles formed through dissolution and re-precipitation tend to be more spherical in morphology (Johnson et al., Reference Johnson, Mecham, Krovi, Caffaro, Aravamudhan, Kovach, Fennell and Mortensen2021). The studies reviewed here show that the weathering process that plastics undergo in the natural environmental can be reproduced relatively well in the laboratory and that the secondary MP and NP formed share many similarities with particles isolated from environmental matrices. The applied techniques and combinations of techniques typically resulted in a mixture of highly irregular particle morphologies, which is a critical property of naturally weathered plastics. The majority of the top-down studies, (mechanical-, UV- and dissolution-based approaches) were able to produce a continuum of particle sizes, including a proportion of particles in the ranges required for a hypothetical sMP and NP test/reference material (i.e., just over 1 μm and <1 μm, respectively; Figure 1). Particles <100 nm are only reported in a few studies, but this can be caused both by the lack of formation and the lack of appropriate characterisation methods for such small sizes. As such, there is potential for further processing of the products to isolate and characterise specific particle size ranges for use as test materials. Across the reported studies, such top-down approaches have been applied to a variety of polymer types, with varying degrees of success being reported. However, it is important to highlight that such approaches are typically limited by very low yields, low reproducibility, the need for costly instrumentation and a significant time investment.
All studies employed some form of particle size and/or size distribution characterisation, for which a range of techniques was applied. In the reviewed studies, nine used DLS and five used SEM, with other reported techniques including nano tracking analysis (NTA), asymmetric flow field flow fractionation (AF4) fitted with either (i) a multi-angle light scattering detector, (ii) static light scattering (SLS) or (iii) inductively coupled plasma-mass spectrometry, laser diffraction or granulometry, transmission electron microscopy and Raman microscopy. Several studies employed a combination of particle size characterisation methods. It should be noted that the different techniques are based on different principles for assuming size and so the final determined values are not always directly comparable. For example, DLS generally provides larger sizes than optical techniques such as SEM, likely due to a combination of (i) presence of surfactants on the particle surface, (ii) calculation of hydrodynamic radii assuming spheres when actual sMP and NP are not spherical and (iii) particle aggregation (Ji et al., Reference Ji, Wang, Wang, Fu, Man and Chen2020; Caldwell et al., Reference Caldwell, Lehner, Balog, Rhême, Gao, Septiadi, Weder, Petri-Fink and Rothen-Rutishauser2021; Johnson et al., Reference Johnson, Mecham, Krovi, Caffaro, Aravamudhan, Kovach, Fennell and Mortensen2021). Conversely, NTA provides lower, possibly more accurate, average particle sizes than DLS (Ekvall et al., Reference Ekvall, Lundqvist, Kelpsiene, Šileikis, Gunnarsson and Cedervall2019).
Influence of fragmentation methods on particle and polymer physicochemical properties
Both naturally occurring environmental degradation mechanisms and those degradation processes simulated in the laboratory have the potential to impact or modify multiple physicochemical properties of plastic materials, including crystallinity, glass transition and phase transition temperatures (Ainali et al., Reference Ainali, Bikiaris and Lambropoulou2021). From the perspective of environmental relevance of test and reference materials, it is important to have a robust characterisation relative to the start materials in order to ensure they reflect the properties of sMP and NP found in the environment. It is only recently, however, that a few studies have started to document the effects of degradation methods on particle properties beyond their size and shape. For example, Lionetto et al. (Reference Lionetto, Esposito Corcione, Rizzo and Maffezzoli2021) investigated if there were changes in the polymer crystallinity when producing PET NPs through a combination of ultra-centrifugal milling and ball milling. The milling process was found to lead to a progressive reduction in the degree of crystallinity and an increase in the proportion of amorphous material, resulting in an overall change in material properties. Considering that the amorphous regions of a polymeric material are often more susceptible to different degradation mechanisms (e.g., UV degradation and microbial degradation) than the crystalline regions, it is likely that some differences are observed between the parent material and the resulting sMP and NP test materials. For the production of environmentally relevant materials in terms of behaviour, this needs to be considered further and compared to eventual changes in the same properties under environmental or simulated environmental degradation (Ainali et al., Reference Ainali, Bikiaris and Lambropoulou2021; Conradie et al., Reference Conradie, Dorfling, Chimphango, Booth, Sørensen and Akdogan2022). It is also worth highlighting that top-down test/reference material production methods have an increased likelihood of preserving the additive chemical profile of the start material, thus increasing its environmental relevance.
Stability of particles in aqueous suspension
Beyond the initial preparation of the sMP and NP test and reference materials, their dispersion stabilisation and longer-term stability in aqueous media is another key consideration that must be addressed. It is well established that common surfactants can be used to maintain small particles in aqueous dispersion, avoiding aggregation at concentrations that are higher than the environmentally realistic concentrations typically used for test and reference material stock solutions (Ji et al., Reference Ji, Wang, Wang, Fu, Man and Chen2020; Johnson et al., Reference Johnson, Mecham, Krovi, Caffaro, Aravamudhan, Kovach, Fennell and Mortensen2021). However, there are some critical drawbacks to using surfactants when the particles are to be utilised in environmental fate and effects assessment. Any particles previously exposed to surfactants are unlikely to behave in a ‘natural’ way in environmental fate studies, whereas the surfactants may influence the outcomes of toxicity studies. Despite these drawbacks, there has been an unfortunate overuse of stabilised PS spheres for environmental fate and effects studies conducted to date (Pradel et al., Reference Pradel, Catrouillet and Gigault2023) and it is critical that research moves towards more realistic assessments.
It is possible to look towards the natural world for inspiration when it comes to the dispersion stabilisation of sMP and NP test/reference materials. Most small particles present in natural aquatic environments, including sMP and NP, can be naturally ‘stabilised’ by the sorption of natural organic matter or through biofilm formation (Junaid and Wang, Reference Junaid and Wang2021; Pradel et al., Reference Pradel, Ferreres, Veclin, El Hadri, Gautier, Grassl and Gigault2021). While several of the studies reviewed herein have used synthetic surfactants to stabilise sMP and NP suspensions (Rodríguez-Hernández et al., Reference Rodríguez-Hernández, Muñoz-Tabares, Aguilar-Guzmán and Vazquez-Duhalt2019; Caldwell et al., Reference Caldwell, Lehner, Balog, Rhême, Gao, Septiadi, Weder, Petri-Fink and Rothen-Rutishauser2021), success in using biosurfactant has also been reported (Balakrishnan et al., Reference Balakrishnan, Déniel, Nicolai, Chassenieux and Lagarde2019). Interestingly, Ji et al. (Reference Ji, Wang, Wang, Fu, Man and Chen2020) found that bovine serum albumin provided better suspension and less agglomeration than SDS, suggesting that natural surfactants are perfectly suitable for this purpose. It is therefore possible to consider producing test and reference sMP and NP materials that are stabilised using different natural surfactants depending on the intended use of the particles in experimental studies. We therefore propose that future work on the development of sMP and NP reference materials should include a focus on using natural surfactants to ensure dispersion and dispersion stability.
From test materials to reference materials – What are the next steps?
To date, the vast majority of environmental fate and effects studies with MP and NP have used either spherical, monodispersed, single-polymer particles from commercial suppliers or single-batch test materials produced using bespoke methods developed by the researchers themselves. Only the first approach realises the possibility for interlaboratory comparison and validation of the methods. Despite some success in recent years to produce more environmentally relevant certified MP reference materials (e.g., the Hawaii Pacific University Center for Marine Debris Research Polymer Kit 1.0 (The Center for Marine Debris Research, 2020), there remains a lack of certified reference materials representing sMP and NP particles (Balakrishnan et al., Reference Balakrishnan, Déniel, Nicolai, Chassenieux and Lagarde2019; Eitzen et al., Reference Eitzen, Paul, Braun, Altmann, Jekel and Ruhl2019; Seghers et al., Reference Seghers, Stefaniak, La Spina, Cella, Mehn, Gilliland, Held, Jacobsson and Emteborg2022). The current review has evaluated recent efforts to produce relevant sMP and NP test materials, with some promising techniques already documented and available for further development and optimisation. However, there remains a large gap to cover in terms of reaching availability of reference materials for these size classes. Some studies have taken initial steps towards documenting the reproducibility of their protocols, as well as the homogeneity of produced samples (von der Esch et al., Reference von der Esch, Lanzinger, Kohles, Schwaferts, Weisser, Hofmann, Glas, Elsner and Ivleva2020), but most protocols presented have been developed and applied only on a case study basis.
Importantly, we recommend that there is a focus on considering the additive chemical profiles of test and reference sMP and NP materials, where these chemicals should be incorporated and robustly characterised. Given the potential impact such chemicals could play in the toxicity of MP and NP emissions, this is a critical aspect that needs to be incorporated into the design and production of test/reference particles. In addition to the highest production volume thermoplastics (e.g., PE, PP, PS, PET, PA), synthetic rubbers (elastomers) are expected to be significant contributors to sMP and NP pollution worldwide. For example, tyre wear particles (TWPs) have been shown to be a major constituent of road dust (PM10 and PM2.5) and represent a priority form of sMP emissions globally (European Commission, 2023). Elastomers are emerging as one of the most toxic groups of polymeric materials, suggesting that there is a need for increased focus on understanding the impacts of their release (Sørensen et al., Reference Sørensen, Gomes, Igartua, Lyngstad, Almeida, Wagner and Booth2023). However, none of the examined papers have investigated the production of elastomer test and reference materials, which are inherently more challenging to produce due to their elasticity reducing the potential for fragmentation. Methods for the production of TWP test materials in the MP size range have been proposed, including the abrasion of rubber vulcanizate in combination with mineral particles and surface solvent treatment for enhancing “stickiness” (Son and S-S, Reference Son and S-S2022), but no reports of methods for sMP and NP size ranges are found. We are aware of several ongoing EU and JPI Oceans funded projects (e.g., POLYRISK, PlasticsFate, ANDROMEDA, EUROqCHARM and PlasticTrace) that are currently dealing with the harmonisation and standardisation of sMP and NP test and reference material production. Furthermore, this is an ongoing focus for well-established producers and suppliers of certified reference materials, such as NIST (US), the EU Joint Research Centre (JRC) and The Bundesanstalt für Materialforschung und -prüfung (BAM, Germany). The development of standardised methods for the reproducible production of MP test and reference materials for use by the scientific community could be of relevance to well-established standards organisations such as the International Organisation for Standardisation, the Organisation for Economic Co-operation and Development and the European Committee for Standardisation.
Conclusions and future needs
This review has summarised and evaluated some of the most promising methods currently available for producing more environmentally relevant sMP and NP test and reference materials, particularly for use in environmental fate and effects assessment studies. Most methods have focussed on conventional thermoplastics, whereas a few papers have addressed biodegradable plastics, reflecting their increasing importance and focus as part of the solution to addressing global plastic pollution. However, elastomers do not currently appear to be a focus, which represents an important gap. A combination of wet and dry grinding/milling appears to be the most promising technique to produce of reasonable quantities of sMP, although yields need to be determined and reported more frequently and the approach can be costly and time consuming. All reported methods appear to need further optimisation to maximise yields and reproducibility, while robust and reproducible fractionation techniques will be needed for isolating specific sMP and NP size fractions. Based on the current state-of-the-art, it remains a question as to whether environmentally realistic NPs can be obtained in desired quantities, but combination protocols incorporating milling, chemical degradation (e.g., oxidation) and sonication followed by filtration may offer the best chance. Furthermore, test/reference sMP and NP stabilisation issues during storage and in aqueous matrices is another challenge that needs to be addressed in the future. It is also important to consider that it may never be possible to produce test/reference sMP and NP materials that truly reflect the complex mixture of partially degraded plastic particles that is present in the environment. However, there needs to be a move towards finding a middle ground where the environmental relevance of test and reference materials used in fate and effects assessment is improved to the point that more reliable risk assessment for MP and NP pollution can be conducted. Given the global economic and societal importance of plastics, combined with the problems of mismanaged waste and plastic pollution, it is important that future policy and regulations are based upon robust science and data. To this end, ongoing global plastic treaty negotiations should play an important role in catalysing the development and use of standardised reference materials in MP and NP risk assessment.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/plc.2024.13.
Data availability statement
All data and information used in the development of this work were taken from published literature cited within the manuscript.
Author contribution
Acquisition and analysis of data: L.S. and M.G.; Conception and design of the work: L.S. and A.M.B.; Drafting and revising: L.S. and A.M.B.; Funding acquisition: L.S. and A.M.B.; Interpretation of data: L.S. and A.M.B.; Methodology: L.S., M.G. and A.M.B.
Financial support
This work has been part of the REVEAL project funded by the Research Council of Norway (Grant Agreement No. 301157), the JPI Oceans-Research Council of Norway funded project Andromeda (Grant Agreement No. 312262), the Plastic Trace project, which received funding from the European Partnership on Metrology, co-financed from the European Union’s Horizon Europe Research and Innovation Programme and by the Participating States (Grant Agreement No. 21GRD07), and the Horizon Europe project SOS-ZEROPOL2030 (Grant Agreement No. 101060213) funded by the European Union. The views and opinions expressed are, however, those of the authors only and do not necessarily reflect those of the European Union. The European Union cannot be held responsible for them.
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.