Diabetes is associated with oxidative stress and inflammation due to hyperglycaemia and hyperlipidaemia. Hyperglycaemia and dyslipidaemia induce the generation of free radicals, inflammatory responses and oxidative stress reactions, leading to the complications and mortality associated with obesity and type 2 diabetes. Hyperglycaemia causes oxidative stress due to increased mitochondrial production of the superoxide anion, non-enzymic glycation of proteins, and glucose auto-oxidation. In the absence of an appropriate compensatory response from the endogenous antioxidant network against gluco- and lipotoxicity caused by hyperglycaemia and hyperlipidaemia under diabetes, oxidative stress becomes marked, leading to activation of the stress-sensitive intracellular signalling pathway(Reference Poitout and Robertson1, Reference Prentki, Joly and El-Assaad2). In addition, hyperglycaemia accelerates the formation of advanced glycation endproducts (AGE) which are proteins produced from non-enzymic glycation reactions(Reference Ahmed3). AGE induce free radical formation, accumulate during the normal ageing process and at accelerated rates during the course of diabetes, and are associated with the pathogenesis of chronic diseases such as arthritis, atherosclerosis, liver cirrhosis and diabetic nephropathy(Reference Goh and Cooper4). In particular, there is a strong correlation between AGE accumulation and the presence and severity of diabetic kidney disease(Reference Monnier, Bautista and Kenny5). Moreover, type 2 diabetic individuals almost invariably show a marked disruption of lipid dynamics, often reflected by elevated levels of circulating NEFA and TAG, together with excess fat deposition in various tissues. Accordingly, to prevent diabetic renal damage associated with structural and functional changes, oxidative stress and/or inflammation, it is important to reduce the development of AGE even in the absence of normalising glucose or lipid levels(Reference Coughlan, Cooper and Thomas6).
Oligonol, which is now available commercially as a new dietary ingredient, is an optimised phenolic product derived from lychee fruit polyphenols containing catechin-type monomers and low-molecular-weight oligomers(Reference Fujii, Nishioka and Wakame7). Based on recent studies, there is accumulating evidence that oligonol can induce some physiological and biochemical alterations in vitro and in vivo, such as the induction of apoptosis in cancer cells(Reference Jo, Lee and Ahn8), antioxidant and anti-inflammatory effects in mice(Reference Kundu, Chang and Fujii9), and beneficial subjective effects on the feeling of fatigue in young athletes(Reference Ohno, Sakurai and Hisajima10). Moreover, the oral administration of oligonol improves the regulation of genes for adipokines in white adipose tissue of mice on administering a high-fat diet(Reference Sakurai, Nishioka and Fujii11). Indeed, the dietary feeding of proanthocyanidins, which comprise oligonol, has been reported to induce a significant attenuation of tissue fat levels, without changing the total body mass of animals compared with non-proanthocyanidin-fed animals(Reference Mittal, Elmets and Katiyar12). However, there is no evidence to support whether or not oligonol has any effect on the regulation of renal lipid metabolism, AGE-induced oxidative stress, and inflammation in the kidney of obesity-induced type 2 diabetes. Therefore, we investigated the effects of oligonol on renal damage induced by hyperglycaemia, and abnormal lipid synthesis and AGE formation were examined in the kidney of db/db mice.
Materials and methods
Oligonol
Oligonol was generated by oligomerising polyphenol polymers derived from lychee fruit. The safety of oligonol as a food, dietary supplement and pharmaceutical additive has already been confirmed(Reference Fujii, Nishioka and Wakame7). Oligonol comprises a polyphenol mixture of 16·0 % monomers (catechin, epicatechin, epicatechin gallate and epigallocatechin gallate) and 13·9 % dimers (procyanidin A1, A2, B1 and B2), while lychee fruit polyphenol comprises a mixture of 6·4 % monomers and 9·8 % dimers. Oligonol is commercially available (Amino Up Chemical Co., Ltd, Sapporo, Japan).
Materials
Protease inhibitor mixture solution, 4,6-dihydroxy-2-mercaptopyrimidine (2-thiobarbituric acid), EDTA, reduced glutathione (GSH) and oxidised glutathione (GSSG) were purchased from Wako Pure Chemical Industries, Ltd (Osaka, Japan). 2′,7′-Dichlorofluorescein diacetate was purchased from Molecular Probes (Eugene, OR, USA). The Bio-Rad protein assay kit and pure nitrocellulose membrane were purchased from Bio-Rad Laboratories (Tokyo, Japan). β-Actin, o-phthalaldehyde, phenylmethylsulfonyl fluoride and N-ethylmaleimide were purchased from Sigma Chemical Co. (St Louis, MO, USA). Rabbit polyclonal antibodies against NF-κBp65, sterol regulatory element-binding protein (SREBP)-1, SREBP-2, PPARα and receptor for AGE (RAGE), and mouse monoclonal antibody against cyclo-oxygenase-2 (COX-2) and inducible NO synthase (iNOS) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Monoclonal anti-N ɛ-(carboxyethyl)lysine (CEL) antibody and polyclonal anti-N ɛ-(carboxymethyl)lysine (CML) antibody were kindly provided by Dr R. Nagai (Kumamoto University, Japan). Goat anti-rabbit and goat anti-mouse IgG horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). ECL Western Blotting Detection Reagents were purchased from Amersham Bioscience (Piscataway, NJ, USA).
Experimental animals and treatment
The ‘Guidelines for Animal Experimentation’ approved by the University of Toyama were followed in the present study (registration no. S-2006 INM-22). Male C57BLKS/J db/db and m/m (misty, non-diabetic) mice, aged 5 weeks, were purchased from Japan SLC Inc. (Hamamatsu, Japan), and housed with free access to laboratory pellet chow (CLEA Japan Inc., Tokyo, Japan), comprising 60·5 % carbohydrates, 24·0 % proteins and 3·5 % lipids, and water. They were maintained in a controlled environment (22 ± 2°C, 50 ± 5 % humidity, 12 h light–12 h dark cycle). After adaptation, glucose levels of blood taken from the tail vein and the body weight were measured, and then db/db mice were divided into three groups. The db/db vehicle group (n 10) was orally administered water, while the other two groups (n 10 per group) were orally administered oligonol every day at a dose of 10 or 20 mg/kg body weight, respectively. The non-diabetic m/m mice (n 6) as a normal group were compared with the diabetic groups. Food and water intakes were determined every day during the experimental period. After administration for 8 weeks, blood samples were collected by the cardiac puncture of anaesthetised mice. Serum was separated immediately after centrifugation. Subsequently, each mouse was perfused with ice-cold physiological saline, and then the kidneys were harvested, snap-frozen in liquid N2 and stored at − 80°C until analysis.
Assay of serum samples
Serum glucose, TAG, total cholesterol and NEFA were measured using commercial kits (Glucose CII-Test, Triglyceride E-Test, Cholesterol E-Test and NEFA C-Test, respectively, from WAKO Pure Chemical Industries, Ltd, Osaka, Japan). For the measurement of serum urea N and creatinine, BUN Kainos and CRE-EN Kainos were obtained from Kainos Laboratory Inc. (Tokyo, Japan). Serum insulin and leptin (Morinaga Institute of Biological Science, Yokohama, Japan), adiponectin (CycLex Co., Ltd, Nagano, Japan) and hexanoyl-lysine (Institute for the Control of Aging, Shizuoka, Japan) levels were measured based on ELISA. The serum reactive oxygen species (ROS) level was determined using the method of Ali et al. (Reference Ali, LeBel and Bondy13), and the 2-thiobarbituric acid-reactive substance (TBARS) level was examined by employing the method of Naito & Yamanaka(Reference Naito and Yamanaka14).
Determination of glucose, TAG and total cholesterol contents in the kidney
To measure the glucose concentration in the kidney, tissue was homogenised with 0·9 % NaCl, 0·15 m-Ba(OH)2 and 5 % ZnSO4 were added, and then it was centrifuged at 1400 g for 15 min at 4°C(Reference Momose, Yano and Ohashi15). The glucose concentration of the supernatant fraction was evaluated using a Wako kit (Glucose CII-Test). Total lipids were extracted with a mixture of chloroform and methanol (2:1, v/v) according to the method of Folch et al. (Reference Folch, Lees and Sloane Stanley16), and TAG and total cholesterol levels were measured using the Wako kits, as described above.
Assay of reactive oxygen species and 2-thiobarbituric acid-reactive substance levels in the kidney
ROS generation was measured by employing the method of Ali et al. (Reference Ali, LeBel and Bondy13). Renal tissue was homogenised on ice with 1 mm-EDTA–50 mm-sodium phosphate buffer (pH 7·4). In brief, 25 mm-2′,7′-dichlorofluorescein diacetate was added to homogenates, and, after 30 min, changes in fluorescence were determined at an excitation wavelength of 486 nm and emission wavelength of 530 nm. TBARS levels were estimated according to the method of Mihara & Uchiyama(Reference Mihara and Uchiyama17).
Determination of reduced and oxidised glutathione levels in the kidney
GSH and GSSG assays were carried out using the method of Hissin & Hilf(Reference Hissin and Hilf18). Renal tissue was homogenised on ice with 1 mm-EDTA–100 mm-sodium phosphate buffer (pH 8·0). Then, 25 % meta-phosphoric acid was added to precipitate protein. The homogenate was centrifuged at 4°C at 100 000 g for 30 min. The supernatant fraction was used for assays of GSH and GSSG. To assay for GSH, the supernatant fraction was diluted with buffer followed by o-phthalaldehyde. For the determination of the GSSG concentration, after preincubation with N-ethylmaleimide for 20 min, 0·1 m-NaOH was used instead of sodium phosphate buffer (pH 8·0). After 15 min at room temperature, fluorescence was estimated at an excitation wavelength of 360 nm and emission wavelength of 460 nm. The protein concentration was measured according to the method of Itzhaki & Gill(Reference Itzhaki and Gill19) using bovine serum albumin as a standard.
Preparation of nuclear and post-nuclear fractions
To prepare nuclear fractions, the kidney was homogenised with ice-cold lysis buffer containing 5 mm-2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)-HCl (pH 7·5), 2 mm-MgCl2, 15 mm-CaCl2 and 1·5 m-sucrose, and then 0·1 m-dithiothreitol (DTT) and protease inhibitor cocktail were added. After samples were centrifuged at 10 500 g for 20 min at 4°C, the pellet was suspended with nuclear extraction buffer (20 mm-2[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (pH 7·9), 1·5 mm-MgCl2, 0·42 m-NaCl, 0·2 mm-EDTA, 25 % (v/v) glycerol, 0·1 m-DTT and protease inhibitor cocktail). After the mixture was placed on ice for 30 min, the nuclear fraction was prepared by centrifugation at 20 500 g for 5 min at 4°C. The post-nuclear fraction was extracted from the kidney of each mouse, as described below. In brief, renal tissue was homogenised with ice-cold lysis buffer (pH 7·4) containing 137 mm-NaCl, 20 mm-2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)-HCl, 1 % Tween 20, 10 % glycerol, 1 mm-phenylmethylsulfonyl fluoride and protease inhibitor mixture. The homogenate was then centrifuged at 2000 g for 10 min at 4°C. The protein concentration of each fraction was determined using the Bio-Rad protein kit with bovine serum albumin as a standard.
Western blot analysis
Post-nuclear protein (40 μg) for RAGE, CEL, CML, COX-2 and iNOS expression and nuclear protein (40 μg) for PPARα, SREBP-1, SREBP-2 and NF-κBp65 were electrophoresed in 8–10 % SDS–polyacrylamide gel. Separated proteins were transferred to a pure nitrocellulose membrane, blocked with 5 % skimmed milk solution for 1 h, and then incubated with primary antibodies overnight at 4°C. After washing of the membrane, it was incubated with goat anti-rabbit or goat anti-mouse IgG horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Each antigen–antibody complex was visualised using ECL Western Blotting Detection Reagents and detected by chemiluminescence with LAS-4000 (Fujifilm, Tokyo, Japan). Band densities were calculated by employing an image analyser ATTO densitograph (ATTO, Tokyo, Japan) and normalised to β-actin. The protein expression levels are presented relative to those of m/m mice (represented as 1).
Statistical analysis
All results are expressed as mean values with their standard errors, and analysed statistically using one-way ANOVA followed by Dunnett's test for individual differences among groups. Values of P < 0·05 were considered significant.
Results
Food and water intake, and body-weight gain
The food and water intakes as well as body-weight gain of the db/db mice after 8 weeks were significantly higher than those of the age-matched m/m mice. There were no changes in food and water intake or body-weight gain among the db/db mouse groups (data not shown).
Haematological analysis
Table 1 shows the serum constituents (glucose, TAG, total cholesterol and NEFA), glucose and lipid metabolism-related hormones (insulin, leptin and adiponectin), renal functional parameters (urea N and creatinine) and biomarkers associated with oxidative stress (ROS, hexanoyl-lysine and TBARS). Except for serum adiponectin, all serum constituents and biomarkers were elevated in db/db vehicle compared with m/m mice. Treatment with oligonol did not alter the serum glucose and leptin concentrations; however, it significantly increased the insulin level. The db/db mice administered oligonol at 20 mg/kg showed significant reductions in the levels of TAG, total cholesterol, NEFA, ROS, hexanoyl-lysine, TBARS, urea N and creatinine. The serum adiponectin level was significantly lower in the db/db vehicle than in the m/m mice, while oligonol administration elevated its reduced concentration. Also, the levels of urea N and creatinine were significantly increased in db/db vehicle compared with m/m mice. However, these increased levels were markedly decreased by treatment with oligonol at 20 mg/kg.
Table 1 Haematological analyses
(Mean values with their standard errors)

m/m, non-diabetic misty mice; Vehicle, db/db vehicle-treated mice; Oligo-10, db/db mice treated with oligonol at 10 mg/kg body weight; Oligo-20, db/db mice treated with oligonol at 20 mg/kg body weight; ROS, reactive oxygen species; TBARS, thiobarbituric acid-reactive substances; MDA, malondialdehyde.
Mean value was significantly different from that of the vehicle group: * P < 0·05, ** P < 0·01, *** P < 0·001.
Biomarkers associated with oxidative stress in the kidney
In db/db vehicle mice, the ROS concentration in the kidney was markedly increased compared with that in m/m mice, but the 10 or 20 mg oligonol treatment reduced this increase to 36 or 75 %, respectively (Table 2). Also, renal TBARS levels were increased by 1·36 times compared with m/m mice (m/m, 1·11; db/db mice, 1·51 nmol/mg protein; P < 0·01). However, the elevated renal TBARS levels were significantly reduced in the oligonol-treated db/db mouse groups, and were lowered nearly to the levels of the m/m mice by treatment with oligonol at 20 mg/kg. Regarding GSH:GSSG ratios, the db/db vehicle group showed a significant reduction compared with the m/m group, which resulted from the marked increase in the GSSG concentration in the kidney. However, oligonol treatment did not significantly alter the renal GSH level and GSH:GSSG ratio (Table 2).
Table 2 Biomarkers associated with oxidative stress in the kidney
(Mean values with their standard errors)
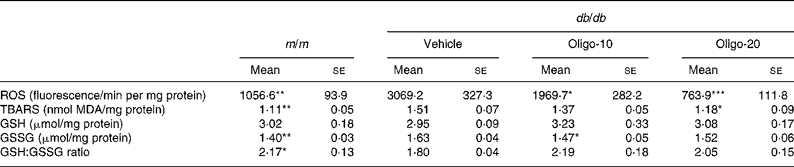
m/m, non-diabetic misty mice; Vehicle, db/db vehicle-treated mice; Oligo-10, db/db mice treated with oligonol at 10 mg/kg body weight; Oligo-20, db/db mice treated with oligonol at 20 mg/kg body weight; ROS, reactive oxygen species; TBARS, thiobarbituric acid-reactive substances; MDA, malondialdehyde; GSH, reduced glutathione; GSSG, oxidised glutathione.
Mean value was significantly different from that of the vehicle group: * P < 0·05, ** P < 0·01, *** P < 0·001.
Kidney weight and renal glucose, TAG and total cholesterol levels
The kidney weights in the three groups of db/db mice were greater than that of the m/m mice (Table 3). The glucose level in the kidney of the db/db vehicle mice showed a more than 2·58-fold increase compared with that of the m/m mice. Also, there was a 1·49-fold increase in the TAG content and a 1·56-fold increase in the cholesterol content of the kidney of the db/db vehicle mice compared with those of the m/m mice. However, these increments in renal TAG and cholesterol levels were significantly reduced by 10 or 20 mg oligonol administration, as shown in Table 3.
Table 3 Kidney weight and renal glucose, TAG and total cholesterol contents
(Mean values with their standard errors)
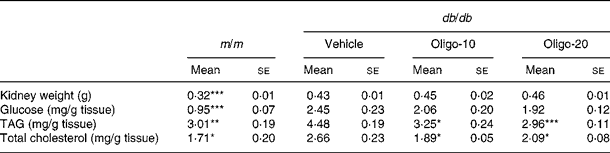
m/m, non-diabetic misty mice; Vehicle, db/db vehicle-treated mice; Oligo-10, db/db mice treated with oligonol at 10 mg/kg body weight; Oligo-20, db/db mice treated with oligonol at 20 mg/kg body weight.
Mean value was significantly different from that of the vehicle group: * P < 0·05, ** P < 0·01, *** P < 0·001.
Protein expression related to lipid metabolism in the kidney
Renal PPARα protein expression decreased in db/db vehicle compared with m/m mice; however, the group treated with oligonol at 20 mg/kg showed an up-regulation of PPARα protein expression (Fig. 1). The protein expressions of renal SREBP-1 and SREBP-2 in db/db mice were significantly higher than those of m/m mice, respectively. The db/db mice administered oligonol at 20 mg/kg, however, showed significantly reduced protein expressions of SREBP-1 but not SREBP-2 compared with db/db vehicle mice (Fig. 1).

Fig. 1 PPARα (a), sterol regulatory element-binding protein (SREBP)-1 (b) and SREBP-2 (c) expressions in renal tissues. m/m, Non-diabetic misty mice; Veh, db/db vehicle-treated mice; Oligo-10, db/db mice treated with oligonol at 10 mg/kg body weight; Oligo-20, db/db mice treated with oligonol at 20 mg/kg body weight. Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the vehicle group: * P < 0·05, ** P < 0·01.
Protein expressions of receptor for advanced glycation endproducts, Nɛ-(carboxyethyl)lysine and Nɛ-(carboxymethyl)lysine in the kidney
To evaluate RAGE, CEL and CML protein expressions in the kidney, we performed Western blot analyses. As shown in Fig. 2, renal RAGE, CEL and CML were elevated in db/db vehicle compared with m/m mice (2·34, 2·69 and 4·36-fold, respectively). However, the oligonol-treated group showed a significant down-regulation of these AGE and their receptors.

Fig. 2 Receptor for advanced glycation endproducts (RAGE) (a), N ɛ-(carboxyethyl)lysine (CEL) (b) and N ɛ-(carboxymethyl)lysine (CML) (c) expressions in renal tissues. m/m, Non-diabetic misty mice; Veh, db/db vehicle-treated mice; Oligo-10, db/db mice treated with oligonol at 10 mg/kg body weight; Oligo-20, db/db mice treated with oligonol at 20 mg/kg body weight. Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the vehicle group: * P < 0·05, ** P < 0·01.
Expression of inflammatory proteins in the kidney
In the db/db vehicle group, NF-κBp65, COX-2 and iNOS protein expressions were significantly up-regulated compared with those of the m/m group (Fig. 3). These increased expressions of proteins related to inflammation and ROS generation in the kidney were markedly reduced by the administration of oligonol, which means the deactivation of NF-κB and down-regulation of NF-κB related to COX-2 and iNOS.

Fig. 3 NF-κBp65 (a), cyclo-oxygenase-2 (COX-2) (b) and inducible NO synthase (iNOS) (c) expressions in renal tissues. m/m, Non-diabetic misty mice; Veh, db/db vehicle-treated mice; Oligo-10, db/db mice treated with oligonol at 10 mg/kg body weight; Oligo-20, db/db mice treated with oligonol at 20 mg/kg body weight. Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the vehicle group: * P < 0·05, ** P < 0·01.
Discussion
Oligonol is a phenolic product derived from lychee fruit extract containing catechin-type monomers and oligomers of proanthocyanidins, produced by a manufacturing process which converts polyphenol polymers into oligomers(Reference Tanaka, Yoshitake and Zhao20). Oligonol is produced by the oligomerisation of polyphenol polymers, typically proanthocyanidins; thus, oligonol delivers higher levels of oligomeric proanthocyanidins compared with fruit and plant sources that generally contain high-molecular-weight proanthocyanidins. Oligonol reflects the phenolic composition of lychee fruit and includes monomers of catechin, epicatechin, epicatechin gallate and epigallocatechin gallate, and oligomers of procyanidin A1, procyanidin A2, procyanidin B1 and procyanidin B2(Reference Sarni-Manchado, Le Roux and Le Guernevé21). These proanthocyanidins have been reported to exhibit beneficial bioactivities in many studies, and so oligonol, a rich source of polyphenol, has been expected to show favourable effects on various chronic diseases. Of course, many beneficial physiological activities of oligonol on antioxidants, anticancer, anti-ageing and anti-inflammation, have been reported, while, in type 2 diabetes, the effects of oligonol on the kidney have not yet been determined. Therefore, we investigated the protective effects of oligonol against renal lipid metabolism, oxidative stress, AGE formation and inflammation in C57BLKS/J db/db mice.
In the present study, the effects of oligonol on serum parameters such as glucose and lipid levels as well as their related hormones were examined. We found that db/db mice showed hyperglycaemia as well as hyperlipidaemia. The administration of oligonol reduced hyperlipidaemia through lowering TAG, total cholesterol and NEFA. However, oligonol treatment did not affect the serum glucose concentration in spite of elevated serum insulin (Table 1). In oligonol-administered db/db mice, the cause of increased insulin secretion is unclear and may be related to the preservation of pancreatic β-cell function by oligonol treatment. In the study of Kanda et al., db/db mice exhibited the apoptosis of pancreatic β-cells which were readily detectable at 8 weeks of age, and decreased cell proliferation at 12 weeks of age with a reduced insulin content in pancreatic islets(Reference Kanda, Shimoda and Tawaramoto22). Additionally, serum adiponectin concentrations were significantly higher in the oligonol-treated than in the vehicle db/db group. Low adiponectin levels are associated with insulin resistance in type 2 diabetic patients and experimental animals(Reference Rabe, Lehrke and Parhofer23). Unfortunately, we cannot measure the insulin sensitivity regarding the influence on target tissues such as muscle and fat. However, other than serum glucose and insulin levels, through oligonol administration, other biofactors such as the lipid profile and oxidative stress were favourable under type 2 diabetic conditions, especially kidney damage. In addition, to investigate the effects of oligonol on renal damage induced by hyperglycaemia and abnormal lipid synthesis, the levels of hyperglycaemia and hyperlipidaemia in the kidneys of db/db mice were also examined. The renal contents of TAG and total cholesterol were significantly decreased by the administration of oligonol (Table 3). These results indicate that the biological activities of oligonol in the serum of db/db mice are associated with lipid metabolism such as synthesis or deposition for energy production.
Hyperglycaemia and elevated NEFA levels result in the generation of ROS, and, consequently, increase oxidative stress. ROS not only directly damage cells by oxidising DNA, proteins and lipids, but also indirectly damage them by activating a variety of stress-sensitive intracellular signalling pathways such as NF-κB, p38 mitogen-activated protein kinase (MAPK), NH2-terminal Jun kinase/stress-activated protein kinase, hexosamines, protein kinase C, AGE/RAGE and others. Activation of these pathways results in the increased expression of numerous gene products that cause cellular damage and play a major role in the aetiology of the later-stage complications of diabetes(Reference Evans, Goldfine and Maddux24). Thus, the up-regulation of endogenous antioxidative systems and suppression of oxidative stress are important factors ameliorating diabetes and its complications. In the present study, we investigated ROS generation and lipid peroxidation, as biomarkers associated with oxidative stress, and also measured GSH and GSSG as indicators of an endogenous antioxidative system. Lipid peroxidation also leads to oxidant production from many molecules, and, thus, amplifies oxidative damage(Reference Niki, Yamamoto and Komuro25). The present results showed that the level of ROS generation and that of lipid peroxidation in the serum and kidney were increased in db/db mice, which implies that these mice show increased oxidative damage due to an elevation of ROS generation induced by hyperglycaemia and hyperlipidaemia. However, oligonol treatment exerted antioxidant activity, promoting decreased serum ROS and TBARS levels with corresponding effects on renal tissue in db/db mice (Table 2). This suggests that the administration of oligonol would ameliorate oxidative stress under type 2 diabetes through the inhibition of ROS generation and lipid peroxidation, and, thus, it would result in the improvement of renal disorders caused by oxidative stress.
Lipid homeostasis is regulated by a family of membrane-bound transcription factors called SREBP. Up-regulations of SREBP-1 and SREBP-2 were reported in leptin-resistant mice such as ob/ob and FVBdb/db mice(Reference Tobe, Suzuki and Aoyama26, Reference Wang, Jiang and Li27). In the present study, the increase in renal SREBP-1 and SREBP-2 in db/db mice was down-regulated by the administration of oligonol. This was probably related to the inhibition of renal TAG and total cholesterol accumulation. Furthermore, PPAR, with three isoforms (α, δ and γ), are also involved in the long-term regulation of lipid metabolism, and their activity is modulated by endogenous lipid-derived ligands. When PPARα is activated, it promotes fatty acid oxidation, ketone body synthesis and glucose sparing(Reference Ferré28) and ameliorates diabetes, insulin resistance, albuminuria, glomerular hypertrophy and mesangial expansion in db/db mice(Reference Park, Zhang and Zhang29). In the present study, the decreased renal PPARα level in db/db mice was significantly increased on oligonol administration. These results clarify the effect of oligonol on regulations of both PPARα and SREBP.
AGE are complex compounds formed via a non-enzymic reaction between reducing sugars and amine residues on proteins, lipids or nucleic acids(Reference Goh and Cooper4). The intracellular production and accumulation of AGE are closely linked to diabetic complications such as neuropathy, retinopathy and nephropathy(Reference Ahmed3, Reference Baynes and Thorpe30). Especially, there is a strong correlation between AGE accumulation and the duration and degree of severity of diabetic kidney disease(Reference Monnier, Bautista and Kenny5). AGE can interact with certain receptors, such as RAGE, to induce intracellular signalling, which leads to enhanced oxidative stress and the production of key pro-inflammatory and prosclerotic cytokines. Recently, attention has been focused on the essential roles of AGE, that is, AGE alter the structure and function of matrix tissue proteins, and AGE-modified proteins stimulate a variety of cellular responses via a specific cell surface receptor, resulting in the expression and activation of pathogenic mediators, for example, the extracellular matrix, oxidative stress, cytokines and growth factors implicated in the development and stimulation of diabetic renal diseases(Reference Yan, Schmidt and Anderson31). In the present study, we performed Western blot analyses of kidney tissue, evaluating AGE actions with receptors related to intracellular responses and the renal AGE level characterised physico-chemically by neither cross-linking nor fluorescence, for example, CEL and CML, identified in ageing and diabetes-related diseases. These products are not only derived from intermediates of glucose metabolism and metabolites of glycolysis, but also serve as general biomarkers of oxidative stress resulting from carbohydrate and lipid oxidation reactions(Reference Koito, Araki and Horiuchi32). CML formation also takes place through glyoxal, which is generated through the auto-oxidation of glucose(Reference Wells-Knecht, Zyzak and Litchfield33), oxidative cleavage of Schiff bases(Reference Glomb and Monnier34) and unsaturated fatty acids(Reference Fu, Requena and Jenkins35). An enhanced oxidative stress status could lead to CML formation, although CML is generally regarded as a glycoxidation product. CML can also be formed through lipid peroxidation and the generation of glycolaldehyde via the myeloperoxidase pathway(Reference Fu, Requena and Jenkins35). Indeed, CML cannot be formed without oxidative stress(Reference Baynes and Thorpe30). Therefore, CML could serve as a general biomarker of oxidative stress resulting from carbohydrate and lipid oxidation reactions. Moreover, recent studies have demonstrated that methylglyoxal is generated through the Embden–Meyerhof and polyol pathways, and rapidly reacts with proteins to form methylglyoxal-derived AGE such as CEL(Reference Koito, Araki and Horiuchi32). CEL is detected in human lens proteins at a concentration similar to that of CML, and its accumulation increases with age in parallel with that of CML(Reference Ahmed, Frye and Degenhardt36). Consequently, the down-regulation of RAGE, CEL and CML expression is important to improve diabetic kidney disease. In the present study, oligonol markedly reduced renal protein levels of RAGE, CEL and CML in the kidney of db/db mice, suggesting that oligonol, with antioxidant activities, has a protective effect against diabetic renal damage caused by RAGE–AGE interactions and complicated complexes of functional protein with CML or CEL.
NF-κB can be activated by a wide array of exogenous and endogenous stimuli including hyperglycaemia, elevated NEFA, ROS, TNF-α, IL-1β, other pro-inflammatory cytokines, AGE-binding RAGE and p38 mitogen-activated protein kinase (MAPK). In particular, AGE trigger the activation of NF-κB via interaction with RAGE, leading to its translocation to the nucleus where it induces transcription, and the promoter region of the RAGE gene contains NF-κB-binding sites, potentially creating a self-perpetuating pathway(Reference Csiszar and Ungvari37). The aberrant regulation of NF-κB is associated with a number of chronic diseases including diabetes and atherosclerosis(Reference Ahmed, Frye and Degenhardt36). NF-κB regulates the expression of a large number of genes, including growth factors, pro-inflammatory cytokines and others(Reference Evans, Goldfine and Maddux38, Reference Celec39). NF-κB is involved in the regulation of COX-2 and iNOS expressions that mediate the inflammatory process(Reference Surh, Chun and Cha40). NF-κB activation induces insulin resistance by lipid/fatty acid infusion and the inhibition of insulin signalling by lipid metabolites such as diacylglycerol and ceramide(Reference Sinha, Perdomo and Brown41). In our Western blotting analysis, experimental type 2 diabetes resulted in the increased expression of NF-κBp65, COX-2 and iNOS proteins, whereas the expression of these three proteins was markedly reduced on oligonol administration. Of note, the protein expressions of NF-κBp65, COX-2 and iNOS were fully recovered by oligonol at a concentration of 20 mg/kg to the levels of m/m mice. These results showed that the anti-inflammatory effects of oligonol may be associated with the down-regulation of COX-2 and iNOS followed by the inhibition of NF-κB transcription-stimulated oxidative stress and AGE–RAGE interaction in the kidney of type 2 diabetic mice.
In summary, oligonol treatment in a type 2 diabetic state improves the serum lipid profile (TAG, total cholesterol and NEFA) and renal function. Also, oligonol ameliorates renal abnormalities with lipid dys-metabolism, oxidative stress, excessive formation of AGE and inflammation. Accordingly, oligonol, a supplier of low-molecular-weight polyphenols, may reduce the risk of type 2 diabetes by the amelioration of metabolic disorders including dyslipidaemia, oxidative stress, as well as inflammatory responses, in part, by the induction of AGE.
Acknowledgements
The present study was supported in part by a Grant-in-Aid (C) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (no. 19500661 to T. Y.). J. S. N., H Y. K., C. H. P. and H. F. conducted the experimental work. T. Y. designed the experiment and wrote the manuscript. The authors declare that there are no conflicts of interest.








