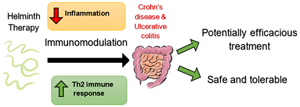
Introduction
Inflammatory bowel disease (IBD) is a group of chronic inflammatory disorders that affect the gastrointestinal tract, characterized by relapsing and remitting episodes of inflammation resulting from an uncontrolled immune-mediated inflammatory response (Malik, Reference Malik2015; Shapiro et al., Reference Shapiro, Subedi and LeLeiko2016). Crohn's disease (CD) and ulcerative colitis (UC) are the two main types of IBD seen in populations (Fakhoury et al., Reference Fakhoury, Negrulj, Mooranian and Al-Salami2014). The cause of IBD is currently unknown, however there has been evidence suggesting that genetic and environmental factors may be linked to its development (Baumgart and Carding, Reference Baumgart and Carding2007).
The incidence of immune-related diseases such as IBD is rising in developing countries. Between 1990 and 2017 the number of people with IBD globally increased from 3.7 million to more than 6.8 million (95% UI 6.4–7.3) – an increase of approximately 83.9% over 27 years and this trend is projected to continue (GBD, 2017 Inflammatory Bowel Disease Collaborators, 2020). The hygiene hypothesis (Strachan, Reference Strachan1989) suggests that this could be due to medical advancements and lifestyle changes reducing our exposure to infections that would have previously been common, and that a link exists between exposure to these infections and modulation of our immune system (Okada et al., Reference Okada, Kuhn, Feillet and Bach2010).
Currently, there is no known medical cure for IBD and therefore there is interest in exploring new avenues of treatment (Fakhoury et al., Reference Fakhoury, Negrulj, Mooranian and Al-Salami2014). Treatments offered at present are limited to medications to control the symptoms of the disease and reduce inflammation, or surgery to remove affected sections of the bowel. Unfortunately, many of the medications cause adverse effects, especially when required at higher doses (Fakhoury et al., Reference Fakhoury, Negrulj, Mooranian and Al-Salami2014). Surgical resection of the bowel can sometimes resolve UC if the entire affected section of the bowel is removed and there are no extra-intestinal symptoms. In CD this is not a curative option and is usually used to manage disease complications (Baumgart and Sandborn, Reference Baumgart and Sandborn2007).
Helminths have co-existed with humans for millions of years and have adapted mechanisms to modulate their host's immune system to support their own survival (Helmby, Reference Helmby2015). Studies in animal models have produced evidence demonstrating the ability of helminths to suppress inflammation (Smallwood et al., Reference Smallwood, Giacomin, Loukas, Mulvenna, Clark and Miles2017; White et al., Reference White, Johnston, Grainger, Konkel, O'Connor, Anderton and Maizels2020; Lothstein and Gause, Reference Lothstein and Gause2021). In animals with helminth infections a polarization towards a Th2 immune response which has a role in suppressing inflammation was identified. Recent research has linked this to the release of damage-associated molecular patterns (DAMPs), which include trefoil factor 2, ATP and chitinase-like proteins, which are released when a helminth invades epithelial barriers (Gause et al., Reference Gause, Rothlin and Loke2020). This leads to an increase in regulatory T cells and certain cytokines, including IL-4, IL-5, IL-10, IL-13 and TGF-β (Ayelign et al., Reference Ayelign, Akalu, Teferi, Molla and Shibabaw2020; Maizels, Reference Maizels2020; Lothstein and Gause, Reference Lothstein and Gause2021) and a decrease in pro-inflammatory cytokines such as IFNγ and TNF (Smallwood et al., Reference Smallwood, Giacomin, Loukas, Mulvenna, Clark and Miles2017; Ayelign et al., Reference Ayelign, Akalu, Teferi, Molla and Shibabaw2020). There is also evidence that helminth infection can suppress the Th1 and Th17 immune responses which have a crucial role in the pathogenesis of autoimmune diseases (Walsh et al., Reference Walsh, Brady, Finlay, Boon and Mills2009; Lothstein and Gause, Reference Lothstein and Gause2021). Therefore, there may be potential for the use of helminths to improve symptoms of immune-related disorders such as IBD (Maruszewska-Cheruiyot et al., Reference Maruszewska-Cheruiyot, Donskow-Lysoniewska and Doligalska2018).
A previous systematic review looking at the use of helminth therapy for inducing remission of IBD only identified two studies that were suitable for the review, and concluded that more evidence was needed (Garg et al., Reference Garg, Croft and Bager2014). This systematic review aimed to re-investigate helminth therapy for IBD and identify new evidence that might allow for a firmer conclusion on the concept of using helminths in UC and CD management.
Methods
Systematic searches were conducted to identify studies that would be suitable for this review. PubMed, Embase, Medline and the Cochrane Central Register of Control Trials were used to carry out searches, and searches were conducted using MeSH headings including helminth, helminth therapy, IBD, UC and CD. Reference lists of studies that met the inclusion criteria were also searched to identify any studies that may have been missed by the database searches. The previous systematic review looking at a similar question regarding helminth therapy and IBD (Garg et al., Reference Garg, Croft and Bager2014) was also examined.
In terms of developing the research question, the PEO (population, exposure, outcome) framework (Khan et al., Reference Khan, Kunz, Kleijnen and Antes2003) was used. A summary of this is outlined in Table 1.
Table 1. Development of the review question

An overview of the inclusion and exclusion criteria for the search results of this review can be found in Table 2. There was no exclusion based on the sex or ethnicity of the participants, and there was no limitation on the date the studies were published as the aim of this review was to provide a comprehensive summary of any available evidence.
Table 2. Inclusion and exclusion criteria

Study selection was conducted in two phases. In the first phase, titles and abstracts were screened. In the second phase, full texts were assessed to determine their eligibility for inclusion. The stages in the PRISMA flow diagram (Fig. 1) (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009) were followed to identify and screen the results of the initial search and to identify studies eligible for inclusion in the review.

Fig. 1. PRISMA flow diagram. The PRISMA diagram (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009) shows the steps of the search and selection process detailed in the ‘Methods’ section of this review.
Data extraction was carried out using a data extraction form which was designed using the Joanna Briggs Institute (JBI) guidance on data extraction (Tufanaru et al., Reference Tufanaru, Munn, Aromataris, Campbell, Hopp, Aromataris and Munnz2020) and the Cochrane Handbook for Systematic Reviews of Interventions (Li et al., Reference Li, Higgins, Deeks, Higgins, Thomas, Chandler, Cumpston, Li, Page and Welch2019). The CASP (Critical Appraisals Skills Programme) randomized control trial (RCT) checklist (Critical Appraisal Skills Programme, 2018) was used to critically appraise the studies that were RCTs, and the methodological index for non-randomised studies (MINORS) assessment tool (Slim et al., Reference Slim, Nini, Forestier, Kwiatkowski, Panis and Chipponi2003) was used to appraise the open-label studies.
Due to there being quite a lot of heterogeneity between some of the studies (i.e. varying study designs, different treatments used and differing methods of measuring the outcomes) a meta-analysis was not suitable. As such, a narrative synthesis was used to consistently summarize all the findings of the studies.
This was conducted by one individual under the supervision and guidance of another experienced in completing systematic reviews.
Results
Search results and study characteristics
The database searches identified 491 records. An additional three studies were identified during a search of the background literature on the subject. After the removal of duplicates, 367 remained for the title and abstract screening. Following this, 331 studies were excluded leaving 36 studies for the phase 2 full-text screening. Screening of the full texts excluded 27 studies, leaving nine that were eligible for inclusion in the review (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003, Reference Summers, Elliott, Urban, Thompson and Weinstock2005a, Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006; Sandborn et al., Reference Sandborn, Elliott, Weinstock, Summers, Landry-Wheeler, Silver, Harnett and Hanauer2013; NCT01576471, 2017; NCT01953354, 2017; Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019). The results of the screening and exclusions/inclusions were documented using the PRISMA flow diagram (Fig. 1) (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009).
Table 3 provides a full summary of the eligible studies in the review. Eight of the eligible studies used live helminth ova/ larvae. Of these, seven used T. suis ova with the dose ranging from 250 T. suis ova (TSO) to 7500 TSO, and one used N. americanus larvae. One study used P28 glutathione-S-transferase (P28GST) (Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019), a protein derived from Schistosoma. Of the nine studies that were included six involved patients with CD (Summers et al., Reference Summers, Elliott, Urban, Thompson and Weinstock2005a; Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006; Sandborn et al., Reference Sandborn, Elliott, Weinstock, Summers, Landry-Wheeler, Silver, Harnett and Hanauer2013; NCT01576471, 2017; Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019), two involved patients with UC (Summers et al., Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; NCT01953354, 2017), and one involved patients with both (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003). Five were double-blind RCTs (Summers et al., Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; Sandborn et al., Reference Sandborn, Elliott, Weinstock, Summers, Landry-Wheeler, Silver, Harnett and Hanauer2013; NCT01576471, 2017; NCT01953354, 2017; Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017), and four were open label studies (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003, Reference Summers, Elliott, Urban, Thompson and Weinstock2005a; Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019). Two of the RCTs were the published results of clinical trials, not fully written articles (NCT01576471, 2017; NCT01953354, 2017). Eight studies looked at the efficacy of the treatment and made at least some comment on the safety (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003, Reference Summers, Elliott, Urban, Thompson and Weinstock2005a, Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006; NCT01576471, 2017; NCT01953354, 2017; Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019), and one looked solely at the safety (Sandborn et al., Reference Sandborn, Elliott, Weinstock, Summers, Landry-Wheeler, Silver, Harnett and Hanauer2013).
Table 3. Summary of studies
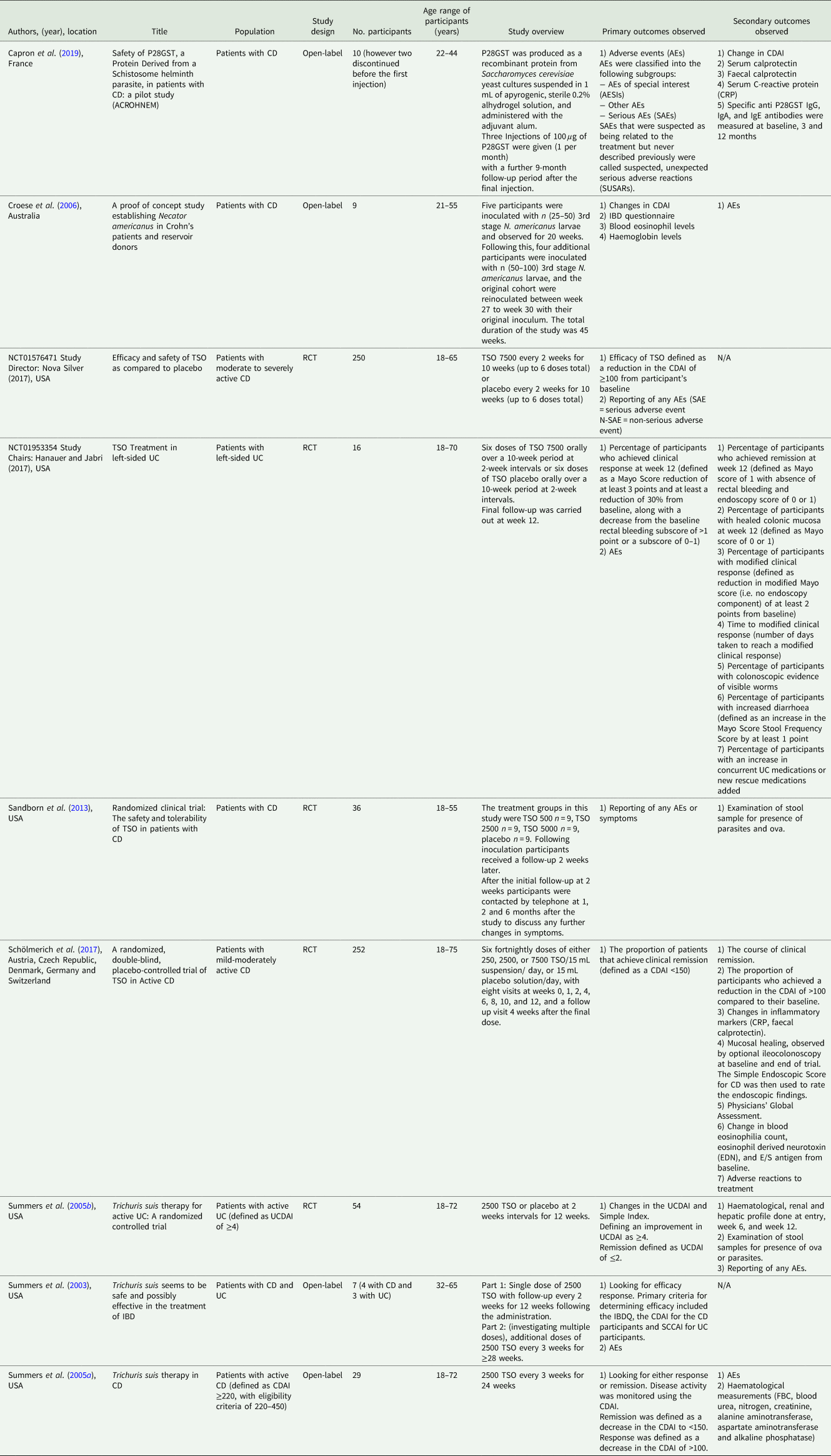
The studies were all published between 2003 and 2019. Six were conducted in the USA (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003, Reference Summers, Elliott, Urban, Thompson and Weinstock2005a, Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; Sandborn et al., Reference Sandborn, Elliott, Weinstock, Summers, Landry-Wheeler, Silver, Harnett and Hanauer2013; NCT01576471, 2017; NCT01953354, 2017), one was in Australia (Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006), and two were in Europe (Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019).
The number of participants taking part in the studies varied between seven participants in the smallest study (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003) and 252 participants in the largest (Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017). The age range across all of the studies was 18–75 years, and all of the studies included a mix of male and female participants. None of the studies appeared to intentionally discriminate between ethnicities however the majority of participants were white/ Caucasian.
Quality appraisal of the studies
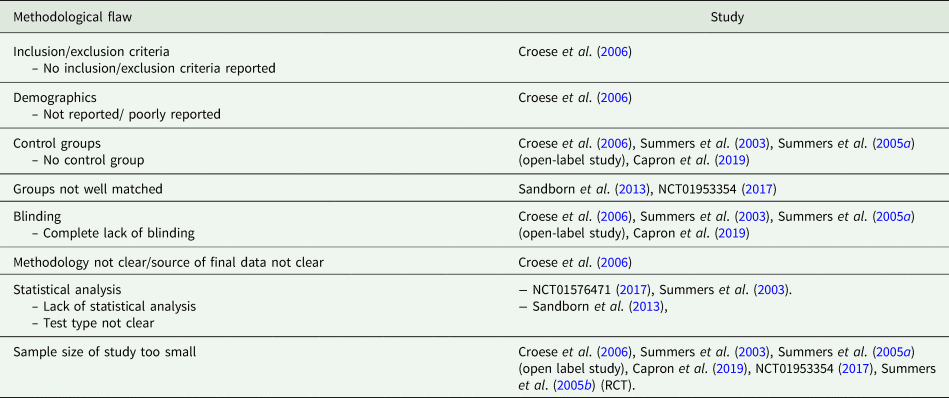
The five RCT's were appraised using the CASP RCT checklist and the MINORS assessment tool was used to appraise the four open-label studies. Table 4 summarizes the methodological flaws identified in the studies. These include areas of potential bias such as whether studies used blinding and control groups, and if the study methodology was explicit.
Table 4. Summary of methodological flaws in the studies
Summary of study findings
To answer the research question of this systematic review the results of the studies are categorized into those regarding efficacy and safety.
Efficacy of treatment
Eight of the nine studies assessed the efficacy of a helminth therapy (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003, Reference Summers, Elliott, Urban, Thompson and Weinstock2005a, Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006; NCT01576471, 2017; NCT01953354, 2017; Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019). Of these, six used Trichuris suis ova (TSO) as the treatment (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003, Reference Summers, Elliott, Urban, Thompson and Weinstock2005a, Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; NCT01576471, 2017; NCT01953354, 2017; Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017), one used Necator americanus larvae (Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006), and one used P28GST (Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019). Five of the studies were looking at patients with CD (Summers et al., Reference Summers, Elliott, Urban, Thompson and Weinstock2005a; Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006; NCT01576471, 2017; Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019), two looked at patients with UC (Summers et al., Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; NCT01953354, 2017), and one looked at both (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003).
Five of the eight concluded that helminth therapy seemed to be effective in patients with CD or UC (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003, Reference Summers, Elliott, Urban, Thompson and Weinstock2005a, Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019). This was mostly measured through improvement in disease activity indexes with some studies investigating additional outcomes. These disease activity indices included the CD activity index (CDAI), the UC disease activity index (UCDAI), the simple clinical colitis activity index (SCCAI) and the IBD quality of life questionnaire (IBDQ). Only one of these studies was an RCT (Summers et al., Reference Summers, Elliott, Urban, Thompson and Weinstock2005b), and as such had control for comparison, but all the studies showed a statistically significant improvement (where statistical analysis was available) (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003, Reference Summers, Elliott, Urban, Thompson and Weinstock2005a, Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019). An additional finding in one of the studies was that maintenance doses were required to sustain the effect of the TSO treatment (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003). This study involved the use of an initial single dose of TSO with 12 weeks of monitoring, followed by an extended trial in which some participants received repeat doses every 3 weeks. The extended trial found that this prolonged the duration of clinical improvement in all four participants who took part. The results from the study using P28GST also indicated the need for repeat dosing, as at the end of the monitoring period (9 months after the final dose), there was no longer a significant difference between the baseline and post-treatment CDAI scores (Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019). This finding suggests that there is a natural clearance from the body in the case of both live and molecular treatment, and that the continued presence of the helminth/helminth derived product is needed to maintain the therapeutic effect.
Three of the eight studies concluded that treatment showed no advantage over placebo, all of which were RCTs looking at TSO treatment, and again used changes in disease activity indices (in this case CDAI and the Mayo score) as the main measure of efficacy (NCT01576471, 2017; NCT01953354, 2017; Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017).
There was some evidence of colonic mucosal healing in one of the studies which seemed to show a notable difference between the treatment group and the placebo (66.7% showed mucosal healing in the treatment group and 16.7% in the placebo group). However, after statistical analysis, this was not found to be statistically significant (P = 0.242) (NCT01953354, 2017).
One study (Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017) which investigated different doses of TSO, ranging from TSO 250 to TSO 7500, did suggest that higher doses may have greater efficacy. With clinical remission in this study defined as CDAI <150, of the TSO 250 group 38.7% achieved remission and in the TSO 7500 group 47.2% achieved remission. However, as this study did not find a statistically significant difference between the treatment groups and the placebo group this finding should not be over-interpreted.
Measurements of inflammatory markers in one study (serum calprotectin, faecal calprotectin and CRP) showed that levels did decrease following the therapy, but when analysed this was not found to be statistically significant (Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019). However, another study that also measured inflammatory markers (CRP and faecal calprotectin) found no clinically significant change took place between the start and end of the study (Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017). It is possible that this may be because the treatments used were different between these two studies.
In the studies that investigated the levels of eosinophils and antibodies specific to the treatment, there was an observed increase (Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006; Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019), which is consistent and expected with parasitic infection (Huang and Appleton, Reference Huang and Appleton2016), These findings suggest that the treatment did exert an effect on the host's immune system. However, when taken with other outcomes measured this was not found to have an effect on the rate of disease remission (Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017). After the completion of the studies, the eosinophil levels decreased after a period of time following the last treatment dose (Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019).
Safety
All of the nine studies commented on adverse events (AEs) experienced during the course of the study.
There was no incidence of mortality in any of the studies. The incidence rate of serious AEs (SAEs) was generally low across all of the studies, while the rate of non-serious AEs (N-SAEs) was higher. It should be noted that in the studies with control groups the rate of SAEs and N-SAEs was relatively similar between the treatment group and the placebo group.
In studies that looked at haematological measures (i.e. full blood count, renal profile, hepatic profile) there were no significant changes observed (Summers et al., Reference Summers, Elliott, Urban, Thompson and Weinstock2005a, Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006), suggesting that the treatments did not have a negative physiological effect on these systems.
The overall conclusion of all the studies was that the treatments could be considered safe and well-tolerated in the treatment groups (Summers et al., Reference Summers, Elliott, Qadir, Urban, Thompson and Weinstock2003, Reference Summers, Elliott, Urban, Thompson and Weinstock2005a, Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006; Sandborn et al., Reference Sandborn, Elliott, Weinstock, Summers, Landry-Wheeler, Silver, Harnett and Hanauer2013; NCT01576471, 2017; NCT01953354, 2017; Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019).
Discussion
Whilst this systematic review identified more studies for inclusion than the similar study conducted in 2014 (Garg et al., Reference Garg, Croft and Bager2014), like the previous review, it still does not reach a conclusive answer in regard to using helminth therapy for CD and UC. Arguably more studies indicated that helminth treatments seemed to be efficacious (five out of eight studies). However, the three that did not find any benefit were all RCTs that had a placebo for comparison and by virtue of their methodology were less likely to be biased than the open-label studies (Burns et al., Reference Burns, Rohrich and Chung2011). Therefore, it seems that there is an almost equal balance of evidence both for and against helminth therapy.
It should be noted though, that all studies concluded that helminth therapy seemed to be safe and tolerable, which gives some support towards using it. IBD can be somewhat varied in its presentation but some of the reported AEs were consistent with symptoms of IBD (particularly the GI symptoms (Seyedian et al., Reference Seyedian, Nokhostin and Malamir2019) but also some extraintestinal symptoms (Levine and Burakoff, Reference Levine and Burakoff2011)), meaning a number of the AEs could have been the result of the disease rather than the treatment. This is supported by the RCT studies which showed a similar number of AEs in the placebo group to the treatment groups (Sandborn et al., Reference Sandborn, Elliott, Weinstock, Summers, Landry-Wheeler, Silver, Harnett and Hanauer2013; NCT01576471, 2017).
As all the studies concluded that the use of helminth therapy was safe and tolerable the need for further research in this area is indicated. An important part of the studies in this review was identifying a helminth/helminth derived product that would be well tolerated in participants and ideally cause minimal symptoms. With regard to the live helminths used in the studies, Trichuris suis does not typically infect humans and exists naturally as a porcine parasite (Cutillas et al., Reference Cutillas, Callejón, de Rojas, Tewes, Ubeda, Ariza and Guevara2009). Though not the usual host of T. suis, cases of cross infections in humans have been documented in the past, but there is no known disease specifically associated with infections in humans (Beer, Reference Beer1976). This fact may be the reason it is an appealing choice for helminth therapy. Necator Americanus is a more unusual choice, as it is a natural human parasite and can produce symptomatic disease (Hotez et al., Reference Hotez, Bethony, Bottazzi, Brooker and Buss2005), but the study that investigated it (Croese et al., Reference Croese, O'neil, Masson, Cooke, Melrose, Pritchard and Speare2006) did so with the rationale that exploiting its long lifespan (3–5 years) (Roberts et al., Reference Roberts, Janovy and Schimdt2008) would be beneficial as it may reduce the need for repeated dosing.
Implications for practice
The results of this review do not provide any evidence to suggest that helminth therapy is better than the current recommended treatments for IBD or that any changes should be made to current practice. This is consistent with existing guidelines which do not presently suggest helminth therapy as a treatment option (Lamb et al., Reference Lamb, Kennedy, Raine, Hendy, Smith, Limdi, Hayee, Lomer, Parkes, Selinger, Barrett, Davies, Bennett, Gittens, Dunlop, Faiz, Fraser, Garrick, Johnston, Parkes, Sanderson, Terry, Gaya, Iqbal, Taylor, Smith, Brookes, Hansen and Hawthorne2019). However, as some of the studies demonstrate a degree of efficacy, there is the potential for it to be offered in the future as an alternative therapy for patients who are not responding to current recommended treatments. Whilst the reported efficacy may be the result of a placebo effect, it could be beneficial to a patient if they felt symptomatic relief. All the studies concluded that helminth therapy could be considered safe, so there is nothing currently to suggest that this could not be attempted. In the future, studies comparing the efficacy of helminth therapy to the current standard treatments (e.g. steroids), or investigating them as a combination therapy, may be of interest.
There may be an issue with patient compliance if helminth therapy did become an available treatment option as not all patients may be comfortable with the idea of being infected with live parasites. Although, it should be noted that there have been cases of patients intentionally infecting themselves with helminths in an attempt to self-medicate their condition (Ahrens, Reference Ahrens2016; Meluban, Reference Meluban2019; Nelson et al., Reference Nelson, Glaser and Tondon2019). The fact that the studies included in this review managed to recruit voluntary participants to trial helminth therapy should also be considered, as it suggests a number of IBD sufferers are willing to try this treatment. Potentially helminth derived products, such as P28GST (Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019), may have more success in terms of appeal.
Strengths
This review provided a comprehensive search of four databases, with an examination of reference lists and a previously published systematic review of a similar topic (Garg et al., Reference Garg, Croft and Bager2014). Only relevant studies were included and the included studies were critically appraised with appraisal tools appropriate for the design of each study.
Limitations
This review was limited by the fact that it only included studies that were available in the English language. This was due to the authors being solely English speakers, and as this study received no funding no translators could be hired. However, it should be noted that the screening process only excluded 6 studies out of a total of 367 due to a language barrier. Therefore, it is possible that relevant studies published in other countries may have been omitted resulting in a potential bias in this review, but, if so, the number is low.
A limitation within some of the studies themselves is the way in which efficacy was measured. There is some evidence suggesting that the CDAI, UCDAI and the Mayo score may not be the most reliable way of tracking disease activity in relation to the assessment of treatment and that they don't always correlate well with endoscopic assessment, inflammation and mucosal healing (Filik et al., Reference Filik, Dagli and Ulker2006; Morris et al., Reference Morris, Stewart, Sandborn, Loftus, Fowler and Jones2013; Mihai et al., Reference Mihai, Prelipcean, Dranga, Gavrilescu, Cardoneanu, Lacatusu and Mihai2018). Whilst all are historically validated tools, they represent more of a view of the patient's subjective well-being than the actual level of mucosal inflammation seen in the disease (Mihai et al., Reference Mihai, Prelipcean, Dranga, Gavrilescu, Cardoneanu, Lacatusu and Mihai2018). There is some belief that the inclusion of inflammatory biomarkers (such as CRP, ESR and faecal calprotectin) might provide a better assessment of disease activity (Morris et al., Reference Morris, Stewart, Sandborn, Loftus, Fowler and Jones2013; Rogler and Biedermann, Reference Rogler and Biedermann2015; Mihai et al., Reference Mihai, Prelipcean, Dranga, Gavrilescu, Cardoneanu, Lacatusu and Mihai2018), which two of the studies in this review did additionally investigate (Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann and Matthes2017; Capron et al., Reference Capron, Beghin, Leclercq, Labreuche, Dendooven, Standaert, Delbeke, Porcherie, Nachury, Boruchowicz, Dupas, Fumery, Paupard, Catteau, Deplanque, Colombel and Desreumaux2019). This suggests that there are additional outcomes that were not considered by all the studies, and that the measure of efficacy used may not always have been truly accurate. Furthermore, many of the disease indices used in the studies in this review have self-reported elements, and due to differences in the judgement of individuals, this could create some variation or bias in reporting.
Another limitation of this review was that database searches and data extraction was only carried out by one individual. As such, there is potential for consistent biases or mistakes throughout the review without a second reviewer to minimize the risk of this with a differing opinion. It should be noted though that this systematic review was completed under the supervision and guidance of another individual with experience in carrying out systematic reviews.
Conclusion
The results of this systematic review suggest that helminth therapy is safe for use and may potentially have some efficacy, however further research (particularly with control groups) is needed to provide stronger evidence for this. Future studies should also consider the use of inflammatory markers as a means of more reliably monitoring disease activity in response to treatment. As it stands, it is difficult to conclusively rule out a placebo effect as the cause of any efficacy seen following treatment. Additional examination of dose strengths in clinical trials could be of interest as only three studies explored this, and of these only two looked at the efficacy of treatment. This also applies to dosing regimens, as this was not particularly explored in the studies in this review. It is possible that products derived from helminths could be more tailored for therapeutic use than living organisms, but as it stands, based on the results of one study, it is not possible to recommend advantages to molecular therapy over the live parasite without further research. P28GST did show promise as an efficacious and safe treatment for CD, so there does appear to be potential in this.
Acknowledgements
We would like to thank the University of Warwick for providing access to its library services.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors
Conflicts of interest
None.
Ethical standards
None.