A 60-year-old woman was admitted to our emergency room with acute-onset chorea of all four extremities (see video in supplementary material). Her medical history was unremarkable apart from hypertension. A neurological examination revealed no abnormalities apart from involuntary movements. Brain magnetic resonance imaging showed acute bilateral infarctions in the head of the caudate nucleus (Figure 1A and B). Magnetic resonance angiography revealed that both anterior cerebral arteries (ACAs) originated from the right internal carotid artery (Figure 1C and D). We started administering oral antiplatelets (aspirin at 100 mg/d and clopidogrel at 75 mg/d) and oral atorvastatin (40 mg/d) for 21 days after the final diagnosis, and the patient’s chorea signs spontaneously improved. The aspirin and atorvastatin continued to be administered after discharge. At the 6-month follow-up assessment, the patient reported no residual symptoms.
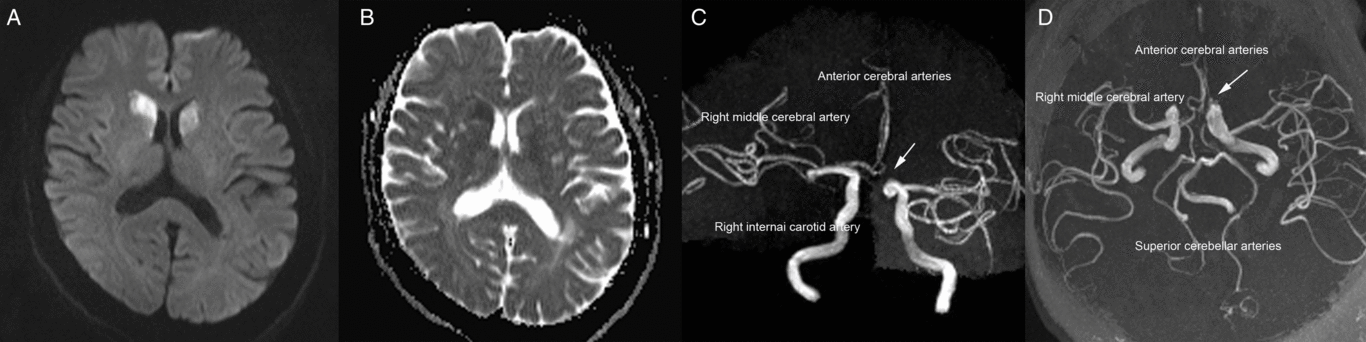
Figure 1: (A) Diffusion-weighted imaging conducted 2 days after symptoms onset revealed a high-intensity signal in the bilateral head of the caudate nucleus. (B) The apparent diffusion coefficient values within the bilateral head of the caudate nucleus were low. (C and D) Magnetic resonance angiography revealed variations in the anterior cerebral arteries (arrows).
Bilateral caudate nucleus infarctions are rare; a retrospective analysis of 240 cases revealed that the average incidence of bilateral caudate nucleus lesions was 1.6%.Reference Bhatia and Marsden1 The formation of this infarction type is mainly related to the vascular anatomy of the caudate head and variations of anterior circulation. Previous studies reported that the caudate head receives its blood flow from three principal sourcesReference Kumral, Evyapan and Balkir2, Reference Fukuoka, Osawa, Ohe, Deguchi, Maeshima and Tanahashi3: (1) the recurrent artery of Heubner, a directly penetrating artery that originates from the proximal A2 segment, A1–A2 junction, or distal A1 segment of the ACA; (2) the anterior lenticulostriate arteries, which originate from the A1 segment of the ACA; and (3) the lateral lenticulostriate arteries, which originate from the middle cerebral artery. Because our patient’s left ACA lacked an A1 segment, the right ACA’s A1 segment bilaterally supplied the recurrent arteries of Heubner and the anterior lenticulostriate arteries. Consequently, occlusion of these arteries is regarded as a cause of bilateral caudate head infarctions.Reference Kumral, Evyapan and Balkir2
Post-stroke movement disorders are diverse.Reference Mehanna and Jankovic4–Reference Suri, Rodriguez-Porcel and Donohue6 A previous studyReference Suri, Rodriguez-Porcel and Donohue6 showed that chorea is the second most common post-ischemic stroke (IS) movement disorder, accounting for approximately 17.4% of all post-IS movement disorders. However, nearly 90% of post-IS chorea cases involve hemichorea, which makes post-IS bilateral chorea a rare occurrence.Reference Caproni and Colosimo5 Previous studies of patients with bilateral caudate head infarctions have reported diverse potential neurobehavioral symptoms such as confusion,Reference Mendez, Adams and Lewandowski7 dementia,Reference Mendez, Adams and Lewandowski7 apathy,Reference Fukuoka, Osawa, Ohe, Deguchi, Maeshima and Tanahashi3, Reference Mendez, Adams and Lewandowski7 delirium,Reference Narumoto, Matsushima and Oka8 disorientation,Reference den Heijer, Ruitenberg, Bakker, Hertzberger and Kerkhoff9 confabulations,Reference den Heijer, Ruitenberg, Bakker, Hertzberger and Kerkhoff9 mutism,Reference Fukuoka, Osawa, Ohe, Deguchi, Maeshima and Tanahashi3 depression,Reference Fukuoka, Osawa, Ohe, Deguchi, Maeshima and Tanahashi3 decreased recent memory,Reference Mrabet, Mrad-Ben Hammouda, Abroug, Smiri and Haddad10 and reduced spontaneity.Reference Mrabet, Mrad-Ben Hammouda, Abroug, Smiri and Haddad10 The most common behavioral disturbance appears to be abulia.Reference Fukuoka, Osawa, Ohe, Deguchi, Maeshima and Tanahashi3 However, in the present case, the patient did not experience any of the aforementioned symptoms or neurobehavioral disturbances other than choreic movements of the four extremities, as vividly demonstrated in the video in supplementary material.
Hemichorea has been classically associated with vascular lesions affecting the contralateral or ipsilateral caudate nucleus, lentiform nucleus, or thalamus.Reference Bhatia and Marsden1, Reference Mehanna and Jankovic4, Reference Caproni and Colosimo5 The bilateral motor cortico-striato-pallido-thalamo-cortical loop and related neural pathways might have been involved in the chorea of all four limbs observed in our patient.Reference Mehanna and Jankovic4
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2019.50.
Acknowledgements
This research was supported by the National Key R&D Program of China (2016YFC1301600), JLUSTIRT (2017TD-12) to Yi Yang.
Disclosures
The authors declare no conflict of interest.
Statement of Authorship
Drafted the manuscript: YQ, HJ, and FLZ. Acquisition of data: YQ. Analysis or interpretation of data: HJ, ZNG, and FLZ. Image and video analysis: JL and HQQ. Study concept and design: YY.



