Introduction
In the face of rapid environmental changes, avoiding extinction requires organisms to respond with behavioural changes, moving to a new geographic region, rapid evolution and/or phenotypic plasticity (Hoffmann and Sgrò, Reference Hoffmann and Sgrò2011; Munday et al., Reference Munday, Warner, Monro, Pandolfi and Marshall2013; Wong and Candolin, Reference Wong and Candolin2015; Radchuk et al., Reference Radchuk, Reed, Teplitsky, van de Pol, Charmantier, Hassall, Adamík, Adriaensen, Ahola, Arcese, Miguel Avilés, Balbontin, Berg, Borras, Burthe, Clobert, Dehnhard, de Lope, Dhondt, Dingemanse, Doi, Eeva, Fickel, Filella, Fossøy, Goodenough, Hall, Hansson, Harris, Hasselquist, Hickler, Joshi, Kharouba, Martínez, Mihoub, Mills, Molina-Morales, Moksnes, Ozgul, Parejo, Pilard, Poisbleau, Rousset, Rödel, Scott, Senar, Stefanescu, Stokke, Kusano, Tarka, Tarwater, Thonicke, Thorley, Wilting, Tryjanowski, Merilä, Sheldon, Pape Møller, Matthysen, Janzen, Dobson, Visser, Beissinger, Courtiol and Kramer-Schadt2019). For organisms with limited behaviours and mobility, phenotypic plasticity has the potential to be particularly important, as it allows for a rapid response without genetic changes. Thus, for many organisms, phenotypic plasticity is expected to play a key role in allowing organisms to cope with rapidly changing environments (Charmantier et al., Reference Charmantier, McCleery, Cole, Perrins, Kruuk and Sheldon2008; Wong and Candolin, Reference Wong and Candolin2015).
Moreover, phenotypic plasticity can facilitate rapid evolutionary processes, including by buying time for genetic adaptations to occur (Mousseau and Fox, Reference Mousseau and Fox1998; Sun et al., Reference Sun, Catherall, Pascoal, Jarrett, Miller, Sheehan and Kilner2020). This can be particularly true when a plastic response to environmental changes can be passed from one generation to the next, something known as transgenerational plasticity or maternal effects (Harmon and Pfennig, Reference Harmon and Pfennig2021). Transgenerational plasticity has been demonstrated in many taxa; often, previous generations ‘prime’ their offspring for environmental conditions that match those of maternal generations (Mousseau and Fox, Reference Mousseau and Fox1998; Burgess and Marshall, Reference Burgess and Marshall2014). If the parental environments are accurate predictors for the environments of offspring, transgenerational plasticity can be adaptive, increasing the fitness of offspring experiencing those environmental conditions (Galloway and Etterson, Reference Galloway and Etterson2007).
Although transgenerational plasticity is increasingly considered as a potentially important mechanism to counteract the negative impacts of environmental changes, most studies have focused on responses of a single species to changing abiotic environments, such as temperature elevation (Shama et al., Reference Shama, Strobel, Mark and Wegner2014) or environmental pollution (Tran et al., Reference Tran, Janssens, Dinh and Stoks2019; Meng et al., Reference Meng, Tran, Delnat and Stoks2021). However, changing environments also have the potential to influence the way organisms interact. Focusing on just one type of interaction, host–parasite interactions, increasing temperatures can alter parasite reproduction, infectivity and prevalence, host resistance and ultimately epidemic dynamics (Harvell et al., Reference Harvell, Mitchell, Ward, Altizer, Dobson, Ostfeld and Samuel2002; Altizer et al., Reference Altizer, Ostfeld, Johnson, Kutz and Harvell2013; Gehman et al., Reference Gehman, Hall and Byers2018). While this likely leads to selection on parasites in many cases, transgenerational plasticity may also play a role in allowing parasites to persist in a rapidly changing environment. There have been some studies of transgenerational plasticity in host–parasite interactions, but most of the attention has been focused on hosts (Gervasi et al., Reference Gervasi, Civitello, Kilvitis and Martin2015; Pigeault et al., Reference Pigeault, Vézilier, Nicot, Gandon and Rivero2015; Michel et al., Reference Michel, Ebert and Hall2016; Nystrand et al., Reference Nystrand, Cassidy and Dowling2016; Roth and Landis, Reference Roth and Landis2017; Paraskevopoulou et al., Reference Paraskevopoulou, Gattis and Ben-Ami2022) rather than on parasites or the overall outcome of host–parasite interactions (Tseng, Reference Tseng2006; Little et al., Reference Little, Birch, Vale and Tseng2007; Shocket et al., Reference Shocket, Vergara, Sickbert, Walsman, Strauss, Hite, Duffy, Cáceres and Hall2018). This means that, currently, there is a major gap in our understanding of how host–parasite interactions and parasite fitness change as environments change, hindering our ability to understand and predict epidemic dynamics in a rapidly changing world.
In addition to its importance in light of anthropogenic climate change, understanding transgenerational plasticity of parasites is important because seasonal changes in environments are common and can influence the outcomes of host–parasite interactions within a generation (Altizer et al., Reference Altizer, Dobson, Hosseini, Hudson, Pascual and Rohani2006; Martinez, Reference Martinez2018). Changing temperatures can have complex impacts on hosts and parasites (Altizer et al., Reference Altizer, Ostfeld, Johnson, Kutz and Harvell2013), making it difficult to predict a priori how parasitism should change seasonally (Altizer et al., Reference Altizer, Dobson, Hosseini, Hudson, Pascual and Rohani2006). One factor that increases the complexity even further is that hosts might encounter parasites that were produced in previous seasons (or even years) under different environmental conditions (Decaestecker et al., Reference Decaestecker, Lefever, de Meester and Ebert2004; Shocket et al., Reference Shocket, Vergara, Sickbert, Walsman, Strauss, Hite, Duffy, Cáceres and Hall2018). Overall, it is clear that changes in temperature can strongly impact host–parasite interactions; in some systems, hosts encounter parasites that were produced at different temperatures; and the conditions under which a parasite develops can impact its infectivity. However, at present, it is not clear whether parasite transgenerational plasticity influences key parasite traits such as virulence.
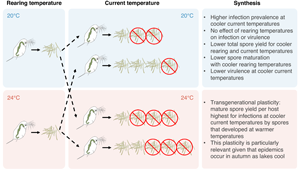
In this study, we tested whether the temperature at which parasite spores develop influences performance of their offspring using a 2-generational laboratory experiment in a zooplankton–fungal system. Metschnikowia bicuspidata is a fungal parasite commonly found infecting the zooplankton host Daphnia dentifera in freshwater lakes in Northern America (Cáceres et al., Reference Cáceres, Tessier, Duffy and Hall2014). In this system, D. dentifera is exposed to M. bicuspidata spores during filter feeding. The ingested needle-shaped spores then penetrate through the gut barrier into the body cavity (Stewart Merrill et al., Reference Stewart Merrill, Hall, Merrill and Cáceres2019), where the spores develop and reproduce, eventually killing the host (Ebert, Reference Ebert2005). Upon host death, the spores of the next generation are released into the environment, where they can be ingested by new hosts to complete the infection cycle (Ebert, Reference Ebert2005). Previous work shows that D. dentifera and M. bicuspidata are likely to encounter each other during autumnal epidemics: infection prevalence tends to increase when temperatures cool from 25°C in the late summer, with infection prevalence peaking at 20°C, and decreasing as temperatures drop during winter (Shocket et al., Reference Shocket, Vergara, Sickbert, Walsman, Strauss, Hite, Duffy, Cáceres and Hall2018). Moreover, it is likely that spores overwinter in the sediment, with some of these becoming resuspended and infecting new hosts in future years (Ebert, Reference Ebert1995; Decaestecker et al., Reference Decaestecker, de Meester and Ebert2002); this means that spores produced at the end of one season (in colder conditions) might infect a host at warmer temperatures in a subsequent year. Thus, we would expect that D. dentifera can be exposed to M. bicuspidata spores derived from warmer or cooler thermal conditions. If transgenerational plasticity exists, we predict that parasites reared at different temperatures should perform differently; if such transgenerational effects are adaptive, we predict that offspring will perform better when their temperature matches that of their parent. In contrast, if transgenerational plasticity is not important, only the current temperatures, rather than rearing temperatures, should influence parasite performance. Prior studies have shown that rearing environments (namely, host genotype and temperature) can influence the infectivity of M. bicuspidata spores (Searle et al., Reference Searle, Ochs, Cáceres, Chiang, Gerardo, Hall and Duffy2015; Shocket et al., Reference Shocket, Vergara, Sickbert, Walsman, Strauss, Hite, Duffy, Cáceres and Hall2018); this shows plasticity of parasites but not transgenerational plasticity, given that the spores from the rearing conditions are the same ones that infect the new hosts. Here, we tested for transgenerational plasticity impacting parasite growth and virulence.
Materials and methods
Experimental design
We conducted a fully factorial experiment by exposing D. dentifera hosts to M. bicuspidata spores: the spores were reared either at cooler (20°C) or warmer temperature (24°C) and then used factorially for exposures at 20 or 24°C. The 4°C elevation of temperatures that we studied is in line with the predicted climate change scenario by the end of this century (Beits et al., Reference Beits, Collins, Hemming, Jones, Lowe and Sanderson2011). It is also well within the range of changes in temperature in the upper mixed layer of lakes that occur during epidemics (Shocket et al., Reference Shocket, Vergara, Sickbert, Walsman, Strauss, Hite, Duffy, Cáceres and Hall2018). In total, this resulted in 4 treatment combinations of rearing and current temperatures, with 40 replicates per treatment. Laboratory stocks of D. dentifera and M. bicuspidata originated from lakes in Barry County in Michigan, USA.
As infections will occur at the ambient temperature, and because hosts will have been reared at that temperature, we reared hosts for this experiment at the 2 focal temperatures. More specifically, we reared D. dentifera at 20 or 24°C in only 2 separate incubators (I-41VL, Percival Scientific, Perry, IA, USA) for 2 generations on a 16 : 8 photoperiod; this means that temperature treatment is confounded with the incubator (as is common in this type of experiment due to logistic constraints). We then collected neonates aged 1–2 days old. Each neonate was maintained individually in a 50 mL beaker filled with 50 mL filtered lake water and was fed thrice a week with phytoplankton food (Ankistrodesmus falcatus, 20 000 cells mL−1).
To create sources of M. bicuspidata spores from different temperatures, we infected D. dentifera individually by adding spores at a density of 145 spores mL−1 at 20 and 24°C. We collected the dead hosts upon natural death, stored them individually in a 1.5 mL tube filled with 100 μL deionized water, and placed the tubes in a refrigerator before use (M. bicuspidata spores die when placed in a freezer; Duffy and Hunsberger, Reference Duffy and Hunsberger2019). All spores used for the experiment were derived from hosts that had died 1–2 months prior to use in infections; these infected hosts were the product of an earlier experiment (Sun et al., Reference Sun, Dziuba, Jaye and Duffy2022). Infected hosts were ground individually using electric pestles for 60 s before we infected new hosts with a well-mixed solution of spores (145 spores mL−1) from each tube at 20 and 24°C. We used a degree-day approach (Vale et al., Reference Vale, Stjernman and Little2008; Manzi et al., Reference Manzi, Agha, Lu, Ben-Ami and Wolinska2020) by adding spores to Daphnia at ages of 6 and 5 days, for 20 and 24°C, respectively, resulting in a 120 degree-day. At exposure, we fed all animals with 20 000 cells mL−1 A. falcatus and maintained them at a 16 : 8 light : dark cycle. Thereafter, all animals were fed with A. falcatus (20 000 cells mL−1) thrice a week. We terminated the experiments when all animals had died. This experiment was conducted in March 2022.
Data collection
To determine virulence, we checked all animals daily for survival and counted the offspring produced, which were then removed from the beakers. We determined the lifetime fecundity of hosts (the total number of offspring) and host lifespan; both lifespan and fecundity tend to be reduced by M. bicuspidata infections (Clay et al., Reference Clay, Dhir, Rudolf and Duffy2019). The dead animals were stored individually in a 1.5 mL tube filled with 100 μL deionized water, and were placed in a refrigerator for subsequent measurement of spore yield, a key component of parasite fitness. Because hosts contain all of the parasite spores that were produced over the course of infection at host death, we quantified spore yield, i.e. the number of spores per host, by grinding dead infected hosts with an electric pellet pestle (Fisher Scientific, Hampton, NH, USA catalogue no.: 12-141-361) for 60 s. A sample (10 μL) of this solution was added to a Neubauer haemocytometer, and we estimated the spore yield by averaging the number of mature spores and total spores (mature + immature spores) from 4 grids. Mature spores can be visually distinguished from immature ones (Ebert, Reference Ebert2005; Stewart Merrill et al., Reference Stewart Merrill, Hall, Merrill and Cáceres2019); mature ascospores were characterized by their needle shape, each containing a dark band in the centre, whereas conidia and needle-shaped spores lacking the central bands and sharp edge were considered immature (see Fig. S1 for examples).
Daphnia that died within 7 days after exposure to parasites were excluded because of early mortality. Any male Daphnia that were misidentified as females in the beginning of the experiment were also excluded. This resulted in a total of 141 individuals (current temperature of 20°C: n = 37 and 37 for rearing temperature of 20 and 24°C, respectively; current temperature of 24°C: n = 35 and 32 for rearing temperature of 20 and 24°C, respectively).
Statistical analyses
To test for the effects of rearing and current temperature on host fitness, we included rearing temperature (20 or 24°C) and current temperature (20 or 24°C) and their interaction as fixed factors. We analysed the lifetime fecundity using a generalized linear mixed model (GLMM) with a Poisson distribution, and analysed the survival with a Cox proportional hazard mixed effect model. In both analyses, the parental source of the spores (i.e. the identity of infected host individual) was included as a random effect. We included this random effect because spores were derived from different host individuals; spores from a single individual were used to infect pairs of new hosts (one at 20°C and one at 24°C), with spores from a single individual used to infect 1–3 pairs of hosts.
We analysed the effects of rearing and current temperatures on parasite fitness in a similar fashion by including rearing and current temperatures and their interaction as fixed factors. We analysed the number of mature spores and total number of spores using GLMMs with a Gaussian distribution; we calculated the natural log of the number of spores plus 1 prior to analyses to meet the requirements of normality for regressions. We analysed the probability of infection and the proportion of spore maturation (the number of mature spores divided by the total number of spores) using GLMMs with a binomial distribution and logit link function. Similarly, the parental source of the spores was included as a random effect.
GLMMs were conducted with the glmer function in the lme4 package (Bates et al., Reference Bates, Maechler, Bolker and Walker2015), whereas the Cox proportional hazard mixed effect model was conducted in the coxme package (Therneau, Reference Therneau2012). We started the analyses by including the interaction terms, with non-significant interactions removed from the models. If significant interaction terms were detected, pairwise post-hoc comparisons were made to assess differences between individual treatments in the emmeans package (Lenth, Reference Lenth2021). All analyses were conducted in R version 4.1.2 (R Development Core Team, 2014).
Results
There was no effect of rearing temperature but a marginally significant effect of current temperature on infection prevalence: infections carried out at warmer temperatures led to a marginally lower infection prevalence than cooler temperatures (χ 2 = 3.29, P = 0.070), regardless of whether the spores were reared at cooler or warmer temperature (Fig. 1A; χ 2 = 0.13, P = 0.721). Among 141 D. dentifera exposed to M. bicuspidata spores, 82.4% (61 out of 74) and 70.1% (47 out of 67) developed terminal infection at current cooler and warmer temperatures, respectively.

Fig. 1. Parasite fitness was affected by both rearing and current temperatures, indicating transgenerational effects of temperature on parasites. Infection prevalence (A) was not as strongly impacted by temperature as was parasite fitness, measured as the number of total spore yield (B), proportion of spore maturation (C) and mature spore yield (D). Means and standard error bars are shown. The box plots show median values, the 25th and 75th percentiles and interquartile ranges. Significant (*) and non-significant (ns) differences between treatments are indicated in panel (D).
For hosts that were successfully infected, both current (χ 2 = 9.40, P = 0.002) and rearing (χ 2 = 5.58, P = 0.018) warmer temperatures increased total spore yield (Fig. 1B). Of the spores produced, a smaller proportion showed maturity when current temperatures were warmer (59.2% at 20°C vs 36.3% at 24°C; χ 2 = 33.22, P < 0.001) and when parasites were reared at cooler temperatures (46.5% at 20°C vs 51.9% at 24°C; χ 2 = 6.74, P = 0.009; Fig. 1C). Moreover, current and rearing temperatures interacted in affecting the mature spore yield per host – i.e. the yield of spores that had reached the developmental stage with the potential to initiate a new infection (current temperatures × rearing temperatures: χ 2 = 4.62, P = 0.032; Fig. 1D). Specifically, when infecting hosts at cooler temperatures, parasites that developed at warmer temperatures produced more mature spores compared to those that developed at cooler temperatures (t = −3.30, P = 0.002), yet there was no such difference between spores that developed at cooler vs warmer temperatures when infecting hosts at warmer temperatures (t = −0.14, P = 0.891; Fig. 1D). For parasites that developed at cooler temperatures, spore yield was similar at both cooler and warmer current temperatures (t = −0.30, P = 0.765; compare blue bars in Fig. 1D). However, parasites reared at warmer temperatures produced fewer mature spores at current warmer temperatures compared to current cooler temperatures (t = 2.73, P = 0.008; compare red bars in Fig. 1D).
In contrast to the results for spore yield and maturation, parasite virulence was influenced by current temperature but not by rearing temperature (Fig. 2). There was no effect of the temperature at which parasites were reared on the total number of host offspring (χ 2 = 1.45, d.f. = 1, P = 0.228). Fecundity was only affected by current temperatures, with fewer host offspring produced at warmer temperatures (χ 2 = 10.67, d.f. = 1, P = 0.001; Fig. 2A). Similar patterns were observed in survival probability, such that there was no effect of rearing temperatures (χ 2 = 1.13, d.f. = 1, P = 0.288) but a significant effect of current temperatures – infected individuals were more likely to die early at warmer temperatures (χ 2 = 76.36, d.f. = 1, P < 0.001; Fig. 2B).

Fig. 2. Virulence was affected by current temperature but not rearing temperature. This held both for virulence measured as the total number of offspring (A) and as survival probability (B). The colours indicate temperatures at which parasites were reared. The box plot shows median values, the 25th and 75th percentiles and interquartile ranges.
Discussion
Transgenerational plasticity has the potential to influence organisms' responses to rapidly changing environments, yet whether transgenerational plasticity of parasites exists, and whether it alters parasite fitness and/or virulence, have been overlooked. By investigating the impacts of temperature on both rearing and current environments, we showed that infection prevalence depended mainly on current temperatures, whereas reproductive success of the parasite was determined by the interplay between the (rearing) temperature at which spores developed and the (current) temperature at which the new infections occurred. Infections by parasites that had been reared at warmer temperatures yielded more mature spores, but only at cooler current temperatures. Thus, our findings demonstrate transgenerational plasticity of parasites in response to changing temperatures but does not provide evidence for adaptive ‘priming’ of parasites.
We found that the prevalence of infection was explained by offspring infection temperatures but not by rearing temperatures; this suggests that M. bicuspidata spores were equally viable, irrespective of previous temperatures. This finding is consistent with previous findings (Little et al., Reference Little, Birch, Vale and Tseng2007) on Daphnia magna and their interactions with the bacterial parasite Pasteuria ramosa, one of the best-studied parasites of Daphnia. A prior study found that current temperatures, rather than parasite rearing temperatures, influenced parasite infectivity, with highest infections at 20°C (Vale et al., Reference Vale, Stjernman and Little2008). Moreover, spore production per host was also highest at this temperature (Vale et al., Reference Vale, Stjernman and Little2008). In another study, optimal performance of the microsporidium parasite Ordospora colligata showed similar patterns (Kunze et al., Reference Kunze, Luijckx, Jackson and Donohue2022), peaking at ~20°C and decreasing at both higher and lower ranges of temperature gradient. Our results are also in line with previous work on the Daphnia–Metschnikowia system in that higher current temperatures reduced parasite infectivity (Shocket et al., Reference Shocket, Vergara, Sickbert, Walsman, Strauss, Hite, Duffy, Cáceres and Hall2018). However, we did not find the same pattern in the effect of rearing temperatures. The earlier study found that higher rearing temperatures increased infectivity (Shocket et al., Reference Shocket, Vergara, Sickbert, Walsman, Strauss, Hite, Duffy, Cáceres and Hall2018), whereas we did not find a significant effect of rearing temperature on infectivity; one possible explanation for this difference is that the earlier study explored rearing temperatures of 15–22°C whereas in our experiment temperature spanned from 20 to 24°C to simulate a shift towards a higher temperature range. Future work considering a wider range of temperatures would help to uncover whether spores reared at intermediate temperatures are most infectious.
Contrary to a previous study which showed that temperature warming can reduce spore production (Vale et al., Reference Vale, Stjernman and Little2008), we found that parasites actually had greater total spore yield (inclusive of both mature and immature spores) in response to warmer current temperatures. Parasites also produced more spores when the spores that began the infection had been reared at warmer temperatures. That is, warming induced parasites to be more productive. However, higher current and rearing temperatures had opposite effects on spore maturation (Fig. 1C): warmer current temperatures substantially reduced spore maturation, but warmer rearing temperatures increased spore maturation. The additive temperature effects of rearing and current temperatures on total spore yield per host, and their contrasting effects on the proportion of spores that matured, mean that warmer rearing temperatures enabled parasites to produce more mature spores than those reared at cooler temperatures, but only in cooler current environments. Yet we found no evidence to suggest that this transgenerational plasticity was adaptive, since the offspring of parasites reared at high temperatures did not have a fitness advantage at current high temperatures (Fig. 1D). These results corroborate previous studies showing the vulnerability of parasites to sustained exposure over generations to high temperatures (Carlson et al., Reference Carlson, Burgio, Dougherty, Phillips, Bueno, Clements, Castaldo, Dallas, Cizauskas, Cumming, Doña, Harris, Jovani, Mironov, Muellerklein, Proctor and Getz2017; Schampera et al., Reference Schampera, Agha, Manzi and Wolinska2022). These findings might explain why seasonal epidemics of M. bicuspidata tend to erupt when lake temperatures gradually cool down in late summer (Shocket et al., Reference Shocket, Vergara, Sickbert, Walsman, Strauss, Hite, Duffy, Cáceres and Hall2018); in these cooling conditions, the transgenerational plasticity we observed would increase parasite fitness.
A potential caveat arises from the fact that the environment a parasite experiences very early in infection can influence parasite fitness (Röder et al., Reference Röder, Rahier and Naisbit2008), and this developmental plasticity could be hard to distinguish from transgenerational plasticity, particularly if the parental conditions influence the likelihood of infection. For example, if more spores successfully infected a host, that might have an impact on the final spore yield, although whether it would lead to more spores (because of a larger initial population size within the host) or fewer (because of resource competition or apparent competition via the immune system) is hard to predict; moreover, given that we know that the parasite shows strongly logistic growth within hosts, differences in initial population size might primarily influence when the parasite reaches its maximal abundance within the host, rather than the final density. While it is difficult to predict how developmental plasticity might impact spore yield, our results related to the proportion of spores that matured do not suggest developmental plasticity. Focusing on the warmer current temperature treatment (where there was a larger, but still non-significant, difference between infection levels for the 2 rearing treatments), spores from the cooler rearing treatment tended towards lower infection levels than those from the warmer rearing treatment (Fig. 1A). However, this lower infection level was associated with a lower proportion of spore maturation (Fig. 1C). We cannot come up with a plausible explanation for why an early infection environment with fewer individuals would reduce spore maturation, so we suspect this result was not driven by developmental plasticity. However, future investigations of transgenerational plasticity in parasites should be designed in a way that allows for disentangling transgenerational effects from developmental plasticity.
While parasite fitness was explained both by the effects of rearing and current temperatures, parasite virulence was exclusively determined by current temperatures. Specifically, parasites were more virulent (i.e. greater reduction in host fecundity and lifespan; Fig. 2) at higher temperatures, when the total number of spores was also higher (i.e. suggesting stronger within-host competition; Fig. 1B). This is in line with previous theoretical work suggesting that parasite virulence should be higher when intraspecific competition is intense (Lively, Reference Lively2006). The absence of an effect of rearing temperatures suggests that parasite transgenerational plasticity had negligible influences on parasite virulence in this system. Instead, parasite virulence was mainly determined by the thermal ecology of host–parasite interactions in current environments, consistent with another recent study (Schampera et al., Reference Schampera, Agha, Manzi and Wolinska2022).
Together, our results highlight the importance of incorporating parasite infection history and the potential for context-dependent transgenerational plasticity. Future research should consider how transgenerational plasticity contributes to parasite adaptation and its consequence on disease transmission, in order to better understand host–parasite interactions in changing environments.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022001056.
Data
The data and code used for this study are available on GitHub (https://github.com/syuanjyunsun/parasite-transgen-exp).
Acknowledgements
We thank members of the Duffy Lab, particularly Kira Monell and Siobhan Calhoun, for logistic support and maintenance of the Daphnia and Metschnikowia cultures. We also thank an anonymous reviewer for very helpful comments on an earlier draft of this manuscript.
Author contributions
S.-J. S., M. K. D. and M. A. D. conceived the study. S.-J. S., M. K. D., K. M. M. and M. A. D. designed the experiments. S.-J. S., M. K. D., K. M. M. and R. N. J. conducted the experiments. S.-J. S. performed data analysis. S.-J. S. wrote the initial draft of the manuscript and all authors contributed to editing.
Financial support
This work was supported by the Gordon and Betty Moore Foundation (GBMF9202; DOI: https://doi.org/10.37807/GBMF9202) and by the US National Science Foundation (DEB-1748729).
Conflict of interest
The authors declare there are no conflicts of interest.