Introduction
Leishmaniasis is a neglected tropical parasitic disease associated with high morbidity and mortality. The World Health Organization (WHO) estimates that 1.3 million new cases occur every year. Brazil is one of the six countries that concentrate over 90% of the people with visceral leishmaniasis (VL), the most serious and fatal form of the disease [1]. According to the Brazilian Ministry of Health, from 2000 to 2016, 398,103 cases of cutaneous leishmaniasis (CL) and 58 300 cases of VL were notified in Brazil [2, 3]. Until 2008, in the south region of Brazil, there were only a few imported cases of human VL reported [4]. However, between 2008 and 2017, 23 autochthonous cases of VL occurred in the State of Rio Grande do Sul, with five deaths [5]. Moreover, between 2009 and 2014, a total of 2251 cases of canine visceral leishmaniasis (CVL) were diagnosed in dogs from cities of Rio Grande do Sul [6]. In Brazil, VL is mainly caused by Leishmania (Leishmania) infantum chagasi [Reference Desjeux7, 8] and domestic dogs are considered the main reservoir for human infection. Leishmania-infected dogs may develop clinical symptoms of the disease, or remain asymptomatic throughout the infection [Reference Ciaramella9, Reference Maia10]. In Brazil, the canine population is estimated at 28 million, including more than 22 million stray dogs [Reference Stevenson11]. However, there is no data about the total number of infected dogs. In order to reduce the dissemination of the disease, one of the strategies is to monitor and control the cases of CVL [Reference Grimaldi12]. Whereas there is no effective treatment that ensures the complete elimination of the parasites, in Brazil the National Program for VL Control adopts the euthanasia of serum reactive canine in its control strategies [13]. This strategy is hardly accepted by the owners of the animals and the studies are controversial in relation to its effectiveness. Therefore, the diagnosis of CVL must be highly accurate, avoiding unnecessary euthanasia [Reference Costa14, Reference Lopes15]. Although a variety of tests including serological, parasitological and molecular methods are available, the diagnosis of CVL is still unsatisfactory [Reference Costa14–Reference Faria and Andrade16]. Serological methods that detect antibodies are the most widely used worldwide. However, their low sensitivity to detect cases with low or absent levels of Leishmania-specific antibodies and their cross-reactivity with other diseases, including Chagas’ disease, babesiosis and ehrlichiosis represent important limitations [Reference Ferreira17, Reference Perez18].
In Brazil, the diagnosis of CVL is carried out following the protocol recommended by the Ministry of Health, which determined the DPP® rapid test, an immunochromatographic assay as the screening test and the enzyme-linked immunosorbent assay (ELISA EIE®) as the confirmatory test [Reference Coura-Vital19]. According to this protocol, only positive samples in the first test should be analysed by ELISA. Considering that in some countries, such as Brazil, the false positive result can lead an uninfected dog to death, and false negative results may cause the maintenance of infected dogs in the population, diagnostic tests must be highly accurate. In the absence of a suitable protocol, a combination of different methods could be a rational way to obtain a more reliable diagnosis [Reference Costa14, Reference Lopes15, Reference Laurenti20]. In this regarding, the Leishmania DNA detection using a conventional polymerase chain reaction (PCR) or real-time PCR has been described as an excellent strategy for a more accurate diagnosis of CVL [Reference Lopes15, Reference Fallah and Khanmohammadi21–Reference Ceccarelli23]. Solcà et al. [Reference Solcà Mda24] showed that the diagnosis of CVL through the amplification of kinetoplast DNA presented the highest rates of sensitivity and specificity in comparison with parasitological and serological methods [Reference Solcà Mda24]. Recently, our group evaluated the prevalence of CVL in the metropolitan area of Porto Alegre using DPP® and ELISA EIE® assays and real-time PCR in samples from asymptomatic dogs and found that there was no agreement between the serological methods, with a prevalence of 4% through real-time PCR [Reference Riboldi25]. Furthermore, some positive samples in the ELISA were not positive in the screening test, reinforcing the need for molecular methods to confirm the infection, especially in non-endemic areas. Based on these issues, this study aimed to evaluate the accuracy of serological tests for CVL in relation to the detection of Leishmania DNA through real-time PCR in samples from symptomatic and asymptomatic dogs from a non-endemic area that was previously assessed by DPP®, ELISA EIE® or both serological tests.
Materials and methods
Clinical samples
Dogs (n = 140) from the non-endemic region in the State of Rio Grande do Sul (municipalities: Canoas, Itaqui, Novo Hamburgo, Portão, Porto Alegre, Santa Cruz do Sul, Santo Antônio da Patrulha, São Borja and São Leopoldo), Southern Brazil. The animals were classified as symptomatic (n = 39) or asymptomatic (n = 101). Symptomatic dogs exhibited clinical signs related to CVL including hyperthermia, nodular reaction, lymphadenopathy, emaciation, anorexia, alopecia, skin and/or ocular lesions, onychogryphosis, epistaxis, polyarthritis, diarrhoea, hepatomegaly, splenomegaly, without direct relation with any other disease [Reference Silva26–Reference Ribeiro28]. All samples included in this study were tested by DPP® and ELISA EIE® [official protocols recommended by the Brazilian Ministry of Health – screening by DPP and confirmation of reactive samples by ELISA] [13], followed by DNA extractions and real-time PCR method to detect the presence of Leishmania sp. DNA.
Ethics
The clinical data and samples from CVL symptomatic dogs, as well as the permission for data use, were provided by the Central Laboratory of the State of Rio Grande do Sul and the study with asymptomatic animals was approved by the Ethics Commission on Animal Use of the Federal University of Health Sciences of Porto Alegre (UFCSPA), under protocol number 118/13.
Laboratory tests
Serological tests
The immunological tests performed were Dual Path Platform (DPP®) and enzyme-linked immunosorbent (ELISA EIE®) (Bio-Manguinhos, FIOCRUZ, Rio de Janeiro, Brazil), distributed by the Ministry of Health (Brazil), and executed as recommended by the manufacturer's instructions at the Centro de Desenvolvimento Científico e Tecnológico and at the Parasitology Laboratory, Laboratório Central do Estado do Rio Grande do Sul, both from Secretaria Estadual da Saúde, Rio Grande do Sul State (SES/RS).
Real-time PCR assay
The DNA was isolated from dog serum samples (200 µl) using the commercial Nucleic Acid and Protein Purification kit (Macherey-Nagel), according to the manufacturer's instructions at the Laboratório de Biologia Molecular from Universidade Luterana do Brasil (ULBRA). The primers 13A (5′-GTG GGG GAG GGG CGT TCT-3′) and 13B (5′-ATT TTA CAC CAA CCC CCA GTT-3′), described by Rodgers et al. [Reference Rodgers, Popper and Wirth29], were used to amplify the DNA. The amplification targeted a 120 pb region of the kDNA minicircles of the genus Leishmania, which is present in multiple copies in a conserved region of the kDNA. The real-time PCR amplification was conducted in a StepOne™ Real Time PCR System (Applied Biosystems) and the amplified products were detected using a SYBR® Green system (Applied Biosystems) as described by Rolim et al. [Reference Rolim30]. The reaction was standardised in a final volume of 20 µl containing 15 µl of the Fast SYBR® Green mastermix, 10 pmol of each primer and 5 µl of the extracted DNA. The amplification conditions were the activation of the enzyme at 95 °C for 20 s, and 40 cycles of denaturation at 95 °C for one second and, annealing/ extension at 61 °C for 20 s. A negative reaction control (PCR mixture containing ultrapure water) was used for each amplification run, with a positive control that consisted of purified Leishmania amazonensis DNA. All samples and controls were run in duplicate. The sample was defined as positive when it had a detectable cycle threshold (C t) and the melting temperature (T m) was the same as for the positive control [Reference Rolim30]. The amplification and dissociation curves were analysed using the StepOne™ equipment software. To access the presence of inhibitors, all samples that tested negative in the PCR were spiked with human DNA and amplified with the β-globin primers PCO3 (5′ACACAACTGTGTTCACTAGC3′) and PCO4 (5′CAACTTCATCCACGTTCACC3′) [Reference Saiki31].
DNA sequencing
The samples with positive results in real-time PCR were amplified by PCR using the same primer set and submitted to DNA sequencing in order to confirm the presence of Leishmania sp. DNA. The sequencing reaction was performed using Big-Dye® Terminator v.3.1 (Applied Biosystems), according to the manufacturer's instructions. The capillary electrophoresis was performed using the ABI3130xl platform (Applied Biosystems). The obtained sequences were edited and analysed with the Lasergene SeqMan software (DNASTAR©, Madison, USA), and the identification of the sequences was performed by comparison with known sequences in GenBank using the BLAST analysis tool (National Center for Biotechnology Information – NCBI).
Statistical analysis
The software IBM SPSS Statistics version 21 was used for the statistical analysis [32], with data presented in frequency and percentage. Chi-square (χ2) test was used to evaluate the association between the diagnostic assays. The agreement between the tests was calculated using Kappa's (κ) coefficient.
Results
The results of the serological and molecular assays for VL of the dogs from the non-endemic area for leishmaniasis are shown in Table 1. Of the 140 samples submitted to serological tests, 60 (42.8%) were reactive in DPP and 59 (42.1%) in ELISA. Only 50 (35.7%) were reactive in both assays (official protocol reactive). Six samples were considered undetermined in ELISA. Of the 140 samples, Leishmania DNA was detected by real-time PCR in 58 (41.4%), in which 39 (67.2%) were also positive in DPP and ELISA, showing moderate agreement between the Brazilian official protocol and the molecular methods (κ = 0.544; CI 95% 0.457–0.630; P < 00 001).
Table 1. Distribution of VL reactive samples of symptomatic and asymptomatic dogs from non-endemic areas according to immunochromatographic, immunoenzimatic and molecular tests

a Six undetermined samples in ELISA were not considered for comparison with real-time PCR.
b Screening by DPP and confirmation of reactive samples by ELISA.
Considering the clinical profile of the dogs (n = 140), 39 were classified as symptomatic (27.9%), and 101 (72.1%) as asymptomatic (Table 1). In the group of symptomatic animals (n = 39), 27 (69.2%) were reactive in DPP and 28 (71.7%) in ELISA. A total of 27 (69.2%) showed reactive results in both assays (Table 1), whereas 20 (74.0%) were also positive in real-time PCR, and seven (25.9%) were negative in this molecular assay (κ = 0.577; CI 95% 0.409–0.744; P < 0001). Twelve samples (30.7%) were non-reactive in both DPP and ELISA, from which 11 (91.6%) were also negative in real-time PCR, and one (8.3%) had a positive result (Table 1). In the group of asymptomatic animals (n = 101), 33 (32.6%) were reactive in DPP and 31 (30.6%) in ELISA (Table 1). A total of 23 (22.7%) were reactive in both assays, whereas 19 (82.6%) were also positive in real-time PCR, and 4 (17.3%) were negative in this molecular assay (κ = 0.490; CI 95% 0.387–0.592; P < 0001). Seventy-eight (77.2%) samples were non-reactive in both DPP and ELISA, from which 60 (76.9%) were also negative by real-time PCR and 18 (23.0%) showed positive results in the molecular assay (Table 1).
Figure 1 shows a comparative performance of serological and molecular assays in symptomatic and asymptomatic dogs from a non-endemic region for VL. In symptomatic animals (N = 39) only one dog non-reactive in both serological assays (right side of the rectangle), presented a positive result in real-time PCR. On the other hand, in the group of asymptomatic animals (N = 101), a total of 20 non-reactive or undetermined samples in serological assays (right side of the rectangle) were considered positive by the molecular method. The real-time PCR was able to detect Leishmania DNA in 21 dogs (15% of the 140) that were not reactive by DPP followed by ELISA [five samples non-reactive by both DPP and ELISA (one symptomatic and four asymptomatics), seven samples reactive only in DPP, seven reactive only in ELISA, and six undetermined in ELISA (of which two were reactive in DPP, all from asymptomatic dogs). Interestingly, from these 21 samples, 20 (95.2%) were from asymptomatic dogs and only one (4.8%) was from a symptomatic animal. Moreover, in the symptomatic dogs, the Leishmania DNA was not amplified in seven samples (17.9%) reactive in both (DPP and ELISA) and one reactive in ELISA (2.6%). In the group of asymptomatic dogs, four (4.0%) were reactive in both (DPP and ELISA), one (1.0%) was reactive in DPP and the parasite's DNA was not detected (Fig. 1). As expected, L. (L.) infantum chagasi was the species involved in canine infections as revealed by DNA sequencing analysis.
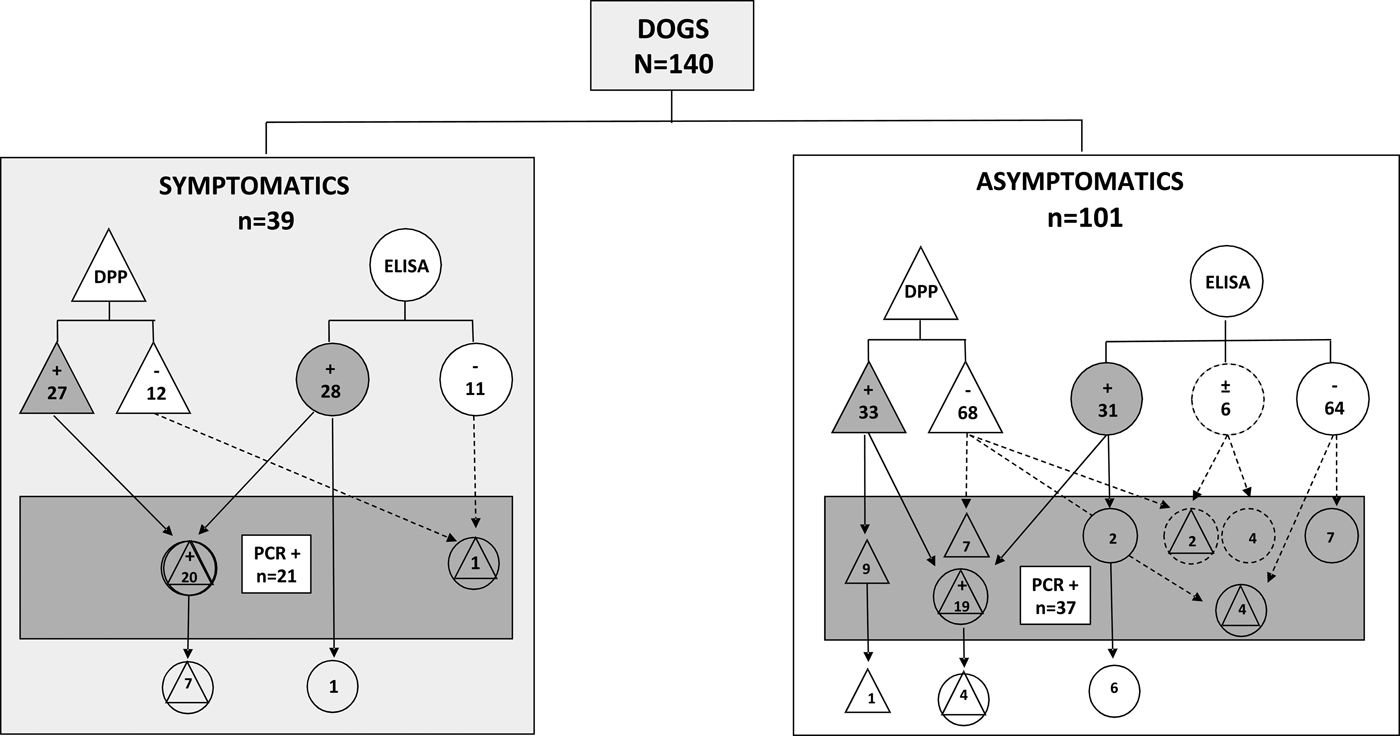
Fig. 1. Distribution of VL reactive samples using serological and molecular methods assessed in serum from symptomatic or asymptomatic dogs from the non-endemic region in Southern Brazil. DPP® assay and their results are represented by triangles. ELISA EIE® assay and their results are represented by circles. Real-time PCR and their results are represented by a rectangle. Results undetermined in ELISA are represented by dotted circles. Reactive results are represented by geometric figures filled in grey.
The homogeneity of the diagnostic (χ2) tests was evaluated according to the clinical profile of the animals (symptomatic or asymptomatic). The test demonstrated that only real-time PCR demonstrated homogeneity (P = 0.096, χ2) in both groups. In contrast, in relation to the serological assays, both DPP and ELISA did not show homogeneity between symptomatic or asymptomatic groups (P < 0.0001, χ2), suggesting a higher diagnostic efficiency in the symptomatic group.
The evaluation of the serological tests for diagnosis of VL in samples of symptomatic and asymptomatic dogs from non-endemic area are presented by values of sensitivity (SE), specificity (SP), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and agreement between tests (κ coefficient) (Table 2). We observed that the sensitivity of serological tests was reduced in the group of asymptomatic animals. The positive likelihood ratio indicated a low to moderate accuracy in both DPP and ELISA in detecting positive dogs in both clinical profile groups. On the other hand, the negative likelihood ratio indicated a low accuracy in asymptomatic dogs in both diagnostic tests, while in symptomatic dogs this rate indicated a great accuracy in both tests (Table 2).
Table 2. Evaluation of immunochromatographic and immunoenzymatic assays for the diagnosis of VL in symptomatic and asymptomatic dogs from a non-endemic region in relation to real-time PCR

SE, Sensitivity; SP, specificity; PLR, Positive likelihood ratio; NLR, Negative likelihood ratio.
a Six undetermined samples in ELISA were not considered for comparison with real-time PCR.
Discussion
In endemic areas for VL, infected dogs are the primary reservoir for zoonotic disease and play an important role in human transmission. According to the World Health Organization, CVL is widespread, with up to 20% of dogs infected in the endemic areas [33]. The state of Rio Grande do Sul, Southern Brazil, is still an area of the low prevalence of leishmaniasis compared with endemic areas in the North of Brazil. However, in the last years, the number of CVL and human VL has increased in Rio Grande do Sul [5, 6]. Moreover, the presence of Lutzomya longipalpis was confirmed in some municipalities of Rio Grande do Sul [5]. Recently our group showed a prevalence of 4% of CVL in the metropolitan area of Porto Alegre using serological assays and real-time PCR as confirmatory [Reference Riboldi25].
Even though serological tests are routinely employed for diagnosing CVL, they have limitations in sensitivity, especially in asymptomatic dogs, and therefore may underestimate the Leishmania infection rates [Reference Grimaldi12]. Despite the high specificity, the serological tests present low capacity to detect Leishmania infection in relation to molecular tests [Reference Lopes15]. Considering the urgency for more accurate tests and the need for comparative data between serological and molecular tests, especially in non-endemic areas for leishmaniasis, this study analysed the presence of Leishmania DNA by real-time PCR in serum from symptomatic and asymptomatic dogs previously assessed by immunochromatographic and ELISA. When we analysed the performance of diagnostic assays using both clinical profiles of symptomatic and asymptomatic dogs (n = 140), we found moderate concordance between serological (DPP and ELISA) and molecular (real-time PCR) tests. Although the real-time PCR presented high homogeneity to diagnose VL in both clinical profiles, the serological tests presented low sensitivity in asymptomatic animals (DPP: 95.2% symptomatic vs. 75.7% in asymptomatic; ELISA: 95.2% symptomatic vs. 65.6% in asymptomatic). The low accuracy found in serological tests (DPP and ELISA) confirms the necessity of better confirmatory laboratory methods, mainly due to the low parasite load and low levels of anti-Leishmania antibody in asymptomatic dogs. Therefore, when the clinical and serological tests are negative, real-time PCR can be used as an alternative [Reference Costa14, Reference Lopes15, Reference Martin-Sanchez34, Reference de Queiroz35]. In the agreement, Grimaldi et al. [Reference Grimaldi12] found that the DPP® rapid test for CVL presented high sensitivity (98%) to identify symptomatic dogs and low sensitivity (47%) to asymptomatic dogs in areas free of VL. However, Laurenti et al. [Reference Laurenti20] showed that in endemic areas the DPP® was able to detect in equal proportions both asymptomatic (92.1%) and symptomatic (89.4%) dogs. These authors also demonstrated that ELISA showed a sensitivity of 89.5% and 91.5% in asymptomatic and symptomatic animals, respectively.
Our data revealed that in the symptomatic dogs, the Leishmania DNA was not amplified in 20.5% of the reactive samples in serological assays against 5% in asymptomatic dogs. It is well known that the false positive results by serological methods may occur due to cross-reactivity, especially with erliquiosis, babesiosis and Chagas’ disease, reducing the accuracy of diagnosis by these methods [Reference Ferreira17, Reference Perez18, Reference Zanette36]. We found that the real-time PCR showed good efficiency to amplify Leishmania DNA in 20 samples negative in immunochromatographic and/or immunoenzymatic assays from asymptomatic dogs, proving the test's efficiency to detect the infection in animals apparently healthy. Francino et al. [Reference Francino37] have described the efficacy of real-time PCR for detecting Leishmania DNA in animals with a low parasite load [Reference Francino37]. In addition, Costa et al. [Reference Costa14] revealed the higher efficacy of real-time PCR in comparison with ELISA to detect cases of Leishmania infection in non-endemic areas [Reference Costa14]. In the agreement, in our study, we found that from a total of 78 samples from asymptomatic dogs non-reactive in both DPP and ELISA, 18 (23.0%) showed positive results in the molecular assay.
The accuracy of serological diagnostic methods depends on several factors, including the protocol performed, the stage of infection at the time of sample collection, and especially the antigen used [Reference Grimaldi12]. Likewise, the accuracy of molecular methods can vary from one technique to another. One strategy to improve the sensitivity of the molecular method is to choose a good target for PCR. The use of kDNA has proven to be efficient by several authors, in view of the number of copies present. Solcà et al. [Reference Solcà Mda24] showed that the real-time PCR using Leishmania kDNA presented the best diagnostic sensitivity for the diagnosis of CVL in endemic areas when compared with parasitological and serological methods [Reference Solcà Mda24].
Conclusion
Visceral diagnosis is still a challenge due to the lack of a sensitive protocol, especially in different prevalence scenarios. This study demonstrated that real-time PCR identified the presence of Leishmania DNA in asymptomatic dogs that had a negative result in serological tests recommended by the official Brazilian protocol for CVL. Taking this into account, our results reinforce that the molecular method is crucial for the confirmation of CVL diagnosis especially in asymptomatic animals from non-endemic regions.
Acknowledgements
The authors would like to thank Fiocruz/Biomanguinhos for the donation of all DPP and ELISA kits. The authors are grateful to the Brazilian agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) for fellowships.
Conflict of interest statement
The authors declare no conflicts of interest.





