Introduction
Recent advances in technology offer a variety of tools for patient imaging immediately before treatment including ultrasound, computed tomography (CT)-on-rails, kilovoltage (kV) cone-beam CT and megavoltage CT (MVCT).Reference Thwaites and Malicki 1 The pre-treatment 3D images are compared with the 3D images obtained previously for planning purpose and the required correction shifts are applied by readjusting the treatment couch position.Reference Dawson and Jaffray 2 This process decreases errors caused by inter-fraction prostate motion with a possibility to reduce the planning target volume (PTV) margin for better sparing of healthy tissues.Reference Adamczyk, Piotrowski and Adamiak 3 , Reference Rivest, Riauka, Murtha and Fallone 4 However, there is an associated cost of increased total time for the treatment, additional radiation dose for imaging and more workload.Reference Ding and Munro 5 Even the most sophisticated immobilisation devices cannot completely eliminate the need for image guidance.Reference White, Yee, Shan, Chung, Man and Cheung 6
Several publications investigated a possibility for reduced image guidance for prostate cancer patients.Reference Kupelian, Lee and Langen 7 – Reference Yeung, Yartsev, Rodrigues and Bauman 9 Kupelian et al. conducted a retrospective study of the residual localisation errors with different imaging scenarios.Reference Kupelian, Lee and Langen 7 The London group proposed to use several initial treatment fractions for establishing the personalised PTV margins for the rest of the treatment.Reference Beldjoudi, Yartsev, Bauman, Battista and van Dyk 8 , Reference Yeung, Yartsev, Rodrigues and Bauman 9
The previous investigations considered two extreme options: either to perform pre-treatment imaging or not. But the image guidance process is performed in two steps: (i) patient positioning on the external marks, CT scan, automatic registration of the planning CT with the just acquired image followed by (ii) an inspection by the radiation therapists of the resulting match and manual correction. The purpose of this study was to evaluate the relative role of the second step and to consider various options for image guidance frequency in relation to body mass index (BMI) of the prostate cancer patients.
Methods
An anonymised database consisting of retrospective data of 6,085 MVCT studies for 216 prostate cancer patients treated on Hi-ART helical tomotherapy units in the London Regional Cancer Program and the Greater Poland Cancer Centre (GPCC) has been created after institutional ethics approvals. The possibility to merge the data for these two centres has been confirmed.Reference Piotrowski, Rodrigues, Bajon and Yartsev 10 In both institutions the patients are asked to empty their bladder and drink 400 mL of water 1 hour before treatment and try to empty their bowels. MVCT image guidance is performed for each patient and each treatment fraction. A conventional double-leg cushion is used in both centres for patient immobilisation.Reference Sexton, Rodrigues, Bauman, Harriman-Duke, Kron and Yartsev 11 Automatic registration of the MVCT studies to the planning kVCT studies by the proprietary software (TomoTherapy Hi-ART version 42) using ‘Bone and Tissue Technique’, ‘Fine Resolution’, ‘Translations Only’ options results in ‘automatic’ shifts values.Reference Boswell, Tomé, Jeraj, Jaradat and Mackie 12 The ‘Bone and Soft Tissues Technique’ with participating voxels of density >0·3 g/cm3 is employed for matching to account for significant prostate gland motion with respect to bony structures.Reference Boswell, Tomé, Jeraj, Jaradat and Mackie 12
The automatic fusion procedure is followed by an inspection of the match by two radiation therapists with position manual shifts (MS) correction if needed. The coincidence of the prostate/rectal wall interface in kVCT and MVCT studies has been used fusion as no fiducial markers were used according to institutional protocols. The sum of automatic and MS for each direction makes the position correction total shifts (TS) as applied clinically. The components of automatic, manual and TS were analysed for mediolateral (x-axis), craniocaudal (y-axis) and anterioposterior (z-axis) directions.
Several options of reduced image guidance procedures have been simulated by the same physicist: (1) MVCT scan, automatic position corrections for each fraction without manual correction—daily automatic correction (DAC) scheme, (2) MVCT scan, automatic and manual position corrections for the first treatment fraction resulting in new external marks on the patient used as a reference position (R1) for the rest of the fractions without imaging guidance—limited image guidance (LIG) scheme based on R1, LIG(R1), (3) and (4) the same but using the average values for shifts obtained in the first three and five fractions to produce new external marks as the references R3 and R5—LIG(R3) and LIG(R5), respectively. Number of the referencing fractions used in this study was established on the basis of the literature.Reference Beldjoudi, Yartsev, Bauman, Battista and van Dyk 8 , Reference Yeung, Yartsev, Rodrigues and Bauman 9 , Reference Broggi, Cozzarini and Fiorino 13
The population-based van Herk marginsReference van Herk, Remeijer, Rasch and Lebesque 14 needed to account for inter-fraction positional error in the remaining treatment were calculated for all simulated image guidance options. Weight and height were collected from the patients’ charts for calculation of the BMI = weight (kg)/height (m2). Correlations between BMI and the positional correction shifts were tested by the Pearson's statistics.Reference Gayen 15 The correlation coefficient (R) was used to evaluate the correlation strength.Reference Cohen 16
The times required for automatic and manual registration components were extracted from the archived patient data treated in GPCC by the in-house software.Reference Piotrowski, Czajka and Bak 17
Results
The average values (−0·7–0·7 mm) and standard deviations (0·4–2·5 mm) of manual corrections were similar in both centres. The distribution of absolute values for manual correction shifts is very similar in two institutions. Larger correction values were detected in anterioposterior (z) direction due to variations in bladder and/or rectal filling.
Table 1 shows the additional (compared with daily IGRT with manual adjustments by radiation therapists) margins required to account for inter-fraction target motion for simulated imaging schedules. The DAC scheme describes the uncertainty introduced by the absence of verification and corrections by the radiation therapists. Reducing the frequency of image guidance procedure leads to significantly larger margins to account for both systematic and random errors in patient position in the remaining treatment fractions.
Table 1 Calculated margins (mm) to account for inter-fraction prostate motion for daily automatic correction (DAC) option and three limited image guidance (LIG) schemes based on one (R1), three (R3) and five (R5) first fractions as a reference

Among 216 patients, 36 were normal, 98 overweight and 82 obese. Table 2 shows the correction shifts for the patients sorted by three BMI rangesReference Keys, Fidanza, Karvonen, Kimura and Taylor 18 —normal (BMI < 25), overweight (25 < BMI < 30) and obese (BMI ≥ 30). Both total and manual correction shifts are generally larger for patients with higher BMI as shown in Figure 1.
Table 2 Percentage of average absolute values <2 mm, between 2 and 4 mm, and >4 mm of total correction shifts in the x, y and z directions for normal (BMI < 25), overweight (25 < BMI < 30) and obese (BMI > 30) patients

Note: Charts present the data for the three referencing options based on the first one (R1), the first three (R3) and the first five (R5) fractions of the treatment.
Abbreviation: BMI, body mass index.

Figure 1 Average absolute values of total registration correction shifts and manual corrections in the x, y and z directions calculated for three reference options (R1), (R3) and (R5) as a function of body mass index (BMI) (kg/m2). From left to right: normal, BMI ≤ 25; overweight, 25 < BMI < 30; obese, BMI ≥ 30.
Abbreviations: TS, total shift; MS, manual shift.
The patients with higher BMI required larger corrections with significant correlations in x and z directions for each referencing scheme (R > 0·3 with p < 0·01 and R > 0·2 with p < 0·03, respectively). The results in Table 1 are collected for all 216 patients without distinction in their BMI values. The impact of the patients’ BMI on the required PTV margins for x and z directions (there was no statistically significant (p > 0·4) shifts/BMI correlation in y direction) is shown in Table 3.
Table 3 Calculated margins (mm) required to account for inter-fraction prostate motion in the x and z directions for normal (BMI < 25), overweight (25 < BMI < 30) and obese (BMI > 30) patients if daily automatic correction (DAC) or limited imaging guidance (LIG) schemes based on the one (R1), three (R3) and five (R5) first fractions as a reference are chosen
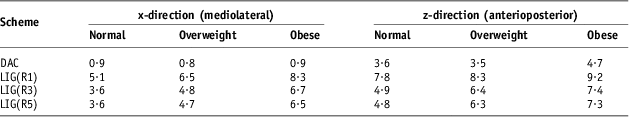
Abbreviation: BMI, body mass index.
Times required for automatic and manual components for position correction for 60 patients treated in GPCC on tomotherapy unit with the prescription dose of 50 Gy in 25 fractions (the remaining dose has been given by brachytherapy according to the institutional protocol) are presented in Figure 2. The average time required for automatic registration procedure (not including MVCT preparation stage that usually takes 2·5 minutes)Reference Piotrowski, Czajka and Bak 17 is below 20 seconds, while inspection of the automatic fusion by the radiation therapists, followed by manual corrections takes between 80 and 230 seconds.

Figure 2 Average values and standard deviations of the times (seconds) required for performing automatic (circles) and manual (dots) registrations for patients grouped by body mass index (BMI) (kg/m2). From left to right: normal, BMI ≤ 25; overweight, 25 < BMI < 30; obese, BMI ≥ 30.
Discussion
We evaluated the role of automatic and manual corrections for patient positioning and simulated different options for image guidance in relation to patients’ BMI. Manual corrections took most of the time in the image-guidance procedure, but the actual correction values were quite small. Manual correction shifts averaged over all fractions and all patients are very close to zero. However, for individual patients, the manual corrections in most cases have the same sign for all fractions. This suggests that some structures (bones, bladder, rectum, etc.) have more impact on automatic matching than the prostate gland. In the commercially available software (TomoTherapy Hi-Art, versions up to 42), all voxels from the total imaged MVCT volume participate in the application of the mutual information algorithm. We propose to modify the fusion software by allowing for selection of a smaller volume for the matching procedure, such as the region around the prostate gland with a reasonable margin. In this case, the automatic registration could be performed faster and with better precision and might eliminate the necessity for manual corrections even for the overweight and obese patients with significant time savings.
In previous publications only total correction shifts were analysed in reduced imaging guidance options.Reference Kupelian, Lee and Langen 7 – Reference Yeung, Yartsev, Rodrigues and Bauman 9 The relative role of automatic and manual corrections is evaluated here for the first time. Manual corrections are on average smaller than the automatic matching results. We calculated the margins needed to correct for inter-fraction prostate motion for different imaging options. Note that these margins are only the components of the total PTV margin that should also include intra-fraction prostate motion, uncertainty of dose distribution calculations and mechanic uncertainties.Reference Bidman, Coffey and Crellin 19 The scenarios with limited number of initial fractions with no image guidance in the remaining fractions: (LIG(R1), LIG(R3), LIG(R5)) were discussed in previous publications.Reference Beldjoudi, Yartsev, Bauman, Battista and van Dyk 8 , Reference Yeung, Yartsev, Rodrigues and Bauman 9 However, these investigationsReference Beldjoudi, Yartsev, Bauman, Battista and van Dyk 8 , Reference Yeung, Yartsev, Rodrigues and Bauman 9 examined the data averaged for all patients without considering their specific features. We have found that including BMI index can provide image guidance options tailored to a particular patient.
We also investigated the possibility (not considered previously) of image guidance option with daily pre-treatment imaging and automatic correction for all treatment fractions (DAC). Limiting the kV/MV CT matching procedure to only automatic correction followed by a quick visual inspection of the result can save considerable time (see Figure 2) for patient with obvious advantages for patient comfort and a real possibility to reduce the in-room time slot. Relatively large standard deviations reflect differences in patient-specific anatomy as well as a confidence level of different radiation therapists. Patients with larger BMI needed more time for manual procedure with a larger uncertainty in the resulting match. The total time for registration tasks summed over all treatment fractions was 2, 6 and 10 minutes for LIG(R1), LIG(R3) and LIG(R5), respectively, because most of the treatment fractions are performed without image guidance. The LIG schemes, in comparison with the DAC for every fraction, dramatically reduce time for total image guidance during treatment, but generally require significantly larger margins (see Table 1).
Relatively strong correlation in the lateral (x) direction with BMI is a result of higher mobility of the belly in overweight and obese patients in this direction. A separation of all patients in the study into three cohorts according to their BMI characteristics allows for calculation of the ‘patient BMI group’-specific margins shown in Table 3 for the lateral and anterioposterior directions. The margins calculated in Table 3 according to the DAC scheme were comparable for different BMI groups while the LIG margins correlated with the patient's BMI values due to larger random errors for heavier patients. Systematic errors were accounted for by using referencing to initial fractions with both automatic and manual corrections.Reference Beldjoudi, Yartsev, Bauman, Battista and van Dyk 8 , Reference Yeung, Yartsev, Rodrigues and Bauman 9 These results demonstrate the importance of taking into account patient-specific features in image guidance for prostate cancer patients. In our opinion, for normal (BMI ≤ 25) patients, the imaging option LIG(R3) is optimal. Usage of the R5 referencing scenario in the LIG scheme does not decrease the margins compared with the R3 option (Table 3) but takes more time while R1 requires large margins with possible detriment for the bladder and/or rectum sparing. Overweight and obese patients will be optimally treated with daily imaging for all fractions followed by automatic correction only with margins from Table 3 for the corresponding group.
Thompson et al. recently investigated the role of patient BMI on intra-fraction prostate motion during fiducial marker image-guided radiotherapy.Reference Thompson, Gill and Thomas 20 Their analysis showed that patients with higher BMI have less intra-fraction displacement of the prostate in superior-inferior dimension compared with the patients with lower BMI. Our results show the opposite tendency for inter-fraction changes and suggest different role of changes in rectal and bladder filling during short radiation treatment and between fractions.
A discussion of preferred option for image-guided radiation therapy involves various techniques and remains a ‘work in progress’. One of the limitations of our study is that effect of dose distribution for the imaging options with different PTV margins for are not evaluated. Ideally, only clinical trials can provide support for the best choice. Given the importance of the delivery of accurate and precise prostate radiotherapy using efficient image-guidance, further investigation into predictive factors for the selection of imaging procedures is warranted.
Conclusions
Several options of the image guidance procedure for prostate cancer patients treated on the helical tomotherapy include performing only automatic corrections or limiting imaging to a couple of initial fractions. BMI was shown to correlate with the magnitude of shifts required for patient setup, especially in the lateral direction. The margins to account for the related uncertainty were calculated for the considered imaging schedules and they may be used clinically depending on the patient BMI. Possible image guidance options have been related to patients’ BMI. Our data show that overweight and obese patients require daily imaging with time saving available by performing automatic kV/MV CT registration only. The patients with normal BMI may be treated with imaging guidance during a few initial treatment fractions.
Acknowledgements
We acknowledge our radiation therapists for sharing their experience.
Financial Support
None.
Conflicts of Interest
None.







