Isoflavones (IFL) are suggested to protect against many chronic diseases including breast, prostate and colorectal cancer, osteoporosis and cardiovascular disorders, as well as menopausal symptomsReference Messina, Persky, Setchell and Barnes1–Reference Uckun, Evans, Forsyth, Waddick, Ahlgren, Chelstrom, Burkhardt, Bolen and Myers7. IFL exposure occurs mainly by the diet through intake of soya products which contain typically a total of 0·01–0·3 % IFL composed of mainly glycosides of genistein (GE), daidzein (DE) and glycitein (GLYE) (Fig. 1) Reference Umphress, Murphy, Franke, Custer and Blitz8–Reference Horn-Ross, Lee, John and Koo11. Strong evidence was provided recently that soya intake protects against breast cancer in adulthoodReference Yan and Spitznagel12 and, particularly, when consumed at early ageReference Shu, Jin, Dai, Wen, Potter, Kushi, Ruan, Gao and Wheng13, Reference Wu, Wan, Hankin, Tseng, Yu and Pike14. Orally administered IFL are believed to be efficiently absorbed by diffusion through the mucosa after conversion of the glycosides to the bioavailable aglycone, which occurs mainly by intestinal bacteriaReference Franke, Custer, Wang and Shi15–Reference Hollman20. Urinary or plasma IFL were found to be reliable biomarkers for soya consumptionReference Seow, Shi, Franke, Hankin, Lee and Yu21–Reference Ritchie, Morton, Deighton, Blake and Cummings27, and urinary appearance of isoflavonoids reflect circulating levels accurately when timing of specimen collection is considered accuratelyReference Franke, Custer and Hundahl16, Reference Franke, Halm, Custer, Tatsumura and Hebshi28.

Fig. 1 Structures and codes of isoflavonoids analysed. (a) Unmetabolised isoflavonoids, (b) metabolised isoflavonoids.
While this is well researched in adults, little is known about the bioavailability of IFL in childrenReference Franke, Custer and Hundahl16–Reference Zubik and Meydani18, Reference Franke and Custer29. Relative to their mothers, urinary IFL excretion rate (UIER) was much lower in infants breast fed from soya-consuming mothers, but higher in babies eating tofuReference Franke, Halm, Custer, Tatsumura and Hebshi28. Urinary analysis revealed that children were more frequently able to convert DE to O-desmethylangolensin (DMA) or equol when they were raised on soya- v. cows' milk-based formula as infants, but this difference was apparent only at very young age and disappeared at age 3–7 yearsReference Hoey, Rowland, Lloyd, Clarke and Wiseman30. In seven up to 4-month-old boys mean total plasma IFL concentrations were 3·7 μmol/l, 20 nmol/l and 16 nmol/l when fed soya-based formula, cows’ milk-based formula, or breast milk, respectively. Urinary IFL concentrations were reported to be lower in these boys than in adults when both were exposed to comparable IFL dosesReference Setchell, Zimmer-Nechemias, Cai and Heubi31, but were not adjusted for body weight (BW). How IFL bioavailability compares between school-aged children and adults, particularly their parents, has so far not been performed in dietary intervention studies. The present study reports on urinary excretion of non-metabolised soya IFL, namely DE, GE and GLYE, and metabolised soya IFL, namely equol, dihydrodaidzein, dihydrogenistein and DMA, to find out whether children experience a higher bioavailability to isoflavonoids when consuming a BW-adjusted dose of soya compared with adults.
Materials and methods
Population
Healthy children between the ages of 3 to 12 years, as well as 17 year olds, and some of their parents were recruited for the present study through cooperation with a local Waldorf school. A total of nineteen adults (sixteen female and three male) and twenty children (twelve female and eight male) completed the study protocol correctly and were included in the analysis (Table 1). Three of the original forty recruited participants were eliminated from the study; two gave an incomplete urine collection and the other had high UIER (>1 nmol/h per kg) at baseline due to soya consumption before starting the study. No participants reported any adverse reactions to soya. Overnight urine (ONU) was collected for 12·13 (sd 0·81) h (Table 1). Subjects were excluded if they had been on antibiotics less than 25 d before participating in the study. Other exclusion criteria were wearing diapers at night, the inability to collect urine for a 12 h overnight period, kidney disease, digestive problems, and an allergy or intolerance to soya. The Waldorf school participants were accustomed to eating soya products, and thus we asked them to avoid, or at least reduce, the consumption of soya on the study day, but only excluded those participants who had a high UIER (>1 nmol/h per kg) at baseline.
Table 1 Participant characteristics
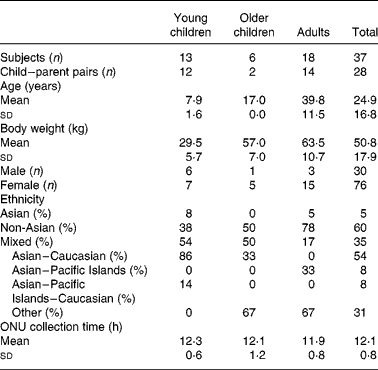
The University of Hawaii Committee on Human Studies approved the study protocol and all consent and assent forms. All parents signed a consent form for themselves when participating and in addition one for their children; participating children 7 years of age and above provided their own additional assent separately.
Study procedures
The participants first learned about the study through word of mouth, then Waldorf school teachers gave them basic information about the study. If the student and/or family were interested, the study staff followed up with details of the study and methods for collecting urine during a visit or phone call to the parents before commencing the study. All participants had to be able to consume soya nuts and to collect an ONU sample. Forty participants received soya nuts in amounts adjusted to their BW (15 g/54·4 kg), supplies for their urine collection, including urine containers with small amounts of boric and ascorbic acid as preservativesReference Franke and Custer29, a worksheet, and a background information questionnaire to complete. Participants were instructed to start the study in the evening by emptying their bladder at approximately 18.00 hours in a container provided to them (baseline urine; BLU) and to consume the soya nuts immediately thereafter. They were asked to collect all following urine voids in large containers until they got up the next morning (ONU). They were also instructed to chill the urine in the coolers provided and to avoid, as much as possible, all other soya products including supplements with soya or IFL from the time they woke up on the study day until the next day. The urine was transported on ice or cool packs to our laboratory where all ONU was mixed and weighed as surrogate for volume determination. Samples of 2 ml BLU and ONU were stored at − 20°C.
On the worksheets, participants recorded their BW and all food consumed the day of the first urine collection, including before and after having eaten the study soya food. They also recorded the time the soya food was consumed (just a few min after providing the spot urine) and the time of the final urine collection in the morning, typically between 05.00 and 07.00 hours. In addition, they reported their current age and ethnicity.
Soya foods used in the study were lightly salted and roasted soya nuts (Revival brand; Physicians Pharmaceuticals, Inc., Kernersville, NC, USA). The IFL composition and content of the nuts was determined by us using HPLC with photodiode array detection without hydrolysis; flavone was used as an internal standard. This method has been validated in a comparison with liquid chromatography-MS-based assaysReference Franke, Hankin, Yu, Maskarinec, Low and Custer32. We checked variations in IFL content caused by crop, manufacturing or other factorsReference Tsukamoto, Shimada, Igita, Kudou, Kokubun, Okubo and Kitamura33 by measuring IFL in each batch received, which resulted in between-batch CV of less than 6 % for total IFL (DE+GE+GLYE+dihydrodaidzein+dihydrogenistein+equol+DMA; Table 2).
Table 2 Isoflavone dose* per 50 kg body weight (BW) in aglycone equivalents†(Mean values and standard deviations)
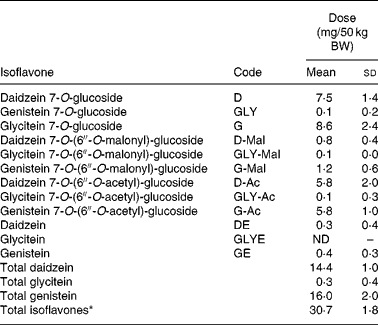
ND, not detected.
* 0·615 (sd 0·036) mg total isoflavones/kg BW equivalent to 0·28 g soya nuts/kg BW or 15 g/120 lbs BW.
† All values obtained by HPLC–photodiode array analysis.
The soya nut serving sizes were controlled for BW to provide an IFL dose of 15 g soya nuts/54·4 kg (120 lbs) which led to a consistent total IFL dose of 0·615 (sd 0·036) mg total IFL/kg BW (mg/kg; Table 2). When the first batch of soya nuts was finished, we started a new batch with IFL composition being very similar (Table 2). Every participant from within each respective age group and from within every family received nuts from the same batch. Thirteen children and thirteen adults ate from the first batch of nuts; six children and five adults ate from the second batch of nuts.
Urinary isoflavonoid analysis
DE, GE, GLYE, equol, dihydrodaidzein, dihydrogenistein and DMA were analysed from urine by HPLC with detection by electrospray ionisation (negative mode) tandem mass spectrometry (ESI-MS)Reference Blair, Appt, Franke and Clarkson34, Reference Franke, Custer, Wilkens, Le Marchand, Nomura, Goodman and Kolonel35. In brief, triply 13C-labelled internal standards of DE, GE, equol and DMA (University of St Andrews, Fife, UK) were added to each specimen hydrolysed with glucuronidase and sulfatase (Roche Applied Sciences, Indianapolis, IN, USA) followed by repeated phase separation with diethyl etherReference Franke, Custer, Wang and Shi15. The combined ether fractions were dried under N2 and redissolved in a 1:1 mixture of acetonitrile–sodium acetate buffer (0·2 m; pH 5). A sample (5–20 μl) of this extract was analysed by LC/ES-MS with a Surveyor TSQ Quantum Ultra triple quadrupole system (ThermoElectron Corp., San Jose, CA, USA) equipped with a Gemini C18 reversed phase column (150 × 2·0 mm; 5 μm) coupled to a Gemini C18 (4·0 × 2.0 mm; 5 μm) direct-connect guard column (Phenomenex, Torrance, CA, USA). The elution, absorbance detection and mass spectrometric measurements were performed as applied previouslyReference Franke, Custer, Wilkens, Le Marchand, Nomura, Goodman and Kolonel35–Reference Adams, Golden, Franke, Potter, Smith and Anthony37. Limits of quantification for all analytes using 1·8 ml urine were 2·5 nm except for dihydrodaidzein and dihydrogenistein (1·5nm) and DMA (5·0 nm). Between-day CV ranged 4–12 % (DE), 5–18 % (GE) and 3–14 % (GLYE).
Urinary creatinine was determined from BLU and ONU with a Roche-Cobas MiraPlus clinical autoanalyser using a kit from Randox Laboratories (Crumlin, Co. Antrim, UK) that is based on a kinetic modification of the Jaffé reaction.
Calculation of hourly urinary isoflavone excretion rate
As previously reported by the present authorsReference Franke, Halm, Custer, Tatsumura and Hebshi38, UIER expressed relative to time (h) is more accurate than expressed relative to creatinine, because in healthy individuals the latter depends mostly on muscle mass, and consequently, largely on BW, sex and ageReference Fomon39, Reference Remer, Neubert and Maser-Gluth40. This is particularly relevant in growing children, not only due to marked changes of muscle mass in absolute terms but also after adjustment for BWReference Remer, Neubert and Maser-Gluth40–Reference Kampmann, Siersbaek-Nielsen, Kristensen and Hansen43. The amount of IFL at baseline present in the ONU collection, although small in all samples, was subtracted from the IFL amount in the ONU sample in order to adjust for background IFL in the ONU sample. Since the time of previous void of BLU was unknown its expression in hourly units was not readily available. Hourly units could, however, be calculated by multiplying the creatinine/h value as available from ONU with the known nmol/creatinine value of BLU separately for each subject. This seemed adequate due to the relatively constant creatinine excretion of healthy individualsReference Remer, Neubert and Maser-Gluth40, Reference Kampmann, Siersbaek-Nielsen, Kristensen and Hansen43. The ONU concentration determined by liquid chromatography–MS (nmol/ml) was multiplied by the weight of the ONU (g) to arrive at absolute amounts (nmol) in the collected urine specimen. This assumed that 1 ml urine weighs approximately 1 g. Although the density of urine is known to be slightly higher than 1, this inaccuracy seems acceptable considering the relatively larger measurement errors connected with urinary volume determinations, and also considering that urine collections per se bear inherent inaccuracies. The absolute amount calculated (nmol) was divided by 12 (for the 12 h of collection) to obtain the hourly excretion rate (nmol/h). This was adjusted if ONU collections deviated from the 12 h urinary collection period (see later). The amount of IFL at baseline contributing to the amount measured in the ONU samples was calculated by applying the known elimination half time of IFL (average T1/2 8 hReference Setchell, Brown, Desai, Zimmer-Nechimias, Wolfe, Jakate, Creutzinger and Heubi17, and using the trapezoid method to calculate area under the curve values in order to arrive at absolute amounts (i.e. mg present in the 12 h period)Reference Franke, Custer and Hundahl16. This value was then subtracted from the measured amount of IFL in the 12 h sample of the ONU collection. If the collection time of the ONU sample deviated from 12 h, adjustment was performed by the known exponential elimination pattern of IFLReference Franke, Custer and Hundahl16, Reference Zubik and Meydani18. For this purpose, we weighted area under the curve of the elimination pattern of each IFL and thereby determined factors by which ONU values would be different for every full hour before and after a 12 h period. The following factors were determined and used to multiply the ONU value for collections of 16, 15, 14, 13, 12, 11, 10, 9 and 8 h: DE (0·825, 0·861, 0·898, 0·945, 1·000, 1·074, 1·16, 1·275 and 1·435); GE (0·871, 0·893, 0·922, 0·955, 1·000, 1·059, 1·132, 1·234 and 1·369); GLYE (0·865, 0·889, 0·915, 0·952, 1·000, 1·072, 1·159, 1·276 and 1·464). Due to the similarity of the factors for the individual IFL, we used averages of these factors of each respective urine collection period for the IFL metabolites. If fractions of hours were collected, then the factors above were considered linearly to the next full hour (closest to 12 h) by interpolation and adjusted as described above. Finally, the 12 h excretion adjusted for differential times of collection and for residual IFL present at baseline was divided by 12 to arrive at the adjusted UIER (nmol/h). This was finally divided by the participant's BW (kg) to give BW-adjusted hourly excretion (nmol/h per kg).
Statistical analysis
Unpaired and paired Student's t tests as well as ANOVA calculations were performed with Excel 2004 for the Macintosh (Microsoft Inc., Redmond, WA, USA). These tests were performed on the original UIER values, as well as on logged values to consider non-normality. Because these tests lead to similar results we present most data on a non-logged basis.
Results
Nineteen of twenty-one children and eighteen of nineteen adults completed the entire protocol correctly. Two children and one adult were excluded due to not collecting the entire 12 h time overnight or having high UIER at baseline. All participants of the present study were given a single BW-adjusted dose of soya nuts (0·28 g nuts/kg BW) which was, according to our HPLC analyses, equivalent to 0·615 (sd 0·036) mg total IFL/kg BW (Table 2). According to the information provided by the participants, all ONU samples considered for the present study were collected within close to the 12 h overnight period, as instructed (Table 1). This was verified by measuring absolute creatinine amount in the ONU specimen. After adjustment of the 12 h IFL amount for the slight deviations from the 12 h collection time, for baseline urinary IFL excretion, and for BW of participants, children, compared with adults, showed a statistically significant higher UIER (P < 0·05 by unpaired t test) for DE (+39 %), GE (+44 %), all non-metabolites (DE+GE+GLYE; +41 %) and total IFL (+32 %) (Fig. 2). When pairs consisting exclusively of children and one of their biological parents (n 15) were compared (mostly their mothers), a similar qualitative and quantitative trend was observed, with higher UIER in children than their parent. This approached significance for all non-metabolites and total IFL (P = 0·06 by paired t test of logged values; data not shown). When we divided the nineteen children into two groups, ages 3–9 years (n 13) and 12–17 years (n 6), we again found that the children had higher UIER than adults, with overall non-significant differences in UIER between the older and younger children. ANOVA analysis revealed that differences in these three groups approached significance for DE (P = 0·09) and all non-metabolites (P = 0·07). For all metabolites (dihydrodaidzein+dihydrogenistein+equol+DMA), we observed lower values in children than in adults (mean of 9 % for all metabolites), but this did not reach significance (P = 0·26 by unpaired t test).

Fig. 2 Isoflavonoid excretion rate of children (□; n 19) and adults (■; n 18) in the overnight urine samples collected for 12 h after consumption of 0·28 g soya nuts/kg body weight. Values are adjusted for baseline excretion and body weight. Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that for adults (P < 0·05; unpaired t test). DE, daidzein; GE, genistein; GLYE, glycitein; DHDE, dihydrodaidzein; DHGE, dihydrogenistein; EQ, equol; DMA, desmethylangolensin; All NM, DE+GE+GLYE; All M, DHDE+DHGE+EQ+DMA; Total IFL, total isoflavonoids (All NM+All M).
Discussion
Although most participants collected the ONU for 12 h (children, 12·03 (sd 0·55) h; adults, 12·13 (sd0·81) h; Table 1) as specified by the protocol, we adjusted for deviations from that period by increasing or decreasing the measured urinary IFL excretion if the period was shorter or longer, respectively. The factors for that adjustment were determined from pharmacokinetic studies on the systemic disappearance of DE, GE and GLYE taking into account a biphasic uptake and exponential elimination patternReference Franke, Custer and Hundahl16, Reference Zubik and Meydani18. The amount of IFL at baseline contributing to the amount measured in the ONU samples was calculated by applying the known elimination half time of IFL (average t {1\over2} 8 hReference Setchell, Brown, Desai, Zimmer-Nechimias, Wolfe, Jakate, Creutzinger and Heubi17, and by using the trapezoid method to calculate area under the curve values in order to arrive at absolute amounts (i.e. mg present in the 12 h period)Reference Franke, Custer and Hundahl16. This amount was then subtracted from the ONU amount in order to arrive at a value that was solely due to the soya nuts consumed for the present study.
The absolute creatinine amount in the ONU specimen was used to determine whether the collections were performed within the declared time periods. The following daily urinary creatinine excretion rates (mg/kg per d) were used for boys and girls, respectivelyReference Remer, Neubert and Maser-Gluth40: age 3 years, 15·2 and 14·5 mg/kg per d; age 4 years, 17·1 and 15·7 mg/kg per d; age 5 years, 17·1 and 15·7 mg/kg per d; age 6 years, 19·3 and 17·9 mg/kg per d; age 7 years, 19·3 and 17·9 mg/kg per d; age 8 years, 19·3 and 17·9 mg/kg per d; age 9–13 years, 20·7 and 18·9 mg/kg per d; age 14–17 years, 23·3 and 20·9 mg/kg per d; for parents, 24·0 mg/kg per d (males) and 22·0 mg/kg per d (females) was usedReference Remer, Neubert and Maser-Gluth40, Reference Kampmann, Siersbaek-Nielsen, Kristensen and Hansen43. We excluded one adult and one child because they were not within an acceptable range of the theoretical age-, sex-, and BW-dependent valuesReference Fomon39, Reference Remer, Neubert and Maser-Gluth40. We found that variations from up to 30 % of that theoretical value could be expected, given the variability in muscle mass, the main determinant of creatinine excretion, as well as renal function, diet, physical exercise, infection, and other factors influencing creatinine excretionReference Remer, Neubert and Maser-Gluth40–Reference Kampmann, Siersbaek-Nielsen, Kristensen and Hansen43. Therefore, we excluded participants with more than 30 % deviation from the theoretical value.
UIER were shown to strongly correlate with circulating IFL levels when the timing of collection was correctly considered and when creatinine-based urinary values were correctly converted to time-based values taking into account BW, sex and ageReference Franke, Custer and Hundahl16, Reference Grace, Taylor and Low24, Reference Ritchie, Morton, Deighton, Blake and Cummings27, Reference Franke, Halm, Custer, Tatsumura and Hebshi28. Thus, urinary IFL analysis can serve as a surrogate for measuring systemic IFL exposure, which avoids invasive blood draws that are particularly difficult to obtain from healthy minorsReference Franke, Halm, Custer, Tatsumura and Hebshi28. Collection of urine over a 24 h period or longer would be ideal, but is often difficult or impossible to perform in human studies for various reasons. In addition, collections of that duration bear inherent risks of missed collections or inclusion of other confounders. A good compromise is the collection of ONU, which is relatively easy to do for participants in the privacy of their homes, resulting in very high complianceReference Franke, Custer, Cerna and Narala9, Reference Maskarinec, Singh, Meng and Franke44. We recommend this particularly for research with children after they reach bladder control, due to the ease for parents to supervise their children in following the protocol.
Consistent with our recent report in infantsReference Franke, Halm, Custer, Tatsumura and Hebshi28 and the previous observation in (pre)pubertal girlsReference Maskarinec, Morimoto, Novotny, Nordt, Stanczyk and Franke45 we discovered in the present study after accurate control of soya and IFL dose, urine collection, and adjustment of confounders that children absorb relatively more IFL than adults. The greater IFL uptake in children could be due to their gut flora that is able to hydrolyse isoflavonoids to the bioavailable aglycone efficiently but does not degrade the aglycones as fast as adults. We proposed this mechanism previously when we observed greater IFL bioavailability after a single high dose of oral antibiotics in a healthy adultReference Franke, Custer and Hundahl16. These findings suggest a higher systemic IFL exposure in children v. adults, particularly when considering that children eat generally much more per kg BW. For example, in Japan where soya consumption is very high, not only relative to Western but also in comparison with other Asian countriesReference Messina, Nagata and Wu46, 2-year-old children consumed almost twice as much soya foods per kg BW compared with adultsReference Nagata, Iwasa, Shiraki, Ueno, Uchiyama, Urata, Sahashi and Shimizu47 (C Nagata, personal communication). The likely high systemic IFL exposures in Asian children may be connected to the low breast and prostate cancer risk in Asian populations (for reviews, see Yan & SpitznagelReference Yan and Spitznagel12, Reference Yan and Spitznagel48) because phyto-oestrogens may act as selective oestrogen receptor modulators which, like steroidal oestrogens, were found to have preventive effects on breast cancer during early periods in life via up regulation of tumour suppressors, increase in breast-cell differentiation and other mechanismsReference Hilakivi-Clarke49–Reference Cabanes, Wang, Olivo, DeAssis, Gustafsson, Khan and Hilakivi-Clarke51. This hypothesis agrees with animal and epidemiological findings of reduced breast cancer risk later in life when soya is consumed during early ageReference Shu, Jin, Dai, Wen, Potter, Kushi, Ruan, Gao and Wheng13, Reference Wu, Wan, Hankin, Tseng, Yu and Pike14, Reference Lamartiniere, Cotroneo, Fritz, Wang, Mentor-Marcel and Elgavish50 and could apply to other beneficial effects of soya consumption. We believe that the IFL exposure to children after soya intake, although higher than in adults, will stay below levels that would give rise to concern regarding adverse effects. Toxic activity is usually observed at much higher IFL levels and adverse effects have not been reported in children of populations with high soya intake.
The present study did not allow for analysis of ethnicity-related influences due to the small numbers in each ethnic group. However, we had fourteen child–parent pairs (about 76 % of our cohort) and thereby tried to keep genetic–ethnic backgrounds similar between the child and the adult group. Analysis of exclusively the fourteen child–parent pairs revealed generally the same outcome as reported for all participants except that significance was not quite reached, probably due to the smaller participant numbers. Similarly, our protocol did not allow for detailed evaluation of IFL metabolites, because the duration of urine sampling after soya exposure was insufficient (only about 12 h) to allow for efficient metabolite formation by the gut bacteria. Longer collection times could have overcome this. However, this was not performed due to reasons described earlier. In our experience, urine collections work best with study participants who collect exclusively overnight, but not for longer periodsReference Franke, Morimoto, Yeh and Maskarinec52, Reference Maskarinec, Oshiro, Morimoto, Hebshi, Novotny and Franke53. Problems in collecting longer than overnight samples, especially collections from children and during the daytime, is hard to achieve, and therefore may result in seriously confounded results mainly due to omissions of collections. It would have also been helpful to include more participants in the study, and ideally from another school since the Waldorf population may not be a representative sample, in particular since many parents reported the family as being habitual soya consumers. However, this population was shown previously to be extremely compliant in soya intervention studies particularly with the involvement of childrenReference Franke, Halm, Custer, Tatsumura and Hebshi38 and avoided therefore many problems usually associated with this kind of trial.
Conclusion
To our knowledge, the present study is the first of its kind to show that not just infantsReference Franke, Halm, Custer, Tatsumura and Hebshi38 but also school-age children take up relatively more IFL than adults. Major strengths of the present study were the consistency of the soya nuts regarding IFL content, the dosing according to BW, the urine collections relative to time with additional adjustment if overnight collections deviated from exactly 12 h, the adjustment to baseline excretion, and the expression of urine excretion relative to BW. In addition, we double-checked completion of the declared collection times by comparison with the theoretical, mainly sex-, BW- and age-dependent creatinine excretionReference Remer, Neubert and Maser-Gluth40, Reference Kesteloot and Joossens41, Reference Kampmann, Siersbaek-Nielsen, Kristensen and Hansen43, Reference Fomon, Haschke, Ziegler and Nelson54. In this way, we were able to obtain accurate data allowing correct comparisons between children and adults which included the identification of non-compliers by biochemical means and the exclusion of those from the analysis. Despite the relatively small number of data in the present study, we obtained robust results considering the insignificant change of all P values when applying logged values.
Acknowledgements
The present study was supported in part by the Revival Company and by the National Cancer Institute (CCSG CA71789). There are no conflicts of interest. We are very grateful for the diligent participation by the children and parents and thank them genuinely for their time and effort. We also appreciate the extraordinary assistance of all study participants, the Honolulu Waldorf School, and the recruitment efforts of Bibiana Potter, Beth Allingham, and Bonnie Ozaki-Jame. Support from the Revival Company for this research is greatly acknowledged. We thank Sandra Hebshi, MS, for assistance in study coordination, Gertraud Maskarinec, MD, PhD, for assistance in statistical evaluations, and Laurie J. Custer, BS, for carrying out liquid chromatography-MS analyses.






