Feeding ruminants a N-reduced diet is preferable for economic and environmental reasons. Dietary crude protein (CP) concentrations of 11−12 % were recommended to meet the requirements of growing goats( 1 ). A reduced dietary N supply was associated with a significant reduction in urinary N excretion( Reference Silanikove 2 ) due to increased expression of renal urea transporters, and thus greater renal urea re-absorption( Reference Starke, Muscher and Hirschhausen 3 ) in goats. In addition, the urea transporting capacity of the ruminal epithelium was increased due to dietary N reduction( Reference Muscher, Schröder and Breves 4 ). By possessing such efficient recycling mechanisms, ruminants such as goats are able to maintain rumen microbes’ N supply, and therefore a sufficient synthesis of microbial protein as the most important source for host protein, provided that energy supply to the rumen flora is also adequate for microbial protein synthesis.
As monogastric species do not have the similar potential to utilise N efficiently, a low-protein diet changes metabolic pathways seriously. A reduction of dietary protein leads to changes in Ca and inorganic phosphate (Pi) homoeostasis in monogastric animals and humans, including decrease in intestinal Ca absorption, reduced urinary Ca excretion and diminished plasma calcitriol and insulin-like growth factor 1 (IGF1) concentrations( Reference Kerstetter, O’Brien and Insogna 5 – Reference Dubois-Ferrière, Brennan and Dayer 8 ).
Despite the N-recycling mechanisms of ruminants, our own previous studies have shown that feeding an N-reduced diet with an adequate Ca supply to young goats resulted in a decrease in plasma calcitriol concentrations( Reference Muscher, Piechotta and Breves 9 ). In addition, the concomitant reduction of dietary N and Ca also resulted in decreased plasma calcitriol concentrations and increased intestinal Ca and Pi absorption associated with decreased expression of the involved transport proteins including the apical transient receptor potential vanilloid channel type 6 (TRPV6), the major intestinal apical Ca channel, and Na+-dependent Pi transporter IIb (SLC34A2, NaPiIIb ), the major intestinal Pi transporter in young goats( Reference Muscher and Huber 10 , Reference Muscher, Wilkens and Mrochen 11 ). In contrast, a solitary dietary Ca reduction stimulated renal calcitriol synthesis and intestinal Ca absorption in adult female goats( Reference Wilkens, Richter and Fraser 12 ). Therefore, it was assumed that calcitriol, as it is known for monogastric animals, is a potent modulator of transcellular intestinal Ca and Pi transport in young goats. In monogastric species, several studies have shown that the expressions of the major intestinal Ca transporting structures such as TRPV6, the intracellular Ca-binding protein D9K ( CaBPD9K ) and the basolateral located plasma membrane Ca2+ ATPase ( PMCA ), as well as the most abundant intestinal Pi transporter NaPiIIb , were regulated by calcitriol( Reference Hattenhauer, Traebert and Murer 13 , Reference Van Cromphaut, Dewerchin and Hoenderop 14 ). Based on the known decrease in plasma calcitriol concentrations in response to an N-reduced diet in young goats, it was hypothesised that a dietary N reduction with a sufficient Ca supply modulates intestinal transcellular Ca and Pi absorption in young goats. Therefore, the aim of the present study was to determine Ca and Pi flux rates of intestinal epithelia in young goats during a single dietary N reduction compared with goats receiving diets sufficient in N and Ca content (control group), diets with reduced Ca content or diets insufficient in N and Ca contents. Therefore, the effects of a reduced dietary N supply on functional mechanisms in caprine intestinal epithelia were characterised. Furthermore, a more detailed characterisation of the modulation of transcellular and paracellular Ca and Pi transport processes in caprine intestinal epithelia was carried out.
In addition, it was assumed that potential changes in intestinal Ca and Pi absorption during dietary N reduction were based on altered expression levels of the described corresponding Ca and Pi-transporting proteins. For this reason, the expressions of TRPV6, CaBPD9K and PMCA, as well of NaPiIIb, in caprine intestinal epithelia were examined. The expression and activity of intestinal basolateral Na+/K+-ATPase, which provides the driving force for Na+-coupled Pi transport, were analysed. The molecular characterisation of an additional intestinal Na+-dependent Pi transporter 1 ( SLC20A1 , PiT1 )( Reference Giral, Caldas and Sutherland 15 ) and the determination of expressions of potential modulators of intestinal Ca and Pi transport, nuclear vitamin D receptor ( VDR )( Reference Schröder, Breves and Pfeffer 16 , Reference Muscher, Hattendorf and Pfeffer 17 ), Ca-sensing receptor ( CaR )( Reference Elfers, Breves and Muscher-Banse 18 ) and insulin-like growth factor 1 receptor ( IGF1-R )( Reference Fatayerji, Mawer and Eastell 19 ), were carried out in goats’ intestinal epithelia, representing further structures that might be affected by the dietary interventions.
Interestingly, in rats, a low-Ca diet induced a stimulation of active transcellular Ca absorption in the ileum( Reference Auchère, Tardivel and Gounelle 20 , Reference Nellans and Kimberg 21 ), which was thought to be incapable of active, vitamin D-dependent Ca transport. Therefore, we hypothesised that a reduction in dietary N and/or Ca supply, as applied in the present study, leads to a shift in intestinal Ca and/or Pi absorption sites, besides the fact that their predominant intestinal absorption sites are present in the proximal and mid-jejunum. Therefore, three different localisations of the goat small intestine were examined.
Methods
The protocols of the animal feeding and handling experiments were approved by the Animal Welfare Commissioner of the University of Veterinary Medicine Hannover (Hannover, Germany) and was in line with the German Animal Welfare Law.
Animals and feeding regimens
A total of twenty-six male, coloured, German goats (about 1-week old) were fed a commercial milk replacer for 6 weeks and were offered wheat straw ad libitum during this period. After weaning, all the animals were maintained on a pelleted control diet containing 21 % CP and 1 % Ca for 1 week to adapt them to the pelleted diet. Subsequently, the goats with an initial weight of 16·1 (sem 1·76) kg were allocated into four feeding regimens: (1) receiving a control diet (21 % CP, 1 % Ca), (2) an N-reduced diet (8 % CP, 1 % Ca), (3) a Ca-reduced diet (22 % CP, 0·4 % Ca) and (4) a combined N- and Ca-reduced diet (8 % CP, 0·3 % Ca) for 6–8 weeks. Goats of the same feeding regimen were housed together in groups of six (N–/Ca+ and N+/Ca–) or seven animals (N+/Ca+ and N-/Ca-) with water available ad libitum. The pelleted concentrates were fed 3 times/d, and the amount per animal was 70 g/kg0·75. In addition, the animals received 25 % of the concentrate weight as chopped wheat straw. To estimate the mean intake of nutrients and minerals per animal, all offered and refused feeds were monitored daily. Animals were weighed weekly.
Diets
The feed content of DM, crude ash, crude fibre, crude fat and CP was determined by Weende analysis (proximate analysis), the standard procedure of the Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten( Reference Naumann and Basler 22 ). The amount of acid-detergent fibre and neutral-detergent fibre was measured by a method described by Van Soest et al.( Reference Van Soest, Robertson and Lewis 23 ). The four diets were isoenergetic containing approximately 12·5 MJ metabolisable energy/kg DM. Table 1 shows the components and composition of the diets. To adjust the weight of reduced N diets, Sipernat 22S, a fine particle silica – which cannot be metabolised and which is commonly used as a digestibility marker due to its inert structure( Reference Sarker, Fournier and Boucher 24 ) – was added.
Table 1 Components and composition of wheat straw and pelleted concentrate dietsFootnote *

BDL, below detection level; CP, crude protein; ND, not detected; ME, metabolisable energy.
* Composition expressed as fed (analysed by the Association of German Agricultural Investigation and Research Center).
† Mineral–vitamin mix per kg: 12·1 g Ca; 1·9 g Na; 2·2 g Mg; 400 mg (1 200 000 IU) vitamin A; 0.3 mg (12 000 IU) vitamin D3; 10 g vitamin E, 6335 mg Zn; 3000 mg Mn; 201 mg Co; 201 mg I; 15 mg Se.
‡ Sipernat type 22S (Evonik Industries AG) is a fine particle silica with high oil absorption capacity. It is widely used as a flow regulator, anti-caking and dusting agent especially in the food and feed industry.
Blood and saliva samples
Blood samples (9 ml each) of the goats were collected shortly before slaughtering by puncturing the vena jugularis with both EDTA-coated and lithium heparinate-coated syringes as well as serum syringes (Sarstedt). Plasma was separated by centrifugation (2000 g at room temperature, 15 min). In addition, saliva samples were collected immediately after blood collection with a sponge from the oral cavity after 5 min of incubation according to the method of Boehnke et al. ( Reference Boehnke, Langner and Weissmann 25 ). Plasma, serum and saliva samples were stored at −20°C for subsequent analysis.
Intestinal tissue, ruminal and abomasal fluid samples
With finishing experimental feeding after 6–8 weeks, goats were slaughtered after captive bolt stunning by exsanguination. To avoid circadian effects, slaughtering was performed always at the same time in the morning. For technical reasons (Ussing chamber experiments), one goat/d was killed. To avoid significant time effects, animals from each feeding group were slaughtered in an alternating manner. Samples of the ruminal and abomasal fluid were collected during slaughtering.
Segments of the proximal and mid-jejunum as well as that of the ileum (50 cm each) were removed within 5-min postmortem, beginning 1-m distal from the pylorus, and were rinsed with ice-cold saline (0·9 % NaCl, w/v).
For RNA isolation, preparation of crude membranes, brush-border membranes (BBM) and nuclear extracts, the mucosa of the middle section of each intestinal segment was stripped off and immediately frozen in liquid N2 and stored at −80°C until further preparation.
For the Ussing chamber experiments, intestinal segments were opened along the mesenteric line, rinsed with ice-cold saline (0·9 %, w/v) and were maintained in a glucose-containing Krebs–Henseleit buffer solution aerated with carbogen (95 % O2−5 % CO2) until the epithelia were mounted in the Ussing chambers.
Incubation of epithelial tissues and measurement of intestinal calcium and inorganic phosphate flux rates in Ussing chambers
After serosal and muscle layers had been stripped from the mucosal layer, the intestinal epithelia were mounted between the two halves of the incubation chambers with an exposed serosal area of 1·13 cm2. Thus, the chambers were separated into serosal and mucosal compartments. On both the sides, the intestinal tissue was incubated with 10 ml of a 38°C warm buffer solution that was continuously aerated with carbogen and maintained at pH 7·4. Both the buffers contained (mm) 113·6.NaCl, 5·4 KCl, 1·2 MgCl2.6H2O, 21·0 NaHCO3, 1·2 CaCl.2H2O, 1·2 NaHPO4.2H2O, 1·2 mannitol, 0·01 indomethacin and additionally 10·0 mm glucose and 7·0 mm-HEPES in the case of the serosal buffer and 20·0 mm-HEPES in the case of the mucosal buffer. After an equilibration time of 20 min, about 148 kBq of 45Ca, 32P and (3H)-mannitol (PerkinElmer GmbH) as radio isotopic tracers was added to each chamber to the serosal or mucosal side; (3H)-mannitol was used as a marker of the paracellular transport( Reference Auchère, Tardivel and Gounelle 20 ). At intervals of 15 min, samples were taken and immediately replaced by equal volumes of the respective buffer solution. Radioactivity of the samples was determined using a liquid scintillation counter (Wallac 1410; PerkinElmer GmbH). Unidirectional Ca, Pi and mannitol flux rates from the mucosal to the serosal (Jms) and from the serosal to the mucosal (Jsm) sides were calculated from the rate of tracer appearance on the unlabelled side using standard equations( Reference Schultz and Zalusky 26 ). To determine net flux rates (Jnet), Jsm were subtracted from respective Jms of paired tissues.
In those chambers containing mid-jejunal epithelia, flux measurements were carried out before and after adding 10mm-Na+-arsenate (Sigma-Aldrich) to the mucosal side in order to characterise the transcellular part of the intestinal Pi transport by competitive inhibition of Na+-dependent Pi transport, with the mid-jejunum representing the major intestinal segment of Na+-dependent Pi transport( Reference Schröder and Breves 27 ).
Biochemical determinations
Plasma urea concentrations were measured using a commercial kit (R-Biopharm; inter-assay CV 3·6 %; intra-assay CV 5·8 %). Ionised Ca concentrations were determined in whole blood samples using an ion-sensitive electrode (Chiron Diagnostics GmbH; inter-assay CV 2 %; intra-assay CV 1 %). Concentrations of total Ca and inorganic Pi were measured colorimetrically in plasma, saliva, ruminal and abomasal fluids by standard spectrometric techniques( Reference Sarkar and Chauhan 28 , Reference Kruse-Jarres 29 ) (inter-assay CV 8·2 % (Ca and Pi); intra-assay CV 4·9 % (Ca), 1·7 % (Pi)). Serum calcidiol concentrations were measured using a competitive ELISA kit (Immundiagnostik AG; inter-assay CV<13·2 %; intra-assay CV<10·7 %). Calcitriol concentrations were measured using a commercial radioreceptor assay kit (Immundiagnostik AG; inter-assay CV<20 and <15 % for samples with calcitriol concentrations of 10 and 60 pg/ml, respectively; intra-assay CV<15 and <10 % for these two concentrations). The calcitriol assay had a detection limit of 2 pg/ml. Both calcidiol and calcitriol assays had been used before to determine respective hormone concentrations in goats( Reference Muscher and Huber 10 , Reference Muscher, Hattendorf and Pfeffer 17 , Reference Widiyono, Huber and Failing 30 ). Total plasma IGF1 and serum concentrations of thyroid hormones triiodthyronine (T3) and thyroxine (T4) were analysed in the Clinic for Cattle, Endocrinology Laboratory, University of Veterinary Medicine, Hannover, Germany, by ELISA and competitive chemiluminescence immunoassays, respectively (inter-assay CV 8·5 % (IGF1); intra-assay CV 3·5 % (IGF1), 7·0–13·2 % (T3), 4·4–10·8 % (T4)).
Total RNA isolation and reverse transcription
Total RNA was isolated using the RNeasy Mini-Kit (Qiagen) according to the manufacturer’s protocol. The RNA concentrations were measured by UV absorbance (BioPhotometer plus; Eppendorf AG). The quality and integrity of the extracted RNA were assessed using an RNA 6000 nanoassay for an Agilent 2100 Bioanalyzer (Agilent Technologies).
Using a random hexamere, oligo-dt primers and TaqMan Reverse-Transcription Reagents (Applied Biosystems), 200 ng of isolated RNA was reverse-transcribed for further analysis according to the manufacturer’s protocol.
Intestinal expressions of TRPV6, CaBPD9K , PMCA, NaPiIIb, PiT1, Na+/K+-ATPase, VDR, CaR and IGF1-R mRNA
For quantification of the expressions of glyceraldehyde-3-phosphate dehydrogenase ( GAPDH ), CaR, PMCA and the TRPV6, caprine-specific TaqMan® primers and probes (Table 2) were purchased from TIB MOLBIOL. Reaction mixtures (20 µl) contained TaqMan Universal PCR Master Mix (Applied Biosystems), 300 nm specific primers, 100 nm specific probe and 16 ng reverse-transcribed complementary DNA (cDNA). The PCR product was amplified (50°C, 2 min; 95°C, 10 min; forty cycles of 95°C, 15 s and 60°C, 1 min) and analysed using a real-time PCR cycler (CFX96TM; Bio-Rad). Expressions of CaBPD9K , IGF1-R, Na+/K+-ATPase, NaPiIIb, PiT1 and VDR were determined using SYBR Green® PCR assays. For CaBPD9K , Na+/K+-ATPase and VDR, specific primers (Table 3) were purchased from Life Technologies, and for IGF1-R, NaPiIIb and PiT1 primers were purchased from TIB MOLBIOL. Reaction mixtures (20 µl) contained KAPA SYBR FAST Universal Master Mix (PEQLAB Biotechnologie GmbH), 200 nm specific primers and 16 ng reverse-transcribed cDNA. PCR products were amplified (95°C, 3 min; forty cycles of 95°C, 10 s and 60°C, 30 s) and detected using a real-time PCR cycler (CFX96TM; Bio-Rad). The thermal profile for melt curve determination began with an incubation of 10 min at 55°C with a gradual increase in temperature (0·5°C/10 s). Absolute copy numbers were determined using calibration curves generated with cloned PCR fragment standards( Reference Wilkens, Kunert-Keil and Brinkmeier 31 ). Specificity of the amplicons was verified by sequencing (GATC) and using NCBI Blast (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Expression of genes of interest were normalised to GAPDH as a constant expressed housekeeping gene. The reactions were carried out twice and included no template control.
Table 2 Primers and probes used for TaqMan assays
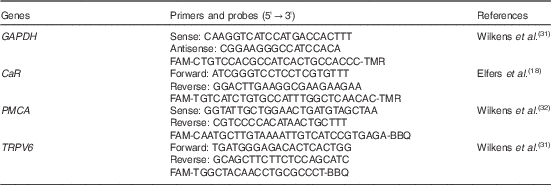
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CaR, Ca-sensing receptor; TRPV6, transient receptor potential vanilloid channel type 6.
Table 3 Primers used for SYBR Green assays

CaBPD9K , Ca-binding protein D9K; IGF1-R, insulin-like growth factor 1 receptor; NaPiIIb, Na+-dependent Pi transporter IIb; PiT1, Na+-dependent Pi transporter 1; VDR, vitamin D receptor.
Intestinal expressions of TRPV6, CaBPD9K , PMCA, NaPiIIb, PiT1, Na+/K+-ATPase, VDR, CaR and IGF1-R protein
The protein expressions of the described transport proteins were exclusively examined in the mid-jejunal epithelia. This was carried out with regard to the results of the Ussing chamber experiments, indicating the mid-jejunum to be the most important intestinal segment for Ca transport. Concerning Pi transport, the mid-jejunum was the segment showing the second highest net flux rates following the ileum in goats, underlying its physiological importance in intestinal Pi transport. Furthermore, our own preliminary experiments have shown protein expressions of TRPV6 and NaPiIIb to be partly below the detection level in the proximal jejunum and ileum.
Mucosa samples of the mid-jejunum were homogenised in an ice-cold homogenisation buffer, and crude membranes as well as cytosolic proteins were isolated as described by Wilkens et al. ( Reference Wilkens, Mrochen and Breves 32 ). For isolating BBM fractions of the intestinal epithelia, a protocol that was previously described by Wilkens et al. ( Reference Wilkens, Kunert-Keil and Brinkmeier 31 ) was used. Nuclear extracts from the caprine mid-jejunum were prepared using a method described by Muscher et al. ( Reference Muscher, Hattendorf and Pfeffer 17 ). Protein concentrations of all the preparations were measured by the Bradford method (Bio-Rad). Immunoblot assays detecting the expressions of CaBPD9K , NaPiIIb, VDR and CaR proteins in the intestinal segments were performed as described elsewhere( Reference Schröder, Goebel and Huber 33 , Reference Huber, Walter and Schröder 34 , Reference Elfers, Breves and Muscher-Banse 18 ). In brief, 15–30 µg of cytosolic preparations (CaBPD9K ), BBM fractions (NaPiIIb), nuclear extracts (VDR) or crude membranes (CaR) were separated by SDS-PAGE (16 % tricine-SDS-gel in the case of CaBPD9K , 8·5 % SDS gel in the case of NaPiIIb, 10 % SDS gel in the case of VDR and CaR) and transferred to nitrocellulose membranes (GE Healthcare) using a tank-blotting system (Bio-Rad). Specificity of the CaBPD9K antibody (Swant; diluted 1:2500 in PBS containing 0·1 % Tween 20 (PBST) and 5 % fat-free milk powder) in goats was validated by cross-reactivity with ovine tissue as reported by the manufacturer. The anti-NaPiIIb (gift from Professor Dr J. Biber, Institute of Physiology, University of Zurich, Switzerland; diluted 1:2000 in PBST) and anti-CaR (Enzo Life Sciences GmbH; diluted 1:250 in PBST containing 1 % bovine serum albumin (BSA; Sigma-Aldrich)) antibodies were successfully pre-incubated with the corresponding antigenic peptide( Reference Huber, Walter and Schröder 34 ) (data not shown). In the case of the VDR antibody (Enzo Life Sciences GmbH; diluted 1:500 in PBST ), alignment with the corresponding recombinant protein verified the specificity of the detected band in caprine intestinal tissue (data not shown). For detecting TRPV6 protein content, BBM fractions (25 µg) were incubated at room temperature in a loading buffer containing 5 mm dithiothreitol (DTT) for 5 min and were separated by 8·5 % SDS-PAGE and transferred to a nitrocellulose membrane. The TRPV6 antibody (Alomone; diluted 1:500 in PBST) was successfully pre-incubated with the corresponding antigenic peptide (data not shown). For abundance of PMCA, 40 µg of crude membrane fractions were separated by 7 % SDS-PAGE and transferred to a nitrocellulose membrane. For Na+/K+-ATPase abundance, 10 µg of crude membranes were heat denatured in a loading buffer containing 5 mm-DTT for 20 min at 70°C and separated by 10 % SDS-PAGE and subsequent transfer onto nitrocellulose membranes. For both antibodies (anti-PMCA (diluted 1:2000 in PBST) and anti-Na+/K+-ATPase (diluted 1:10 000 in PBST) from Enzo Life Sciences), cross-reactivity with ovine tissue was reported by the manufacturer. For detecting PiT1 (anti-PiT1; Thermo Fisher Scientific; diluted 1:500 in PBST) and IGF1-R (anti-IGF1-R; New England Biolabs; diluted 1:500 in Tris-buffered saline (TBS) containing 0·1 % Tween (TBST) and 5 % BSA), 40 µg of BBM fractions (PiT1) or 50 µg of crude membrane fractions (IGF1-R) were incubated in loading dye containing 5 mm-DTT and separated on an 8·5 % SDS-PAGE without previous heating in the case of PiT1 and after heat denaturation (70°C, 20 min) in the case of crude membrane fractions for IGF1-R detection.
Membranes were blocked overnight at 4°C in PBST and 5 % fat-free milk powder. In the case of IGF1-R, PBST was replaced by TBST. Immunodetection of electrotransferred proteins was performed according to standard procedures. After washing with PBST, or with TBST in the case of IGF1-R, and incubating with the corresponding secondary antibody, the bound antibody was visualised using enhanced chemiluminescence (SuperSignal; Thermo Fisher Scientific) according to the manufacturer’s protocol and ChemiDoc system (Bio-Rad).
Quantification of proteins was carried out using Quantity One software 4.4 and Image Lab 5.2.1 software (Bio-Rad). Values of the investigated proteins were normalised to the amount of β-actin (anti-β-actin, AC-15; Sigma-Aldrich) in the case of TRPV6, PMCA, NaPiIIb, PiT1, Na+/K+-ATPase, VDR, CaR and IGF1-R. In case of CaBPD9K , GAPDH (anti-GAPDH; Merck Millipore) as the internal standard with a stable expression level was used.
Measurement of intestinal Na+/K+-ATPase activity
For measuring the activity of the basolateral located Na+/K+-ATPase, 0·5 g of stripped intestinal epithelial tissue from the proximal and mid-jejunum as well as from the ileum was homogenised in 5 ml ice-cold homogenisation buffer containing 20 mm-Tris base, 250 mm-sucrose, 5 mm-sulphuric acid, 5 mm-ethylene glycol tetraacetic acid and 0·8 m-phenylmethanesulphonyl fluoride. After centrifugation (10 min, 600 g , 4°C) and re-suspension of the supernatant, Na+/K+-ATPase activity was measured following the method of Mircheff & Wright( Reference Mircheff and Wright 35 ).
Statistical analysis
All the data are given as means with their standard errors if not stated otherwise and number of animals (n). Data were analysed using GraphPad Prism version 6.05 (GraphPad Software; www.graphpad.com) by two-way ANOVA with Tukey’s multiple comparisons test.
Potential relationships between the measured parameters were analysed by Pearson’s correlation and linear regression. For comparison of Ca and Pi net flux rates, as well as of Na+/K+-ATPase activity in the different intestinal segments, one-way ANOVA was used; P<0·05 was set to be significantly different, and P<0·1 was used to define trends.
Results
Intake, body weight and daily weight gain
The animals were clinically healthy throughout the study. Mean daily DM, concentrate, N, Ca and Pi intakes were estimated from group mean values for each animal. Feed efficiency was calculated as the difference between the final and initial weight divided by the estimated individual feed intake during this time period. The results are summarised in Table 4.
Table 4 Mean daily intakes of DM, concentrate, nitrogen, calcium and inorganic phosphate and feed efficiency of growing goats receiving a nitrogen and/or calcium-reduced diet (Mean values; number of animals)
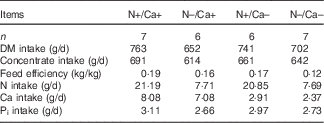
Daily energy supply and Pi supply of all the feeding groups and Ca supply in the (N+/Ca+) and (N–/Ca+) groups covered the recommendations of the Society of Nutrition Physiology (GfE) for young ruminating goat kids( 1 ).
Daily weight gain was not affected by one of the dietary interventions and ranged from 0·15 to 0·18 (sem 0·04) kg/d (Table 5). Goats receiving an N-reduced diet had a significant lower final body weight of about 11 % compared with goats in the (N+/Ca+) and (N+/Ca–) group (Table 5).
Table 5 Effects of a reduced nitrogen and/or calcium diet on initial and final body weight and weight gain of young goats (Mean values with their pooled standard errors; number of animals)
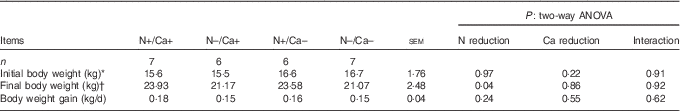
* Initial body weight was determined as the weight at the beginning of experimental feeding at the age of 8 weeks.
† Final body weight was determined as the weight at the time of slaughter at the age of 12–17 weeks.
Blood parameters
The following paragraphs describe the results of the different blood parameters for the different feeding regimens, whereby results are described in detail where differences between the feeding regimens were determined. All the data are summarised in Table 6.
Table 6 Effects of a reduced nitrogen and/or calcium diet on blood parameters of young goats (Mean values with their pooled standard errors; n 5−7 animals)

IGF1, insulin-like growth factor 1; T3, triiodthyronine; T4, thyroxine.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different; Tukey’s multiple comparisons test (P<0·05).
Plasma urea concentrations decreased significantly due to dietary N reduction. Both total and ionised plasma Ca concentrations were significantly reduced in goats fed a reduced N diet and remained unchanged due to dietary Ca reduction. Plasma Pi concentrations were increased when dietary N supply was reduced and remained unaffected by dietary Ca reduction. Plasma concentrations of calcidiol increased (P<0·001), whereas plasma calcitriol concentrations decreased (P=0·04) when dietary N was reduced (Table 6). In contrast, dietary Ca reduction tended to decrease plasma calcidiol concentrations (P=0·06; Table 6) and resulted in a significant increase in plasma calcitriol concentrations (P<0·001; Table 6). Plasma IGF1 concentrations showed a significant decrease in goats fed the N-reduced diet. Serum concentrations of T4 significantly increased in goats fed the N-reduced diet and did not change due to a different dietary Ca supply.
Concentrations of inorganic phosphate and total calcium in saliva, ruminal and abomasal fluids
Salivary Ca concentrations remained unaffected by a reduction of dietary N and Ca, respectively (Table 7), whereas salivary Pi concentrations followed a trend of dropping due to the N-reduced feeding regimen and significantly increased due to dietary Ca reduction (Table 7). Concentrations of Pi and Ca in ruminal fluids remained unaffected by any of the feeding regimens (Table 7). In abomasal fluids, concentrations of Pi and Ca were significantly reduced due to dietary N reduction, whereby the reduction of dietary Ca content and a combined reduction of N and Ca even led to a greater decrease in the Ca concentrations and to an increase in Pi concentrations in abomasal fluids (Table 7).
Table 7 Effects of a reduced nitrogen and/or calcium diet on calcium and inorganic phosphate concentrations in saliva, ruminal and abomasal fluid in young goats (Mean values with their pooled standard errors; n 4–7 animals)
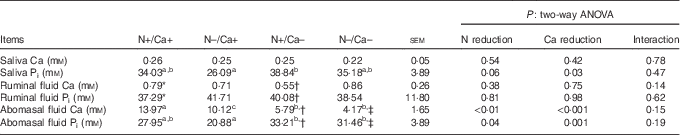
a,b,c Mean values within a row with unlike superscript letters were significantly different; Tukey’s multiple comparisons test (P<0·05).
* For one animal, it was not possible to obtain ruminal fluid at the time point of slaughter (n 6).
† For two animals, it was not possible to obtain ruminal and abomasal fluids at the time of slaughter (n 4).
‡ For one animal, it was not possible to obtain abomasal fluid at the time of slaughter (n 6).
Flux rates of calcium and inorganic phosphate across intestinal epithelia
Net flux rates (Jnet) of Ca were the highest in the mid-jejunum (average of 22·61 nmol/cm2×h), followed by the proximal jejunum (average of 19·08 nmol/cm2×h) and the ileum (average of 2·20 nmol/cm2×h), with significant differences between the proximal jejunum and the ileum (P<0·01; data not shown) as well as between the mid-jejunum and the ileum (P<0·001; data not shown). In all the three intestinal segments investigated, an N-reduced diet led to a significant decrease in Ca Jnet flux rates (Table 8). In the proximal jejunum, the Jms flux rates of Ca remained unaffected by a dietary N reduction, whereas Jsm flux rates were elevated due to the N-reduced feeding regimen, resulting in decreased Jnet flux rates (Table 8). In the mid-jejunum, the decrease in Ca Jnet flux rates was due to significantly lower Ca Jms flux rates and unchanged Ca Jsm flux rates (Table 8). Furthermore, in the mid-jejunum, the reduction in dietary Ca content resulted in an increase in Jms flux rates of Ca without affecting the corresponding Jsm flux rates, leading to an increase in Ca Jnet flux rates (Table 8). In the ileum, due to a significant interaction of N and Ca, the stimulating effect of a Ca-reduced diet on Ca Jnet flux rates seen in the N+/Ca– group, which was the only group showing significant Jnet flux rates of Ca, was no longer detectable in the combined reduced feeding group (N–/Ca–; Table 8). Single dietary N reduction led to (numerically) reduced Ca absorption in this intestinal segment, which was not statistically significant different from the control group (N+/Ca+; Table 8).
Table 8 Calcium, inorganic phosphate and mannitol (Man) flux rates of the different intestinal epithelia of young goats as affected by different dietary nitrogen and calcium supplyFootnote † (Mean values with their pooled standard errors; n 5−7 animals)

a,b Mean values within a row with unlike superscript letters were significantly different; Tukey’s multiple comparisons test (P<0·05).
* Significantly different from zero (one sample t test).
† Jms: mucosal-to-serosal flux rate in nmol/cm2×h; Jsm: serosal-to-mucosal flux rate in nmol/cm2×h; net flux rates in nmol/cm2×h (Jnet=Jms–Jsm).
The Na+-dependent Pi transport was measured in all the three intestinal segments too, and was the highest in the ileum (average of 293·2 nmol/cm2×h), followed by the mid-jejunum (average of 113·5 nmol/cm2×h) and proximal jejunum (average of 17·43 nmol/cm2×h). Net flux rates of Pi were significantly higher in the ileum compared with the proximal and mid-jejunum (P<0·001; data not shown) as well as in the mid-jejunum compared with the proximal jejunum (P<0·01; data not shown). The Jnet Pi flux rates remained unaffected by dietary N reduction in the three intestinal segments investigated, whereas the reduction of dietary Ca content led to a significant decrease in Jnet flux rates of Pi in the proximal (P=0·03) and mid-jejunum (P=0·03) (Table 8). The decrease in Pi net flux rates in the proximal and mid-jejunum was due to a significant decrease in unidirectional Jms flux rates of Pi (P=0·03 and P=0·02), whereas Jsm flux rates were not influenced (Table 8). The addition of Na+-arsenate to the mucosal side led to a 60 % decrease in Jnet Pi in the mid-jejunum, irrespective of the feeding regimen (data not shown).
The applied feeding regimens had no impact on mannitol flux rates in any of the three intestinal segments investigated (Table 8). Significant correlations between Jsm mannitol and Jsm Ca flux rates (Fig. 1(a)–(c)) as well as between Jsm mannitol and Jsm Pi flux rates (Fig. 2(a)–(c)) in all the three intestinal segments investigated are shown by linear regression. A positive correlation could also be shown between Jms flux rates of Ca and mannitol in the ileum (P=0·008, r 0·52; Fig. 1(c)) and between Jms flux rates of Pi and mannitol in the proximal jejunum (P<0·001; r 0·74, Fig. 2(a)). A weak positive correlation was shown for Ca Jms flux rates with corresponding mannitol flux rates in the mid-jejunum (P=0·03; r 0·42, Fig. 1(b)) and for Jms of Pi and mannitol flux rates in the mid-jejunum (P=0·04; r 0·40, Fig. 2(b)). No correlation could be detected between Ca Jms flux rates and corresponding mannitol flux rates in the proximal jejunum (data not shown) or for the Jms of Pi and mannitol flux rates in the ileum (data not shown). In the proximal and mid-jejunum, Jnet flux rates of Pi correlated negatively with calcitriol plasma concentrations (data not shown; P(prox.)=0·01, r −0·50; P (mid)=0·048, r −0·40).

Fig. 1 Linear regression of unidirectional mucosal-to-serosal (Jms; ![]() of (N+/Ca+), (N–/Ca+) and (N–/Ca–); Jms;
of (N+/Ca+), (N–/Ca+) and (N–/Ca–); Jms; ![]() of (N+/Ca–), (Jms;
of (N+/Ca–), (Jms; ![]() ,
, ![]() ), (Jms;
), (Jms; ![]() ,
, ![]() ) or serosal-to-mucosal (Jsm;
) or serosal-to-mucosal (Jsm; ![]() ,
, ![]() ) flux rates of calcium with the corresponding mannitol flux rates in proximal jejunum (a) (Jsm Ca=(0·89±0·14), Jsm mannitol=(8·30±3·79); r
2 0·64, P<0·001), mid-jejunum (b) (Jsm Ca=(1·12±0·06), Jsm mannitol=(2·51±1·42); r
2=0·94, P<0·001; Jms Ca=(0·83±0·37), Jms mannitol=(19·16±15·08); r
2=0·18, P=0·03) and ileum (c) (Jsm Ca=(1·06±0·22), Jsm mannitol=(7·03±3·07); r
2=0·49, P<0·001; Jms Ca=(0·86±0·29), Jms mannitol=(9·40±4·89); r
2=0·27, P=0·008) of goats fed different nitrogen and calcium supply. Calculations are only given when significance was obtained by linear regression. Regression line is only presented when Pearson’s r>0·50.
) flux rates of calcium with the corresponding mannitol flux rates in proximal jejunum (a) (Jsm Ca=(0·89±0·14), Jsm mannitol=(8·30±3·79); r
2 0·64, P<0·001), mid-jejunum (b) (Jsm Ca=(1·12±0·06), Jsm mannitol=(2·51±1·42); r
2=0·94, P<0·001; Jms Ca=(0·83±0·37), Jms mannitol=(19·16±15·08); r
2=0·18, P=0·03) and ileum (c) (Jsm Ca=(1·06±0·22), Jsm mannitol=(7·03±3·07); r
2=0·49, P<0·001; Jms Ca=(0·86±0·29), Jms mannitol=(9·40±4·89); r
2=0·27, P=0·008) of goats fed different nitrogen and calcium supply. Calculations are only given when significance was obtained by linear regression. Regression line is only presented when Pearson’s r>0·50.

Fig. 2 Linear regression of unidirectional mucosal to serosal (Jms; ![]() ,
, ![]() ) or serosal to mucosal (Jsm;
) or serosal to mucosal (Jsm; ![]() ,
, ![]() ) flux rates of inorganic phosphate (Pi) with the corresponding mannitol flux rates in proximal jejunum (a) (Jsm Pi=(0·71±0·08), Jsm mannitol=(2·68±2·26); r
2=0·76, P<0·001), Jms Pi=1·04±0·20), Jms mannitol=(4·17±6·42); r
2=0·55, P<0·001), mid-jejunum (b) (Jsm Pi=(0·98±0·04), Jsm mannitol=−(3·49±0·97); r
2=0·96, P<0·001; Jms Pi=(2·74±1·29), Jms mannitol=(28·09±53·00); r
2=0·16, P=0·04) and ileum (c) (Jsm Pi=(0·76±0·07), Jsm mannitol=−(2·21±0·98); r
2=0·83, P<0·001) of goats fed different nitrogen and calcium supply. Calculations are only given when significance was obtained by linear regression. Regression line is only presented when Pearson’s r>0·50.
) flux rates of inorganic phosphate (Pi) with the corresponding mannitol flux rates in proximal jejunum (a) (Jsm Pi=(0·71±0·08), Jsm mannitol=(2·68±2·26); r
2=0·76, P<0·001), Jms Pi=1·04±0·20), Jms mannitol=(4·17±6·42); r
2=0·55, P<0·001), mid-jejunum (b) (Jsm Pi=(0·98±0·04), Jsm mannitol=−(3·49±0·97); r
2=0·96, P<0·001; Jms Pi=(2·74±1·29), Jms mannitol=(28·09±53·00); r
2=0·16, P=0·04) and ileum (c) (Jsm Pi=(0·76±0·07), Jsm mannitol=−(2·21±0·98); r
2=0·83, P<0·001) of goats fed different nitrogen and calcium supply. Calculations are only given when significance was obtained by linear regression. Regression line is only presented when Pearson’s r>0·50.
Intestinal expressions of TRPV6, CaBPD9K , PMCA, NaPiIIb, PiT1, Na+/K+-ATPase, VDR, CaR and IGF1-R mRNA
The integrity of the isolated RNA of all intestinal epithelia, which were used for quantitative PCR, expressed as RNA integrity number was at least 8·5 (data not shown).
In all the three intestinal segments investigated, the combination of dietary N and Ca reduction (N–/Ca–) led to a lower expression of TRPV6 mRNA in comparison with the N+/Ca– group (Tables 9–11), and therefore withdrew the expression-stimulating effect detected in the N+/Ca– group compared with the N+/Ca+ group. This interaction between the two feeding regimens tended to be significant in the proximal jejunum (Table 9) and was significant in the mid-jejunum and ileum (Tables 10 and 11). Due to this interacting effect, the mRNA expression of TRPV6 was not significantly reduced in the N–/Ca+ group compared with the N+/Ca+ group in the intestinal segments investigated, although numerically TRPV6 mRNA expression was lower in the N–/Ca+ group (Tables 9–11). In contrast, in all the three intestinal segments investigated, animals fed the Ca-reduced diet (N+/Ca–) showed significantly higher expression levels of TRPV6 mRNA compared with the control group (Tables 9–11). For the mRNA expression of intracellular CaBPD9K , a reducing effect of dietary N reduction could be detected in the proximal and mid-jejunum (Tables 9 and 10). In the ileum, an interacting effect of N and Ca reduction was detectable, leading to the withdrawal of the expression-stimulating effect of dietary Ca reduction on CaBPD9K mRNA expression in the combined reduction group (N–/Ca–; Table 11). Expression of the basolateral located PMCA was decreased in the mid-jejunum due to the reduction of dietary N (P=0·05; Table 10), and it tended to increase as a result of dietary Ca reduction (P=0·06; Table 10). There was no detectable effect of the different diets on PMCA expression in the proximal jejunum and ileum (Tables 9 and 11).
Table 9 Relative amounts of CaBPD9K , CaR, IGF1-R, Na+/K+-ATPase, NaPiIIb, PiT1, PMCA, TRPV6 and VDR mRNA expression normalised to GAPDH in the proximal jejunum of goats fed a nitrogen- and/or calcium-reduced diet (Mean values with their pooled standard errors; number of animals)

GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TRPV6, transient receptor potential vanilloid channel type 6; CaBPD9K , Ca-binding protein D9K; NaPiIIb, Na+-dependent Pi transporter IIb; PiT1, Na+-dependent Pi transporter 1; VDR, vitamin D receptor; CaR, Ca-sensing receptor; IGF1-R, insulin-like growth factor 1 receptor.
a,b,c Mean values within a row with unlike superscript letters were significantly different; Tukey’s multiple comparisons test (P<0·05).
Table 10 Relative amounts of CaBPD9K , CaR, IGF1-R, Na+/K+-ATPase, NaPiIIb, PiT1, PMCA, TRPV6 and VDR mRNA expression normalised to GAPDH in the mid-jejunum of goats fed a nitrogen- and/or calcium-reduced diet (Mean values with their pooled standard errors; number of animals)
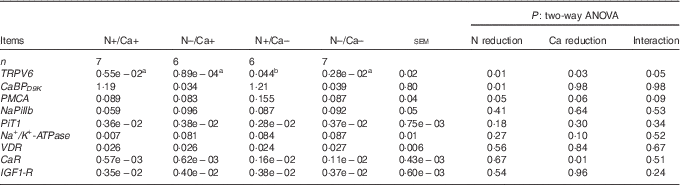
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TRPV6, transient receptor potential vanilloid channel type 6; CaBPD9K , Ca-binding protein D9K; NaPiIIb, Na+-dependent Pi transporter IIb; PiT1, Na+ -dependent Pi transporter 1; VDR, vitamin D receptor; CaR, Ca-sensing receptor; IGF1-R, insulin-like growth factor 1 receptor.
a,b Mean values within a row with unlike superscript letters were significantly different; Tukey’s multiple comparisons test (P<0·05).
Table 11 Relative amounts of CaBPD9K , CaR, IGF1-R, Na+/K+-ATPase, NaPiIIb, PiT1, PMCA, TRPV6 and VDR mRNA expression normalised to GAPDH in the ileum of goats fed a nitrogen- and/or calcium-reduced diet (Mean values with their pooled standard errors; number of animals)
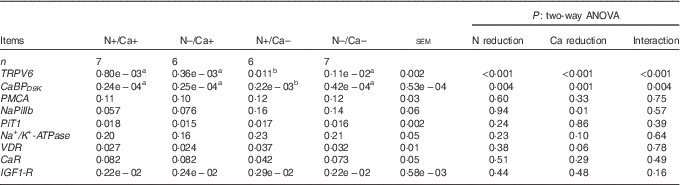
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TRPV6, transient receptor potential vanilloid channel type 6; CaBPD9K , Ca-binding protein D9K; NaPiIIb, Na+-dependent Pi transporter IIb; PiT1, Na+-dependent Pi transporter 1; VDR, vitamin D receptor; CaR, Ca-sensing receptor; IGF1-R, insulin-like growth factor 1 receptor.
a,b Mean values within a row with unlike superscript letters were significantly different; Tukey’s multiple comparisons test (P<0·05).
The mRNA expression of NaPiIIb was unaffected by a dietary reduction of N and/or Ca in the proximal and mid-jejunum (Tables 9 and 10), but increased in the ileum due to dietary Ca reduction (P=0·01; Table 11). A reduction of dietary N and/or Ca had no impact on the mRNA expression of the other potential PiT1 in the intestinal segments investigated (Tables 9–11). Expression patterns of the Na+/K+-ATPase differed between the feeding groups in the ileum, where a Ca-reduced diet tended to increase the mRNA expression (P=0·10; Table 11). In the case of VDR mRNA expression, a stimulating effect could be detected in the ileum due to a dietary Ca reduction (P=0·06; Table 11), but no effect was identifiable in the proximal and mid-jejunum (Tables 9 and 10). The mRNA expression of CaR increased in the proximal and mid-jejunum in the Ca-reduced feeding groups (Tables 9 and 10) but remained unaffected in the ileum (Table 11). No effect of the diets was detectable concerning mRNA expression of the intestinal IGF1-R in the intestinal segments investigated (Tables 9–11).
Intestinal expressions of TRPV6, CaBPD9K , PMCA, NaPiIIb, PiT1, Na+/K+-ATPase, VDR, CaR and IGF1-R protein in the mid-jejunum
Regarding the protein expression of TRPV6, a significant interaction of dietary N and Ca reduction was detectable, whereas N+/Ca– goats showed increased TRPV6 protein expression, demonstrated as a significant effect of dietary Ca reduction, but in combination with a concomitant N reduction (N–/Ca–) this stimulating effect was withdrawn (Fig. 3(a)).

Fig. 3 Semi quantification of (a) transient receptor potential vanilloid channel type 6 (TRPV6), (b) Ca-binding protein D9K (CaBPD9K
), (c) plasma membrane Ca2+ ATPase (PMCA), (d) Na+-dependent Pi transporter IIb (NaPiIIb), (e) Na+-dependent Pi transporter 1 (PiT1), (f) Na+/K+-ATPase, (g) vitamin D receptor (VDR), (h) Ca-sensing receptor (CaR) and (i) insulin-like growth factor 1 receptor (IGF1-R) protein expression in mid-jejunum of goats receiving a dietary nitrogen and/or calcium reduction. Values are means, with their standard errors represented by vertical bars. * Significant effects between the single groups revealed by Tukey’s multiple comparisons test after two-way ANOVA (P<0·05). GAPDH, glyceraldehyde-3-phosphate dehydrogenase (![]() , Ca+;
, Ca+; ![]() , Ca−).
, Ca−).
For protein expressions of both the intracellular CaBPD9K and the basolateral PMCA, a decreased expression could be detected in cytosol preparations (CaBPD9K ) and crude membranes (PMCA), respectively, due to the N-reduced feeding (Fig. 3(b) and (c)).
The reduction of dietary N and Ca led to a lower expression of NaPiIIb in the N–/Ca– group compared with the N+/Ca– and the N–/Ca+ groups (not statistically significant), and therefore to a significant interaction of both N- and Ca-reduced feeding regimens (P=0·01). Individually, dietary N or Ca reduction had no impact on the protein expression of NaPiIIb (Fig. 3(d)). Protein expression of PiT1 was not affected by the diets (Fig. 3(e)). Protein expression of basolateral Na+/K+-ATPase was reduced when dietary Ca content was reduced (Fig. 3(f)), whereas a dietary N reduction had no impact on the protein expression of the Na+/K+-ATPase. Expression of VDR protein was not affected by dietary N and/or Ca reduction (Fig. 3(g)). Protein expression of CaR was significantly reduced in goats fed N-reduced diets (Fig. 3(h)), whereas no effect of dietary Ca could be detected. The IGF1-R protein expression showed no differences between the feeding groups (Fig. 3(i)).
Intestinal Na+/K+-ATPase activity
Activity of the basolateral located Na+/K+-ATPase in the proximal jejunum, mid-jejunum and ileum are presented in Table 12. The Na+/K+-ATPase activity diminished in the mid-jejunum due to dietary N reduction (P=0·02), whereas there was no effect of the diets in the proximal jejunum and the ileum. In principle, the activity of the Na+/K+-ATPase in the proximal and mid-jejunum was significantly higher compared with the activity in the ileum (P<0·001; data not shown).
Table 12 Intestinal Na+/K+ ATPase activity in young goats as affected by different dietary nitrogen and calcium supply (Mean values with their pooled standard errors; n 6−7 animals)

Discussion
The aim of the present study was to determine separately the effects of dietary N and Ca reduction on intestinal Ca and Pi absorption in young goats and the potential overlapping effects of a concomitant reduction of N and Ca, as well as the characterisation of the underlying molecular mechanisms. It has been shown for the first time that a dietary N reduction under adequate dietary Ca supply modulates intestinal Ca absorption in the caprine small intestine.
The limitations of the present study were the group feeding of animals and a reduction of final body weights of the goats fed the N-reduced diets. Nevertheless, energy supply was sufficient for all animals independent of the feeding group according to the recommendations of the GfE. Similar weight gain as well as unaffected plasma T3 concentrations indicated sufficient energy intake of all goats, with T3 representing an energy-dependent hormone, which dropped due to energy deprivation in adult sheep( Reference Todini 36 ). Elevation of plasma T4 concentrations due to dietary N reduction cannot be explained yet, but also indicates sufficient energy supply of the animals( Reference Todini 36 ).
In all the three caprine intestinal segments investigated, with the proximal and mid-jejunum representing the major absorption sites for Ca in small ruminants( Reference Muscher, Wilkens and Mrochen 11 , Reference Schröder, Rittmann and Pfeffer 37 ), Ca net flux rates diminished due to the N-reduced diet. As net flux rates of mannitol, a marker of paracellular transport( Reference Auchère, Tardivel and Gounelle 20 ), remained unchanged due to dietary interventions, the N-reduced diet with or without additional Ca reduction most probably led to a decrease of active transcellular Ca transport in the small intestine. This assumption is also corroborated by the fact that Jms flux rates of Ca did not or only weakly correlate with corresponding mannitol flux rates in the proximal and mid-jejunum (Fig. 1(a) and (b)). This is comparable with the results from studies on rats fed protein-reduced diets, which had a significantly decreased uptake of Ca into intestinal BBM vesicles (BBMV), potentially based on altered expressions of apical Ca channels( Reference Gaffney-Stomberg, Sun and Cucchi 7 ). In contrast, in the N+/Ca– group, active transcellular Ca absorption was stimulated in the mid-jejunum and ileum similar to goats fed the Ca-restricted diet( Reference Wilkens, Richter and Fraser 12 ) and in rats fed a low-Ca diet( Reference Auchère, Tardivel and Gounelle 20 , Reference Nellans and Kimberg 21 ). The fact that in the ileum only Jnet Ca of the N+/Ca– group was significantly different from zero (Table 8) indicated the increase in epithelial transporting capacity, and therefore an extended absorption site for Ca at least in vitro due to this dietary intervention. Plasma Ca concentrations demonstrated that goats fed an adequate N supply were able to compensate for the low Ca intake by increasing absorption efficiency, in contrast to goats fed the N-reduced diet, showing significantly reduced total and ionised plasma Ca concentrations (Table 6), which was seen in young goats fed N- and Ca-reduced diets too( Reference Muscher, Piechotta and Breves 9 , Reference Muscher and Huber 10 ).
In former studies by Muscher et al. ( Reference Muscher, Wilkens and Mrochen 11 ), a simultaneous dietary N and Ca reduction led to an increase in intestinal Ca absorption in the mid-jejunum of young goats. This may have been due to a lower body weight gain, and therefore lower Ca requirement of goats, in the control group in this former study, which was then stimulated by reducing the amounts of Ca and N.
The underlying molecular mechanisms for the changes in intestinal Ca absorption during dietary change could be due to the altered expressions of the involved transporting molecules of the transcellular Ca transport. A reduction of dietary N led to decreased expression of the apical Ca channel TRPV6 revealed by two-way ANOVA and in combination with a reduced dietary Ca supply led to significantly decreased expression compared with solitary Ca reduction, whereas the latter stimulated TRPV6 expression in all the intestinal segments. These findings were in accordance with results from studies with young goats fed a simultaneous N- and Ca-reduced diet, showing reduced TRPV6 expression in mid-jejunum( Reference Muscher, Wilkens and Mrochen 11 ), and adult goats on a Ca-restricted diet having increased expression levels of TRPV6 ( Reference Wilkens, Richter and Fraser 12 ). Expression of the intracellular CaBPD9K diminished in all the intestinal segments due to the reduced dietary N content. The almost missing effect of a dietary Ca reduction on CaBPD9K expression in the anterior parts of the small intestine had been shown in adult goats fed a Ca-restricted diet too( Reference Wilkens, Richter and Fraser 12 ). This, therefore, indicates that CaBPD9K is not the rate-limiting step of transcellular Ca transport or that increased expression of TRPV6 was not associated with an exceeding Ca influx into the enterocyte, requiring an increased intracellular buffer capacity. The expression of the basolateral PMCA was reduced due to N-reduced feeding exclusively in the mid-jejunum, which can partly explain the reduced Ca absorption in this intestinal segment. A single dietary Ca reduction tended to increase PMCA expression in the mid-jejunum, comparable with results of adult goats fed Ca-restricted diets, showing no change in PMCA expression in the jejunum( Reference Wilkens, Richter and Fraser 12 ). It might be that changes in pump activity or an activation of additional extrusion systems, such as the Na+ Ca exchanger 1, which was shown to be expressed in sheep intestinal epithelia( Reference Wilkens, Kunert-Keil and Brinkmeier 31 ), contribute to basolateral Ca extrusion.
All the described changes of expression patterns can be explained by reduced plasma calcitriol concentrations in goats fed N-reduced diets, assuming calcitriol-mediated regulation of expression via the VDR and vitamin D responsive elements (VDRE) similar to monogastric species( Reference Hattenhauer, Traebert and Murer 13 , Reference Meyer, Watanuki and Kim 38 , Reference Darwish and DeLuca 39 ). In addition, changes in the expression of VDR are an important mechanism to modulate the responsiveness of target tissues of calcitriol( Reference Chen, Li and Ye 40 ). Therefore, the slightly (not statistically significant) increased VDR mRNA expression in ileal tissues of N+/Ca– goats in the present study, probably based on increased calcitriol concentrations as reported for ileal tissues of rats after treatment with calcitriol( Reference Khan, Dragt and Porte 41 ), might be one reason for the increased expression of Ca-transporting proteins and the transporting capacity in the ileum in these animals.
Even the concomitant reduction of N and Ca led to a decrease in calcitriol content (not statistically significant) compared with solitary dietary Ca reduction and additionally to increased calcidiol concentrations. This is in line with our former studies( Reference Muscher, Piechotta and Breves 9 – Reference Muscher, Wilkens and Mrochen 11 ). The decrease in plasma calcitriol concentrations in goats fed N-reduced diets could be based on diminished plasma IGF1 concentrations (Table 6). IGF1 is a potential modulator of renal 1α-hydroxylase expression and/or activity( Reference Nesbitt and Drezner 42 , Reference Gómez 43 ), and therefore conversion of calcidiol to calcitriol. By binding to the renal IGF1-R, which had been detected in bovine and ovine renal tissues( Reference Brennan, Gopalakrishnan and Kurlak 44 , Reference Ohashi, Rosen and Smith 45 ), IGF1 increased 1α-hydroxylase activity in a Ca-dependent manner in cell cultures( Reference Menaa, Vrtovsnik and Friedlander 46 ).
A possible link between mineral homoeostasis and protein metabolism is CaR, expressed in the basolateral membrane of the small intestine of monogastric species( Reference Chattopadhyay, Cheng and Rogers 47 , Reference Conigrave, Quinn and Brown 48 ). In this study, expression of CaR mRNA was increased in the proximal and mid-jejunum during dietary Ca reduction, and protein expression in the mid-jejunum was decreased due to dietary N reduction. Thus far it is not known whether the intestinal CaR is able to modulate intestinal Ca absorption as CaR in the kidneys( Reference Topala, Schoeber and Searchfield 49 ). In rats, an up-regulation of CaR could be shown in the parathyroid glands and kidneys through a supraphysiological calcitriol application( Reference Brown, Zhong and Finch 50 ). Furthermore, a VDRE was identified in the promoter region of the human CaR ( Reference Canaff and Hendy 51 ). Assuming the presence of VDRE in caprine CaR too, this could be an explanation for the changes in expression of intestinal CaR in the present study and potential evidence of a modulating effect of CaR on intestinal Ca absorption.
Due to the unchanged IGF1-R expression, it is an unlikely candidate linking protein and Ca metabolism in young goats. More likely, decreased plasma IGF1 concentrations could be associated with decreased intestinal Ca absorption in the goats fed the N-reduced diets in the present study, assuming a positive correlation between these two parameters as it was shown in men( Reference Fatayerji, Mawer and Eastell 19 ) with an unknown underlying molecular mechanism.
Reduced intestinal Jnet Pi flux rates in the proximal and mid-jejunum during dietary Ca reduction were based on a decrease in active transcellular and paracellular Pi absorption. This was verified by a positive correlation between Jms Pi and Jms mannitol in both the intestinal segments (Fig. 2(a) and (b)). The highest Pi absorption, which was mainly transcellular, indicated by the missing correlation between Jms Pi and mannitol (Fig. 2(c)), could be measured in the ileum independently from the feeding regimen, which was shown for adult sheep too( Reference Schröder, Käppner and Failing 52 ). This might be explained by an ileal pH of 8·0, which led to a shift in the equilibrium constant of Pi to a more divalent Pi (HPO4 2–), which is preferably transported by NaPiIIb. In our previous study, Jnet Pi were increased in the mid-jejunum of young goats due to a simultaneous reduction of dietary N and Ca( Reference Muscher, Wilkens and Mrochen 11 ). The discrepancy between these former results and the present data could be based on about 50 % lower dietary P feed content, and therefore lower daily P intake of the goats in the previous study, which might have stimulated intestinal Pi absorption. This was further supported by low but potentially adapted equal plasma Pi concentrations in all goats in the previous study, which may indicate that in the N–/Ca– group the stimulated Pi absorption was a compensatory mechanism to restore previously lower Pi plasma concentrations.
In the present study, goats fed Ca-reduced diets showed no change in plasma Pi concentrations (Table 6), indicating that the remaining intestinal Pi absorption probably was sufficient or compensatory mechanisms such as releasing Pi and Ca from bones were induced (K Elfers, A Liesegang, MR Wilkens, G Breves and AS Muscher-Banse, unpublished results), and therefore high intestinal Pi absorption was not required. Decreased intestinal Pi absorption based on sufficient Pi mobilisation from bone to maintain physiological Pi plasma concentrations has already been shown in goats, whose Ca homoeostasis was challenged by lactation (J Richter, B Schröder and MR Wilkens, unpublished results).
Transcellular Pi transport in the small intestine of goats was mainly mediated by a Na+-dependent Pi co-transport mechanism( Reference Schröder and Breves 27 , Reference Huber, Walter and Schröder 34 , Reference Schröder, Käppner and Failing 52 ), which was confirmed by successful inhibition with Na+-arsenate. Decreased Pi flux rates in the proximal and mid-jejunum during dietary Ca reduction were not based on changes in NaPiIIb expression. An additional likewise Na+-coupled Pi transporter is PiT1, which was expressed in rats’ duodenal and jejunal BBM( Reference Giral, Caldas and Sutherland 15 ) and has been shown for the first time in caprine intestinal epithelia in the present study. The expression of PiT1 was not changed in any intestinal segment investigated, and therefore seemed not to be responsible for reduced Jnet Pi during Ca reduction. It could be speculated that in the proximal and mid-jejunum decreased Pi net flux rates were potentially based on changes in activity of NaPiIIb and/or PiT1. Both NaPiIIb and PiT1 depend on an intracellular-directed Na+-gradient that is generated by the basolateral Na+/K+-ATPase. However, Na+/K+-ATPase mRNA expression remained constant in all the three intestinal segments independently from dietary changes (Tables 9–11), and although Na+/K+-ATPase protein expression was decreased due to dietary Ca reduction in the mid-jejunum (Fig. 3(f)) no effects on activity were observed. Therefore, reduced intestinal Pi absorption in the proximal and mid-jejunum during dietary Ca reduction was probably not based on modulation of Na+/K+-ATPase. In goats maintained on dietary N reduction, Na+/K+-ATPase activity diminished in the mid-jejunum. This was previously shown in rats fed low-protein diets and was supposed to be connected with lower amounts of apical Na+-coupled transport processes( Reference Novotna, Pacha and Heller 53 ). In the present study, no changes in intestinal Pi flux rates during N reduction could be observed, indicating that changes in pump activity did not result in a smaller Na+-gradient, and thus a lower driving force for apical, Na+-coupled transport mechanisms. However, the decreased pump activity might also be explained by a shift to more Na+-independent intestinal amino acid absorption( Reference Munck and Munck 54 ) during dietary N reduction, and therefore a decreased necessity for ATP-driven Na+-extrusion from the cell. This is supported by the fact that during dietary N reduction changes in plasma amino acid composition were observed in young goats (AS Muscher-Banse and K Huber, unpublished results)( Reference Muscher-Banse, Piechotta and Schröder 55 ), potentially indicating altered ruminal microbial protein production, and therefore altered intestinal amino acid composition and uptake.
In contrast to monogastric species, where protein abundance of NaPiIIb was up-regulated by calcitriol( Reference Hattenhauer, Traebert and Murer 13 , Reference Murer, Forster and Biber 56 ), in the anterior parts of the small intestine of goats in this study, NaPiIIb expression was not stimulated by calcitriol, which was in accordance with our previous studies( Reference Huber, Walter and Schröder 34 ). Even more contrary to monogastric species, increased plasma calcitriol concentrations of the goats fed Ca-reduced diets in the present study were associated with reduced intestinal Pi absorption in the proximal and mid-jejunum. This was confirmed by the negative correlation between Jnet Pi and plasma calcitriol concentrations, indicating a regulatory relationship between these parameters. Regarding regulation of PiT1 expression in monogastric species, unchanged expression of PiT1 in the intestine during a Pi-restricted diet and high plasma calcitriol concentrations was shown in rats( Reference Giral, Caldas and Sutherland 15 ). Decreased protein expression of the Na+/K+-ATPase in the mid-jejunum of goats fed Ca-reduced diets could be explained by increased plasma calcitriol concentrations in these animals, as it was shown in murine intestinal epithelia after calcitriol administration( Reference Kutuzova and DeLuca 57 ). Taking into account the involvement of the Na+/K+-ATPase in the formation of tight-junction proteins, an inhibition of the Na+/K+-ATPase might have led to increased epithelial permeability, comparable with studies in cell culture( Reference Rajasekaran, Hu and Gopal 58 ). A greater permeability of the epithelium might have increased paracellular Ca absorption, and therefore the overall amount of intestinal Ca absorption in the goats fed Ca-reduced diets in the present study.
In summary, the results of the present study showed that feeding an N-reduced diet to young goats diminished intestinal transcellular Ca absorption due to reduced expression of Ca-transporting structures. Modulation of expression levels were at least in part based on a decrease in calcitriol plasma concentrations during this feeding regimen. An extension of Ca absorption capacity into the ileum during Ca-reduced feeding could be determined. Effects of dietary N reduction became particularly obvious during a concomitant Ca reduction, which did not stimulate intestinal Ca absorption to a level which would normalise plasma Ca concentrations. Involvement of CaR, VDR as well as IGF1-R seemed not to play a role in mediating the effects of an N-reduced diet or an N- and Ca-reduced diet in young goats. Reduced intestinal Pi absorption during dietary Ca reduction in proximal and mid-jejunum did not affect plasma Pi concentrations and was not based on the altered expression of apical Pi transporters NaPiIIb or PiT1 or modulation of Na+/K+-ATPase.
Therefore, it can be concluded that, although goats are able to recycle N efficiently, and therefore able to cope with a reduced dietary N supply, this dietary intervention, especially in combination with a reduced dietary Ca content, impaired intestinal Ca absorption in a calcitriol-dependent manner leading to decreased plasma Ca concentrations. Therefore, during the life period investigated in the present study, which is characterised by intensive growth, and therefore a special need for Ca and P of the animals, a sufficient dietary N supply has to be ensured. Further investigations are needed to clarify whether, for example, alterations in the binding affinity of CaR or VDR or modulated synthesis of the Vitamin D-binding protein could be involved in the described functional and molecular changes. In addition, Pi uptake into BBMV could give information about modulation of NaPiIIb and/or PiT1 activity. Furthermore, the impact on the paracellular transport of intestinal Ca and Pi absorption has to be considered, and examination of the involved tight-junction proteins could provide an additional explanation for the changes in intestinal Ca absorption during dietary N and/or Ca reduction in young goats.
Acknowledgements
The authors thank B. Schröder, K. H. Südekum, M. Burmester, K. Hustedt, B. Leppich and K. Kiri for their technical assistance and advice. The authors also thank M. Piechotta (Clinic for Cattle, Endocrinology Laboratory, University of Veterinary Medicine Hanover, Germany) for performing the assays of IGFI, T3 and T4 and J. Biber for supplying the NaPiIIb antibody (Institute of Physiology, University of Zurich-Irchel, Zurich, Switzerland). Furthermore, the authors thank Frances Sherwood-Brock for proofreading the manuscript.
The project was supported by the German Research Foundation (DFG; grant number Mu 3585/1-1). The DFG had no role in the design, analysis or writing of this article.
A. S. M.-B. designed the experiments; A. S. M.-B., M. R. W. and K. E. conducted the research; A. S. M.-B., K. E. and M. R. W. analysed the data and K. E. and A. S. M.-B. wrote the paper. All authors discussed the results and commented on them in the manuscript.
There are no conflicts of interest.

















