INTRODUCTION
Streptococcus pneumoniae (Pneumococcus or Pnc) is commonly acquired through close contact. After acquisition, individuals frequently carry the organism asymptomatically in the nasopharynx. Some new carriers develop clinical disease ranging from more common otitis media and pneumonia, to less common meningitis and septicaemia.
Previous studies have highlighted a number of risk factors for Pnc carriage including: exposure in the family [Reference Leino1], number of siblings [Reference Coles2], smoking [Reference Coles2], age (some studies suggest increased risk for infants aged <6 months [Reference Coles2]), presence of older children in the household [Reference Leino1], female gender [Reference Coles2] and day-care attendance [Reference Neto3]. The relationship between Pnc carriage and disease is highlighted by reports of clusters of serious Pnc disease in various closed settings [Reference Tan, Ostrawski and Bresnitz4–6]. Factors associated with clearance of carriage include recent antibiotic consumption [Reference Sanchez5, 7–Reference Schrag9]. However, rapid re-colonization by non-susceptible Pnc isolates following antibiotic treatment has also been reported [Reference Dagan8].
The aim of our study was to identify the important risk factors for acquisition of Pnc carriage in a household setting with small children in the United Kingdom and assess the impact of antibiotic therapy on Pnc carriage in this setting. We used data collected during a previously published observational longitudinal carriage study undertaken in a family setting in the United Kingdom undertaken before the introduction of Pnc conjugate vaccine into the childhood vaccination programme [Reference Hussain10]. A longitudinal design had originally been selected to enable estimation of transmission parameters such as duration of carriage and incidence of infection [Reference Leino1, Reference Coles2, Reference Hussain10–Reference Ekdahl13]. Pnc carriage was measured in children and their families monthly over a 10-month period (October 2001 to July 2002). Risk-factor data for Pnc carriage were measured. These included individual risk factors such as age, sex, day-care attendance (age <5 years) and smoking (both individual and in the household) and household risk factors such as family size and number of siblings. Time-varying risk factors were also measured at each visit by a study nurse: antibiotic use (since last visit) at each time-point and the presence of any Pnc carriage in the family.
MATERIALS AND METHODS
Data collection
Details of the study design and participants have been described elsewhere [Reference Hussain10]. Informed consent to participate was obtained from all participants including parents or guardians of minors. The study obtained appropriate ethical approval. In brief, index children from birth to age 3 years and members of their household were recruited from primary health-care child registers in four general practices in Hertfordshire, UK. Following recruitment of a family, a study nurse administered a pre-tested, structured, detailed questionnaire at the initial visit to each family member. Both household and individual data were gathered from all household members including information on age, gender, medical history and recent clinical symptoms, day-care attendance and smoking.
Nasopharyngeal swabs were collected from all family members at approximately monthly intervals on ten occasions between October 2001 and July 2002. A shorter questionnaire was administered at each monthly visit to each family member to gather individual information on various issues including use of antibiotics since the previous swab and changes in day-care attendance. Swabs were tested for Pnc at the Respiratory and Systemic Infection Laboratory, Health Protection Agency Centre for Infections using standard methods, including serotyping and antimicrobial susceptibility testing as described elsewhere [Reference Hussain10].
Antibiotics were not included in the analyses if they were exclusively used for urinary tract infections, e.g. nitrofurantoin [British National Formulary (BNF) 5.1.8], or have anti-anaerobe activity only in particular, metronidazole (BNF 5.1.10), topical antibiotics (excluding ophthalmic preparations, which might influence nasopharyngeal carriage) (BNF 13.10) or drugs acting on the ear (BNF 12.1). Due to the small number of individuals using antibiotics at each swab month, we did not have statistical power to consider antibiotic classes separately.
Statistical methods
For analysis the outcome was carriage (yes/no) at each month for each individual. The main risk factors of interest were as follows: age at the start of the study (<2, 2–4, 5–17, ⩾18 years); gender; day-care attendance by children (none, 1–19 h, ⩾20 h); smoking (no, passive, yes) where passive means a non-smoker who lives in a household with a smoker; number of children in the household (1, ⩾2); use of antibiotic since the previous visit (yes/no); Pnc carriage at the previous visit; and carriage by another family member at the previous visit. Other risk factors considered were: calendar month of swab; carriage by another family member at the time of the visit; antibiotic use 2 months previously; and total family size.
The data were analysed using multilevel models with individual- and family-level random effects. The models were fitted in Stata version 8.0 [14] using the gllamm (generalized linear latent and mixed models) function. The outcome was binary so a logit link function was used to generate odds ratios. Individual- and family-level random effects were modelled as following a Normal distribution. Risk factors were treated as fixed effects. The model was therefore as follows:
For swab i, individual j and family k the probability of carriage P ijk is given by

The values of σ12 and σ22 indicate the residual variability due to individual- and family-level variation.
Crude carriage was initially calculated according to each risk factor. Univariable analysis was then performed by fitting gllamm with the individual and family random effects and each individual risk factor. For smoking only those aged ⩾18 years were included. For day-care attendance only those aged ⩽4 years were included. Finally a multivariable gllamm was fitted including all the main risk factors of interest. The individual- and family-level random-effects estimates were also calculated. Interactions between significant factors in the multivariable model were also examined.
RESULTS
At least one swab was provided by 489 individuals (four individuals with no swabs were excluded). Fifty-four percent of the individuals were male. Thirty-seven percent were children aged <5 years, 14% were aged 5–17 years and 49% were ⩾18 years. Amongst adults, 54 (29%) were cigarette smokers. Only one individual aged <18 years reported smoking. The proportion of children attending day-care centres increased with age (46% of those aged <2 years to 84% for those aged 2–4 years). All children aged >5 years except one attended school.
Carriage results were available for 3753 swabs taken from 489 individuals from 121 households. The number of swab results per individuals were as follows: one swab 40 persons, two swabs 40 persons, three swabs 12 persons, four swabs 11 persons, five swabs 10 persons, six swabs 10 persons, seven swabs 19 persons, eight swabs 31 persons, nine swabs 98 persons and ten swabs 218 persons. Only 19 individuals had gaps of >2 months between successive swab results.
No cases of invasive Pnc disease were reported.
Twenty-five percent (n=932/3753) of all swabs were positive for Pnc carriage. This remained relatively constant throughout the study period (Fig. 1 a). The proportion of isolates resistant to erythromycin was 10% and 4% were penicillin-non-susceptible (as previously described). Approximately half (52·6%) of all antibiotics were prescribed for the 0–4 years age group, 9·6% for 5–17 years and about 37·8% for six individuals ⩾18 years. The most commonly used antibiotics were penicillins (53%) followed by macrolides (17%), chloramphemicol (ophthalmic preparation) (7%) and cephalosporins (4%) (Fig. 1 b). Penicillin use was more frequent in the winter months (swab months 1–5).
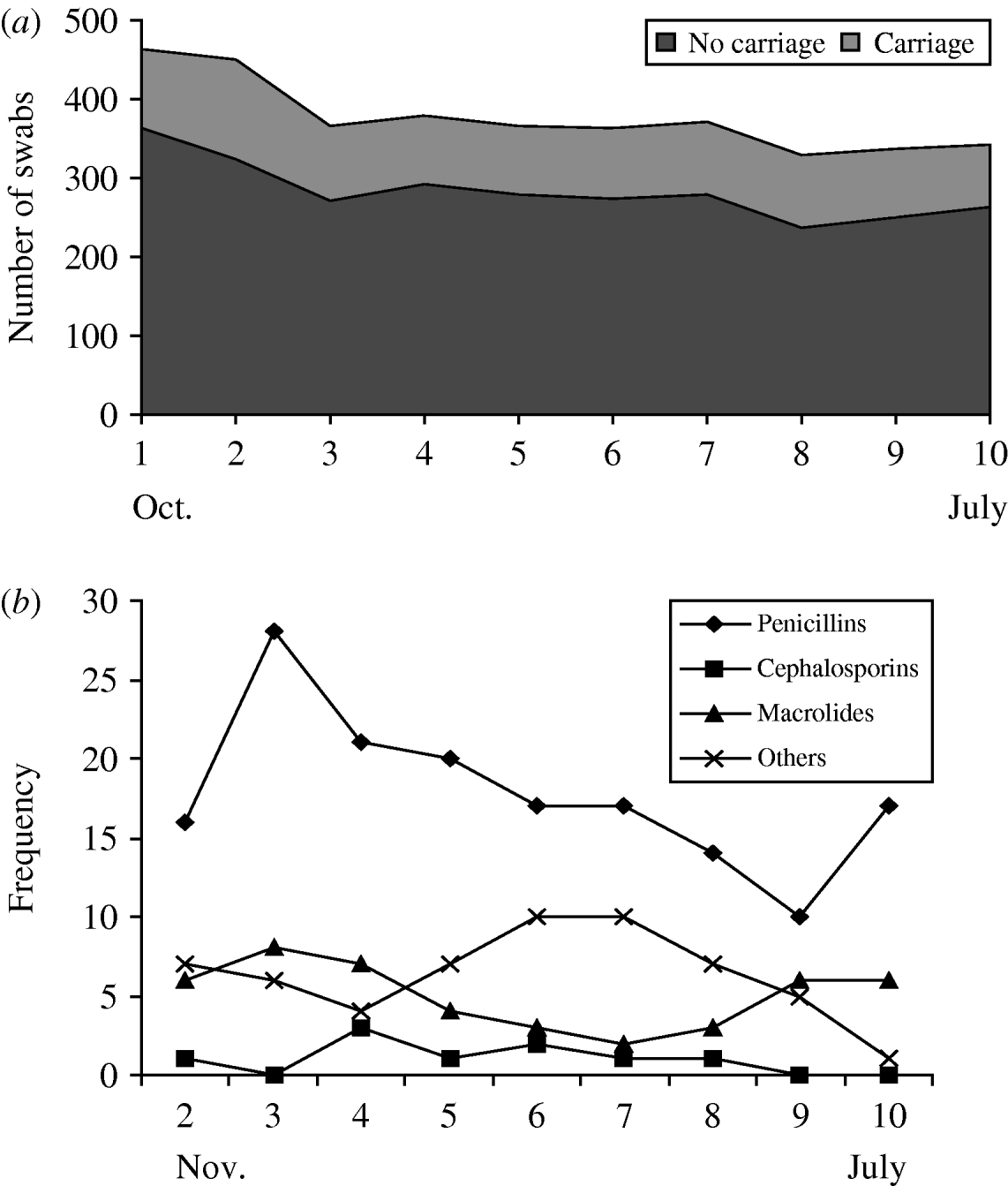
Fig. 1. Streptococcus pneumoniae (Pnc) carriage and use of antibiotics by swab month. (a) Pnc carriage by swab month, (b) antibiotic use by swab month.
Risk factors for carriage
Table 1 shows the odd ratios for Pnc carriage by different risk factors. The univariable analysis showed that crude carriage drops from 55·5% in those aged 0 or 1 year to 7·7% in those aged ⩾18 years. Crude carriage was 25·9% when no previous antibiotics had been used compared to 18·3% when antibiotics had been used.
Table 1. Crude carriage and gllamm univariable and multivariable analysis results
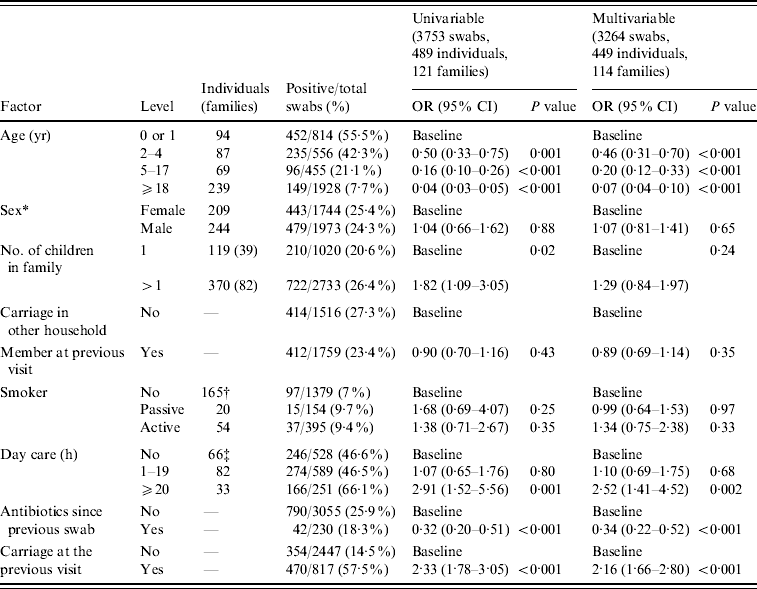
* Sex unknown for 36 individuals all of whom only had one swab taken.
† For crude carriage and univariable analysis only those aged ⩽4 years included.
‡ For crude carriage and univariable analysis only those aged ⩾18 years included.
Individual level variability σ12=0·42.
Family level variability σ22=0·48.
The univariable and multivariable results were fairly similar indicating little confounding, the only exception being number of children in the family, which was no longer significant on multivariable analysis because households with more children would be expected to have higher crude carriage due to the younger age profile. The multivariable analysis results showed that carriage significantly decreased with age. There was no effect of sex or smoking (either active or passive) on carriage. Day-care attendance >20 h a week was associated with increased carriage but those attending for <20 h had similar carriage to those not attending day care. Antibiotic use since the previous swab was associated with a reduced risk of carriage. Individuals who carried Pnc at the previous swab were more likely to carry at a subsequent swab. There was no evidence of any interaction between use of antibiotics, age and day-care attendance.
Although no measured family-level variables were significant in the multivariable model the family-level random effect was of similar size to the individual-level random effect. This indicates that the variability not accounted for in the model is explained as much by the overall level of carriage in the family as an individual's tendency to carry and this would be best explained by family transmission. An analysis looking at carriage by a family member at the same visit showed that this was associated with an increased risk of carriage, but this was not included in the model because it is not possible to determine the direction of transmission (individual to family or family to individual).
Additional univariable analyses looking at calendar month of swab and family size showed no effect of these factors. Moreover, antibiotic use 2 months previously was not associated with a reduced carriage level with crude carriage at 28·9% – similar to the overall crude carriage level of 25·9%.
DISCUSSION
This paper presents the results of a risk-factor analysis of a hierarchical longitudinal Pnc carriage study undertaken in a UK setting. Few studies such as this have previously been published due to the challenge of both successfully collecting the carriage and risk-factor data and then analysing this type of complex dataset. Our study highlighted that day-care attendance for >20 h per week was an important risk factor for Pnc carriage. Antibiotic consumption significantly reduced the risk of carriage at the following visit. These findings are generally congruent with the findings from the few studies that have been undertaken [Reference Leino1–Reference Neto3].
Our study showed that antibiotic use results in a two-third reduction in risk of carriage the following month. The reduction of risk lasted approximately 1 month after taking an antibiotic. This finding is similar to previously published prospective studies [Reference Varon15] among populations where Pnc antimicrobial resistance levels are low, as in this study population. Our study had insufficient statistical power to explore the effectiveness of different types of antibiotic or the potential role of antibiotic consumption in the acquisition of penicillin-resistanct Pnc, for which there is increasing evidence [Reference Dagan16–Reference Melander18].
We demonstrated that day-care attendance was a risk factor for Pnc carriage and presumably represents a route for the introduction of Pnc into a household. In addition, we observed a probable dose–response effect, with an increase in the effect size with the amount of time spent in day care. This strengthens the causal evidence of this association. Although day-care attendance as a risk factor for carriage has been reported previously [Reference Neto3], another study from Finland has not replicated this finding. Besides factors such as study design and method of analysis, the discrepancy with the Finnish findings may be explained by differences in day-care attendance: in Finland, where extended parental leave results in children starting day care when they are older [Reference Leino1]. Other potential day-care-related risk factors for carriage that might be important but were not measured in our study include size of day-care groups [Reference Rosen11], proportion of the community attending and age profile of those attending [Reference Huang, Finkelstein and Lipsitch19].
We found that individuals who were most likely to carry were those who had carried in the previous month. This probably reflects the same carriage episode, rather than re-infection: indeed mean duration of carriage in this study has previously been reported to be about 50 days in young children [Reference Melegaro, Gay and Medley20]. Carriage was also more likely if another household member had carried at the same visit, but not the previous visit. This association may be due to the individual transmitting Pnc to the family member rather than risk from the family member, so the direction of causality is not clear.
We found that young age was a risk factor for Pnc carriage. This matches findings from a study undertaken in India [Reference Coles2]. However, a longitudinal household study in Finland found carriage in the first 6 months of life was unusual [Reference Leino1], and made several suggestions including maternal protection or smaller family size or age of starting day care. We only had a few infants aged <6 months in our study, but nine of 20 samples in this age group were shown to carry Pnc. Further work needs to be undertaken to describe these international differences in first age of acquisition and to gain a better understanding as to why they exist, e.g. the role of day-care attendance, breast-feeding practice.
Few studies have assessed the influence of household structure, which is critical to understand the dynamics of these close-contact infections [Reference Leino1, Reference Hussain10, Reference Sleeman12]. Various analytical approaches have been used to analyse these complex longitudinal datasets and to identify risk factors including marginal logistic regression (MLR) [Reference Leino1], MLR plus an association model [Reference Ekholm, Jokinen and Kilpi21], case crossover [Reference Reintjes22], multilevel random effects or Hierarchical Generalized Linear Models (HGLMs) [Reference Lee23] and Bayesian modelling [Reference Smith24]. Statistical models for longitudinal data need to adjust for dependence between repeated measurements [Reference Twisk25]. However, in addition to these within-subject correlations, it is potentially important to also adjust for family clustering due to higher contact rates (resulting in higher transmission) within households and estimate both the individual- and household-level variance components. We have successfully achieved this and found that there is indeed significant family-level variability – meaning that some families tend to carry more than others, although we did not identify specific family-level risk factors for carriage. Other household carriage studies have highlighted the role of the household in acquisition of carriage such as in Finland where family size is small [Reference Leino1] and in India, where family size is larger [Reference Coles2].
There are several potential weaknesses to our study. The study was undertaken in households with young children sampled from primary-care registers in Hertfordshire, so generalization needs to be undertaken cautiously. Lack of sensitivity of the nasopharyngeal swabbing method has been previously highlighted for detection of Neisseria meningitidis. This possibly applies also to Pnc [Reference Trotter and Gay26] and subsequent misclassification could potentially bias study results towards the null value. Furthermore, the dataset although adequately powered to address the primary objectives of the study related to Pnc carriage, had insufficient power to evaluate risk factors for acquisition of penicillin-resistant Pnc in a low-prevalence settings such as the United Kingdom – and in particular any impact of previous antibiotic consumption on carriage. Information collected on use of antibiotics since the previous swab was not standardized, and although we attempted to classify antibiotics by British National Formulary (BNF) chapter, it is possible that we inadvertently included topical preparations or antibiotics used for the ear (which would not have an affect on Pnc carriage). This may have led to an underestimate of the effect of antibiotics.
In conclusion, we demonstrated that day-care attendance is an important risk factor for Pnc carriage in families with young children in the United Kingdom. Furthermore, in individuals in households with young children, taking antibiotics significantly reduces the risk of Pnc carriage the following month. This suggests that if reduction in carriage (and thus transmission) in a household setting was needed, antibiotics could play a role.
ACKNOWLEDGEMENTS
We thank study nurses Amanda Tew, Mary Clark and Lisa Williams who were responsible for recruiting and following up the study participants; Usha Gungabissoon and Teresa Gibbs of the HPA Centre for Infections Immunisation Department who were responsible for data entry and database management, Professor Elizabeth Miller for her support and advice and Androulla Efstratiou, Karen Broughton, R. Talukda, S. Martin and other staff of RSIL for support and advice before during and after the study. Thanks are also due to Hanna Nohynek, Tuija Leino and Kari Auranen from the Pnc-Euro project team at the National Public Health Institute, Finland who provided helpful advice in the design of the study, and Professor Young Jo Lee, Seoul National University, South Korea for his helpful advice on the HGLM methodology. Oliver Morgan was funded by the NHS London Deanery of Postgraduate Dental and Medical Education. The study was part of the ‘Pnc-Euro’ project funded by the European Union (QLG4-CT-2000-00640).
DECLARATION OF INTEREST
None.




