Depression is now rated as the most disabling health condition worldwide,1 and affects up to 9.3% of community-dwelling elderly people.Reference Luppa, Sikorski, Luck, Ehreke, Konnopka and Wiese2 Between 2015 and 2050, the proportion of the global population aged ≥60 years will increase from 12 to 22%.3 This suggests that the number of people with late-life depression (LLD) will more than double by 2050. Patients with LLD are significantly burdened with psychiatric comorbidities such as anxiety disorders,Reference Manetti, Hoertel, Le Strat, Schuster, Lemogne and Limosin4 as well as a 1.5- to 2-fold increased risk of developing a subsequent ischemic heart event.Reference Gleason, Pierce, Walker and Warnock5 The relationship between LLD and cardiovascular disease is likely complex and bidirectional, which may be mediated by impairment in the autonomic control of the heart.Reference Brown, Karmakar, Gray, Jindal, Lim and Bryant6 Such a risk can be quantified by measuring heart rate variability (HRV), with a recent meta-analyses showing that low-frequency heart rate variability (LF HRV) is significantly lower in patients with LLD.Reference Brown, Karmakar, Gray, Jindal, Lim and Bryant6 Current treatments for LLD, which include antidepressants, have been found to be inadequate, with modest response rates of 44%Reference Nelson, Delucchi and Schneider7 and high rates of adverse events.Reference Kok and Reynolds8 Additionally, some antidepressants have been linked to worsening of autonomic impairment associated with LLD.Reference Nezafati, Vojdanparast and Nezafati9 Non-pharmacological interventions, particularly meditation-based and automatic self-transcending meditation (ASTM) interventions, are of interest. This particular category of meditation offers ease of learning, and may have beneficial effects on depressive symptoms, likely through its stress-alleviating response.Reference Walton, Schneider, Nidich, Salerno, Nordstrom and Bairey Merz10 Additionally, ASTM may offer benefits on cardiovascular health, as per recent recommendations published by the American Heart Association, who concluded that transcendental meditation, a form of ASTM, may be considered for use in clinical practice to lower blood pressure, whereas all other meditation techniques, including mindfulness, could not be recommended at this time.Reference Brook, Appel, Rubenfire, Ogedegbe, Bisognano and Elliott11 Such a reduction in blood pressure may be mediated via the baroreflex control of the vagal-sympathetic autonomic tone. This autonomic tone can be easily measured by modern HRV recording systems.
Sahaj Samadhi meditation (SSM) is a less well-known meditation technique also belonging to the ASTM category, which has not yet been investigated in the LLD population. SSM may have a beneficial effect on depression symptoms as well as HRV,Reference Vasudev12 but this requires further validation. We describe the first randomised controlled trial (RCT) of SSM versus treatment as usual (TAU) in patients suffering from LLD. We hypothesized that people in the SSM arm would have a significant improvement in HRV as well as depressive symptoms, compared with the TAU arm.
Method
Study design and participants
We conducted a single-centre, assessor-blinded RCT comparing the effects of a 12-week SSM arm with TAU. Ethical approval was received from the Research and Ethics Board of Western University (Ontario, Canada) in April 2014 (approval no. 104936). Informed consent was obtained from all participants after they had a clear understanding of study procedures. The study was registered at Clinicaltrials.gov (identifier: NCT02149810) on 18 July 2013. After trial registration, beginning on 2 June 2014, we recruited 83 participants (Table 1) suffering from a mild to moderate major depressive episode, as confirmed by an independent trained rater. If there were any concerns about the presence or lack of this diagnosis, clarification was sought from either of two experienced geriatric psychiatrist investigators (A.V. and A.M.B.).
Table 1 Demographic and clinical characteristics of the intervention (SSM) and control (TAU) group
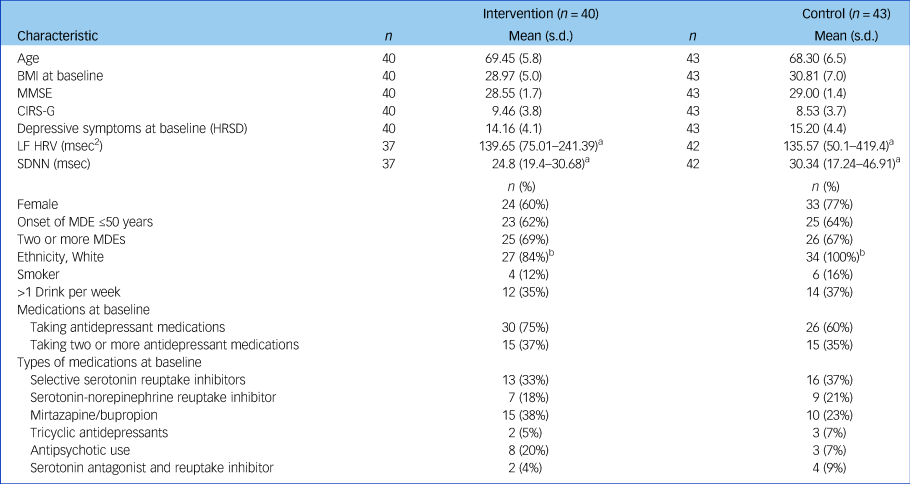
There were 7 cases missing data on MDE onset, 8 for frequency of MDEs, 12 for smoking status and CIRS-G, 11 for drinking status, 1 for BMI and 2 for MMSE.
BMI, body mass index; CIRS-G, Cumulative Illness Rating Scale for Geriatrics; HRSD, Hamilton Rating Scale for Depression; HRV, heart rate variability; LF HRV, low-frequency heart rate variability; MDE, major depressive episode; MMSE, Mini-Mental State Examination; SDNN, s.d. of the normalised N-N interval; SSM, Sahaj Samadhi meditation; TAU, treatment as usual;.
a. Baseline HRV values are reported as median (interquartile range) because the data was skewed.
b. With the exception of ethnicity (Fisher's exact test P = 0.023), there were no significant differences between conditions on any demographic or clinical variable listed.
Recruitment strategy consisted of advertisements placed in community centres and libraries in London (Ontario, Canada) and surrounding areas, with the headline ‘A Meditation Study for Seniors looking for Relief from Feeling Low and Depressed’. We screened 270 potential participants from June 2014 to August 2016, through physician referrals and recruitment posters. Of these, 95 met the study criteria and were enrolled and randomised. A final sample of 83 participants completed the study procedures (Fig. 1).

Fig. 1 Participant recruitment, enrolment, follow-up and analysis.
Inclusion/exclusion criteria
Inclusion criteria included men and women aged 60–85 years, with a current major depressive episode of mild or moderate severity, as confirmed by the Mini Neuropsychiatric InterviewReference Lecrubier, Sheehan D, Weiller, Amorim, Bonora and Harnett Sheehan13 and a Hamilton Rating Scale for Depression (HRSD; 17-item) score >8 and <22.Reference Zimmerman, Martinez, Young, Chelminski and Dalrymple14 Other inclusion criteria included being of stable physical health status, with no severe cardiac episode in the past 12 months; being able to sit comfortably for 30–45 min without any major pain or discomfort; being able to hear and follow verbal instructions with eyes closed; and being willing and able to attend four initial SSM training sessions and 75% of follow-up appointments as an out-patient.
We excluded patients who were participating in other studies or who regularly practiced any other type of meditation, including mindfulness or breath-based techniques; had been diagnosed with a stroke, transient ischemic attack, heart disease or a seizure in the past year; suffered a head injury within the past 6 months; scored <24 on the Mini-Mental State Examination (MMSE);Reference Folstein, Folstein and McHugh15 had experienced active suicidal thoughts and/or plan (as assessed by a clinical interview) at any stage of the study; or had other significant mental health diagnoses (including substance dependence, post-traumatic stress disorder, obsessive–compulsive disorder and major neurocognitive disorder). Supportive psychotherapy, as provided by participants' case managers and/or their geriatric psychiatrists, was allowed.
Randomisation, blinding and allocation concealment
Participants were randomised by an independent clerical staff using computer-generated randomisation. The outcome assessors and investigators were kept blind to the participants' allocation status. The randomisation sequence code was kept safe in the clerical staffs' locked cabinet for the duration of the trial. The only theoretical exception for the code to be broken was any serious adverse event and need for subsequent participant withdrawal. The randomisation code was broken after the 12-week follow-up assessment point for all study participants. An independent statistician performed the statistical analyses.
Study intervention
SSM training was offered in groups of four or more participants by certified teachers from our collaborating partner, the Art of Living Foundation, under the supervision of their Research Director (R.I.N.). The instructional phase consisted of four 90–120 min sessions on consecutive days. On day 1, participants met with the instructor in a group format to learn about the intervention. Following this, participants met with the instructor individually and received the two essential components of SSM. Participants received both a sound (mantra) specifically chosen for the individual to facilitate the process of settling the mind to quieter levels, and a precise yet effortless method of using the mantra. Together these components have been found to enable the mind to shift from its usual active thinking level to a fully alert yet silent state of awareness (for further details see Supplementary File 1 available at https://doi.org/10.1192/bjp.2018.265).
On days 2–4 the correct practice and understanding of meditation was reinforced. This was followed by once weekly 60 min follow-up sessions, for 11 subsequent weeks. Participants were required to attend 75% of follow-ups and practice SSM at home for 20 min, twice daily over the study period. Participants recorded their practice frequency in a daily practice log. Participants in the SSM group continued to receive their TAU, including antidepressant medications and/or non-structured supportive therapy.
TAU control
During the first 12 weeks of the study, TAU participants continued to receive their TAU, including antidepressant medications and/or non-structured supportive therapy. Following the 12-week study assessment, TAU participants were offered the opportunity to attend the 4-day instructional phase of SSM. They were not offered the 11 weeks of SSM follow-up, nor did they attend a week 24 study assessment.
HRV and autonomic measures
The primary outcome measures were changes in HRV. On reviewing the relevant literature in the field, we chose to include both a time domain measure of HRV, i.e. s.d. of the normalised N-N interval (SDNN), as well as one frequency domain measure (i.e. LF HRV) as our primary outcome. Both measures are well-validated markers of autonomic health, as suggested by a recent meta-analysis of autonomic data collected in patients with LLD, as well as in patients with or without a previous history of cardiovascular disease.Reference Brown, Karmakar, Gray, Jindal, Lim and Bryant6, Reference Hillebrand, Gast, de Mutsert, Swenne, Jukema and Middeldorp16
Participants underwent a cardiovascular assessment at baseline and 12-week follow-up. Data was collected by a trained nurse blinded to treatment allocation. Participants were made comfortable on a bed for 10 min followed by a further 10 min to collect data by standard electrocardiogram (Powerlab; ADInstruments, Colorado, USA). Manual blood pressure was estimated by a sphygmomanometer, from the average of three baseline recordings. A strap placed behind the back of participants was adhered to a bellows with Velcro to measure respiratory excursions. All HRV measures were calculated by the embedded LabChart 7 software, including frequency domain measures: LF HRV (collected at 0.04–0.15 Hz), high-frequency HRV (collected at 0.15–0.4 Hz) and time domain measures of HRV, such as s.d. of the N-N interval, the interbeat intervals from which all artifacts have been removed (SDNN), on electrocardiogram, root-mean-square of successive differences and number of R-R intervals differing by >50 msec from adjacent intervals.
Depression symptom outcomes
The secondary outcome of this trial was changes on the HRSD, rated by a trained research assistant. The HRSD includes 17 items, with each item scored from 0 to 4, with a total possible score ranging from 0 to 52; scores of ≥24 indicate severe depression, 17–23 indicate moderate depression, 8–16 indicate mild depression and 0–7 indicate no depressive symptoms.Reference Zimmerman, Martinez, Young, Chelminski and Dalrymple14
Exploratory mental health outcomes included Clinical Global Impression Improvement (CGI-I) scoreReference Busner and Targum17 and anxiety symptoms were determined by the Geriatric Anxiety Inventory (GAI),Reference Pachana, Byrne, Siddle, Koloski, Harley and Arnold18 physical activity was determined by the Physical Activity Scale for the Elderly (PASE),Reference Washburn, Smith, Jette and Janney19 quality of life was measured by the Quality of Life Profile Seniors Version (QOLPSV)Reference Raphael, Brown, Renwick, Cava, Weir and Heathcote20 and side-effects were determined by the Toronto Side Effects Scale (TSES).Reference Vanderkooy, Kennedy and Bagby21
Other demographic and clinical characteristics
All participants provided demographic information, had their height and weight checked, were interviewed for comorbid medical conditions (Cumulative Illness Rating Scale – Geriatrics; CIRS-G)Reference Parmelee, Thuras, Katz and Lawton22 and completed a screening cognitive examination (MMSE).Reference Folstein, Folstein and McHugh15
The rater-assessed depression scales (HRSD) as well as the self-reported mental health measures listed above, were collected at weeks 4, 8 and 12 for all participants.
Statistical analyses
Analyses were conducted on a PC running SAS v9.4 (SAS Institute Inc. Cary, USA) on a Windows 7 Platform. Demographic and clinical characteristics of the intervention (SSM) and control (TAU) condition were compared by independent samples t-tests and χ 2 or Fisher's exact test for continuous and categorical data, respectively.
Linear mixed models (described below), controlling for baseline score, were used to compare the SSM and TAU groups' change score from baseline to 12-week follow-up on SDNN and LF HRV (primary outcome) and depression severity (HRSD) (secondary outcome). Other exploratory outcomes were evaluated in a similar way. Linear mixed models were also used to compare the percent change in HRSD scores, whereas generalised linear models were used to compare the proportion of patients who responded to the intervention (≥50% decrease from baseline on the HRSD, defined a priori) and the proportion of patients who achieved remission (scores ≤7 on the HRSD, defined a priori) at the end of intervention (week 12). Linear mixed models with variance components covariance structure were used in comparing HRSD scores between groups (SSM and TAU), time (week 0, 4, 8 and 12) and group × time interaction. All HRV-related measures were natural log-transformed. Cohen's d was calculated by dividing the mean difference in change scores between the two groups by the s.d. of the control groups' change score.
Following established guidelines,Reference Lohr, Schochet and Sanders23 the linear mixed models accounted for the partially nested design, which allowed clustering in the intervention group (because of participants experiencing a group-based intervention, added as a random effect) and no clustering in the control group. Using this method, different hierarchical structures were fit for the two groups. PROC MIXED was used for continuous outcome variables, and PROC GLIMMIX was used for binary outcome variables. Restricted maximum likelihood estimates were used and the d.f. were adjusted with the Satterthwaite method. Residual and influence diagnostics were checked and indicated the models fit the data well. The intraclass correlation, a statistical measure of the effect of clustering on change scores (from baseline to 12 weeks), in SDNN and LF HRV was <0.001, and for depressive symptoms according to HRSD score, was 0.306. The level of statistical significance was set at α = 0.05. Complete-case analysis was used, although there were no missing data for primary and secondary outcomes. Four participants (one TAU, three SSM) were missing HRV and autonomic measures at baseline and 12-week follow-up.
Results
Data from 83 participants was included in the final analyses (Table 1 and Fig. 1). At baseline, there were no significant differences between SSM and TAU groups in any of the HRV- or mood-related measures. The CIRS-G indicated that the majority of participants in this study did not have severe pre-existing cardiovascular issues: 74% reported ‘no problems’ with heart and 51% reported no vascular function issues, whereas 9 and 10% reported ‘current mild problem’ or ‘past significant problem’, respectively. No participants in the study withdrew because of adverse effects, and therefore the investigators remained blinded to all participants' allocation status throughout the study. See Table 1 and Supplementary Table 1 for further details regarding baseline participant characteristics.
For the primary outcome measure, change in HRV (SDNN and LF HRV) from baseline to week 12, there were no significant differences between SSM and TAU groups. SDNN for the SSM group decreased by −0.006 (P = 0.92), whereas the TAU group decreased by −0.116 (P = 0.11) and the mean difference was 0.109 (95% CI −0.09 to 0.31, P = 0.28, Cohen's d = 0.19). Similarly, LF HRV for the SSM group increased by 0.017 (P = 0.92), whereas the TAU group decreased by −0.017 (P = 0.92) and the mean difference was 0.034 (95% CI −0.44 to 0.51, P = 0.89, Cohen's d = 0.03; see Table 2). Similarly, for the exploratory cardiovascular outcomes of high-frequency HRV, root-mean-square of successive differences, number of R-R intervals differing by >50 msec from adjacent intervals, systolic and diastolic blood pressure and respiratory rate, there were no significant differences between groups (see Supplementary Table 3).
Table 2 Estimated change score for the intervention (SSM) and control (TAU) group and associated 95% CI

HRSD, Hamilton Rating Scale for Depression; LF HRV, low-frequency heart rate variability; SDNN, s.d. of the normalised N-N interval; SSM, Sahaj Samadhi meditation; TAU, treatment as usual. Bold indicates statistically significant results.
a. Mean difference represents the difference in change score between the two groups. For example, the decline in HRSD scores among the treatment group was 2.66 points greater relative to the control group.
b. Unless otherwise specified, smaller values denote greater improvement. LF HRV values are natural log-transformed.
However, participants in the SSM group had a significant improvement in depressive symptoms (HRSD) over this period compared with the TAU group (2.66-point greater reduction relative to TAU; 95% CI 0.26–5.05, P = 0.030, Cohen's d = 0.61) (Table 2). This finding was replicated in comparing HRSD scores over the four time points among the two groups, finding a significant group × time interaction (P = 0.006), with a statistical difference between groups evident at the 8-week (2.83-point difference, s.e. 1.05, P = 0.008) and 12-week (3.62-point difference, s.e. 1.13, P = 0.002) follow-up assessment (Fig. 2).
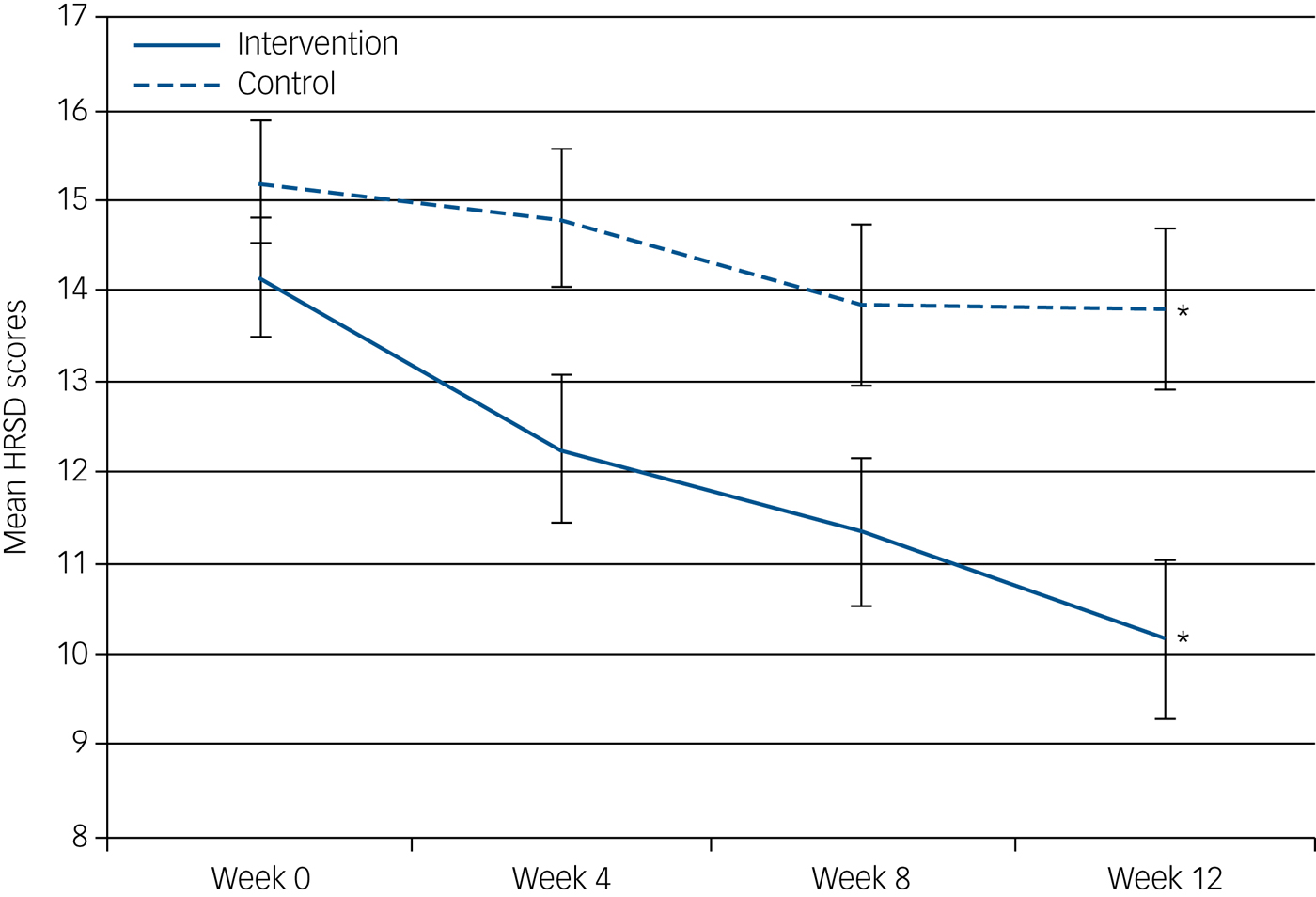
Fig. 2 Mean HRSD scores with s.e. for the SSM and TAU arms. Mean depressive symptoms (HRSD scores) of the intervention and control group at each time point. *Statistically significant difference between groups at 12-week follow-up.
Furthermore, the SSM group had a more than three times increased odds of response (>50% reduction on HRSD depressive symptoms) compared with the TAU group (30.0 v. 11.6%; odds ratio, 3.26; 95% CI 1.01–10.53; P = 0.049). Similarly, the SSM group had a threefold higher odds of achieving remission compared with the TAU group (40.0 v. 16.3%; odds ratio, 3.36; 95% CI 1.06–10.64; P = 0.040; see Supplementary Table 5). Supplementary Table 5 also shows the percent reduction, response rate and remission rate of patients at 4- and 8-week follow-up.
On the exploratory mental health-related measures, SSM was associated with greater improvements in anxiety (GAI; P = 0.021) and clinical global impression of depression severity (CGI-I; P = 0.018) scores, compared with TAU. SSM and TAU groups did not differ significantly on the exploratory outcome measures of physical activity (PASE; P = 0.22), quality of life (QOLPSV; P = 0.72) and adverse effects (TSES; P = 0.99).
Discussion
This is a pivotal RCT reporting the safety and efficacy of SSM, a novel intervention for the treatment of LLD. We found that SSM did not significantly improve any of the HRV variables in this population. However, the intervention significantly improved depressive symptoms. The significant and clinically meaningful effect of SSM on depression in this geriatric sample is relevant, given the relative lack of effective, scalable and well-tolerated treatments for LLD.
Based on a thorough literature search, to the best of our knowledge, there are no published reports of any meditation technique on HRV in patients with LLD. However, we did find published reports of mindfulness-based stress reduction (MBSR) in younger non-depressed samples (this included yoga exercises, breathing exercises, a few minutes of mindfulness meditation and mantra chanting),Reference Nijjar, Puppala, Dickinson, Duval, Duprez and Kreitzer24 as well as non-pharmacological interventions such as exercise in LLD participants,Reference Toni, Belvederi Murri, Piepoli, Zanetidou, Cabassi and Squatrito25 and yoga-based interventions offering beneficial responses on HRV in younger depressed and non-depressed samples.Reference Chu, Wu, Lin, Chang, Lin and Yang26 Such a deficit in literature regarding the role of non-pharmacological interventions on HRV in patients with LLD needs to be addressed in the future because this sample of patients are particularly prone to developing a subsequent cardiovascular event compared with younger populations.Reference Pocklington27
There could be a number of reasons why SSM was not associated with a statistically significant improvement in the HRV measures in our study. The majority of our study participants (64%) were taking antidepressants at the time of enrolment, which, through their independent effects on HRV,Reference Sgoifo, Carnevali, Alfonso and Amore28 could have led to signal dilution in this sample. Similarly, vasoactive medications,Reference Kaufman, Bosner, Bigger, Stein, Kleiger and Rolnitzky29 as well as common age-related conditions such as hypertension, diabetes mellitus and atherosclerosis, are all known to reduce HRV.Reference Istenes, Körei, Putz, Németh, Martos and Keresztes30 Such factors likely contributed to a relatively low and heterogeneous baseline HRV values, making any improvement in HRV difficult to detect. Additionally, HRV is a complex, non-linear system that should be measured by autonomic modulation rather than the typical linear time domain measures used in adult populations.Reference Nicolini, Ciulla, De Asmundis, Magrini and Brugada31 Future studies should seek to include autonomic modulation measurement, if and when it becomes readily available.
The most significant finding of this study was that, compared with TAU, SSM substantially reduced depressive symptoms. So far as we are aware, there has also not been any previous published trial of any meditation technique belonging to the larger category of ASTM, to which SSM and transcendental meditation belong, in patients with LLD. In addition, we could not find any other comparable RCT of any other purely meditation based intervention for the treatment of clinically diagnosed LLD. For the sake of comparison, the observed odds of remission in depressive symptoms in our study (odds ratio, 3.4; 95% CI 1.1–10.6) was higher than the odds of remission (odds ratio, 2.0; 95% CI 1.1–3.7) observed in an RCT that compared a placebo to venlafaxine augmentation with aripiprazole in patients with LLD.Reference Lenze, Mulsant, Blumberger, Karp, Newcomer and Anderson32
Based on our findings, SSM joins a small but growing group of non-pharmacological mind-body interventions available for the treatment of LLD. In a two-centre RCT study, older patients with either an anxiety or depressive disorder (including dysthymia, n = 103) were offered either MBSR or a health education control.Reference Wetherell, Hershey, Hickman, Tate, Dixon and Bower33 Participants in the MBSR group received mindfulness meditation as well as light yoga for eight sessions. The study authors reported an effect size of Cohen's d = 0.46 on their patient-reported outcome measurement information system depression outcome. Because of the heterogeneity of their clinical sample, different outcome measures and addition of yoga as an essential component of their meditation-based intervention, findings from this studyReference Wetherell, Hershey, Hickman, Tate, Dixon and Bower33 are difficult to compare with ours. The effect size on depressive symptoms in our study (Cohen's d = 0.61) is comparable with one of the most commonly adopted forms of non-pharmacological therapy, cognitive–behavioural therapy (from recent reviews, mean Cohen's d = 0.72 in older adults).Reference Wuthrich and Rapee34 In another study, Lavretsky et al Reference Lavretsky, Alstein, Olmstead, Ercoli, Riparetti-Brown and Cyr35 stabilised their group of participants with LLD (n = 112) receiving escitalopram, and then randomised to tai chi or health education. Depression response criteria was set at a lower threshold, i.e. 30% fall in HRSD scores, compared with our criteria of 50% fall in HRSD scores. Pre-intervention, Lavretsky et al's participants had a mean HRSD of 8.2, compared with our HRSD mean of 14.16. Lavretsky et al Reference Lavretsky, Alstein, Olmstead, Ercoli, Riparetti-Brown and Cyr35 found 65% achieved remission with HRSD scores of ≤6, compared with our study, where 40% achieved remission with HRSD scores of ≤7.
SSM was found to be a well-liked augmentation strategy based on its excellent tolerability and no drop-outs due to intervention-related adverse effects. SSM, belonging to the category of ASTM, may be a particularly well-suited form of meditation for an LLD population. ASTM's cognitive process entails relaxed attention to a mantra and is relatively effortless, requiring neither concentration nor sustained vigilant attention. This distinguishes it from other meditation categories. For example, open monitoring meditations (e.g. mindfulness meditation) involve moment-to-moment sustained and non-reactive attention to one's emotions, thoughts, bodily sensations and environment.Reference Lutz, Slagter, Dunne and Davidson36 The third major category of meditation, focused attention, involves sustained and vigilant attention to a particular object such as a candle, a mantra, one's breath or a specific emotion, such as in loving kindness meditation.Reference Lutz, Slagter, Dunne and Davidson36, Reference Lutz, Greischar, Rawlings, Ricard and Davidson37
Overall, the treatment of LLD remains complex and challenging in routine clinical care, where the majority of patients with LLD are offered antidepressants, with as many as 27% of older patients discontinuing antidepressants because of adverse events.Reference Nelson, Delucchi and Schneider7
SSM offers a broad range of other benefits, including being a standardised and manualised mind-body intervention and a scalable group therapy. SSM could theoretically offer health cost-benefits compared with TAU: SSM can be delivered in a group setting of up to 15 participants per certified non-clinician instructor, which would free up the availability of clinical staff for management of other significant mental healthcare issues. Whether this could translate into significant cost benefits compared with traditional psychotherapeutic interventions (e.g. cognitive–behavioural therapy) for the augmentation treatment of LLD remains to be examined.
Strength and limitations
Strengths of this study included its RCT design, often lacking in meditation research.Reference Sedlmeier, Eberth, Schwarz, Zimmermann, Haarig and Jaeger38 The sample size of this study was reasonable and similar to other innovative behavioural trials for geriatric mental illness.Reference Lavretsky, Alstein, Olmstead, Ercoli, Riparetti-Brown and Cyr35 We used validated biological assessments as well as objective and self-report measures for depression and other mental health domains. Nonetheless, there are some limitations to this study. This study lacked an active comparator to control for variables such as instructor attention, socialising in a group atmosphere and leaving a residence for the study intervention. To reduce expectation bias, future RCTs with an active comparator arm should present the control arm to participants as being something of equivalent value and benefit as SSM. Finally, although the study was powered to detect small and large changes in depression, our study, like other previous studies, may have been underpowered to assess the HRV changes as well as the potential long-term cardiovascular beneficial effects of this intervention.Reference Quintana39 Future studies could also consider stratifying by presence of antidepressant medication and/or vasoactive medications, as well as presence of diabetes, as such factors could moderate the response on HRV.
Future directions
In conclusion, a 12-week intervention of adjunctive SSM may have beneficial effects on depression and mood-related symptoms in patients with LLD. This study's findings provide support for the possible implementation of SSM as an adjunct to routine clinical care. Further investigation of the long-term effects of SSM on depression outcomes are needed. Additionally, future studies could investigate whether SSM's effects are due to meditation itself or non-specific factors by including an active control group. The possibility of a dose-response relationship of SSM practice needs to be assessed in further studies. Cost–benefit analyses are needed to confirm the potential for scalability of this intervention. Lastly, enriching the sample of patients with LLD with a recent history of a cardiovascular event would likely increase the likelihood of detecting a change in HRV with SSM.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2018.265.
Funding
This study was supported by the Innovation Fund of the Alternative Funding plan of the Academic Health Sciences Centres of Ontario, London, Ontario, Canada and the Schulich Research Opportunity Project, London, Ontario, Canada.
Acknowledgements
We would like to thank all the participants in this study who kindly offered their time and effort to participate in this study. We would also like to thank the Lawson Health Research Institute, London Health Sciences Centre and Parkwood Institute Mental Health Care for the use of their buildings and facilities to conduct this study. We would also like to thank a number of undergraduate students who have contributed in participant recruitment, data entry and clean-up.







eLetters
No eLetters have been published for this article.