Introduction
About 50% of the world's land area comprises pastures. With high variations in botanical composition and yield, legumes form an important fraction of these pastures in tropical and subtropical climates, particularly in arid and semiarid ecosystems (Muir et al., Reference Muir, Santos, Cunha, Dubeux, Lira Júnior, Souza and Souza2019). Forage legumes have been used to improve the nutritional status of ruminants (Braga et al., Reference Braga, Ramos, Carvalho, Fonseca, Fernandes and Fernandes2020; Diniz et al., Reference Diniz, Santos, Verás, Cunha, Simões Neto, Souza, Dubeux, Abreu and Ferreira2021; Mpanza and Hassen, Reference Mpanza and Hassen2023). These plants can establish symbiotic relationships with diazotrophic microorganisms, allowing for biological nitrogen (N) fixation. This symbiotic interaction leads to N accumulation in plant tissues and a high forage concentration of crude protein (CP) (Castro-Montoya and Dickhoefer, Reference Castro-Montoya and Dickhoefer2020). Furthermore, ingesting forage with better nutritional quality reduces animal methane production (Schilde et al., Reference Schilde, Soosten, Hüther, Meyer, Zeyner and Dänicke2021; Mpanza and Hassen, Reference Mpanza and Hassen2023).
Stylosanthes genus is one of the most economically important cultivated forage legumes. This genus consists of several species native to tropical (Costa et al., Reference Costa, Fracetto, Fracetto, Souza, Santos and Lira Júnior2018), subtropical (Carvalho et al., Reference Carvalho, Barreto, Prado, Habermann, Martinez and Branco2020) and temperate regions of America (Calles and Schultze-Kraft, Reference Calles and Schultze-Kraft2016), Africa (Muraina et al., Reference Muraina, Olanite, Onifade, Ojo, Ewetola, Akinlolu and Akintunde2017) and Southeast Asia (Luo et al., Reference Luo, Liu, Zhang, Wang, Chen, Luo, Liu and Liu2020). The genus Stylosanthes consists of species with production cycles ranging from perennial to annual or biannual, which can be used for animal nutrition and soil improvement (Schultze-Kraft et al., Reference Schultze-Kraft, Rao, Peters, Clements, Bai and Liu2018). Additionally, owing to their well-developed root systems, they are drought-tolerant and adapted to low-fertility soils (Liu et al., Reference Liu, Huang, Hu, Jia, Li, Luo, Liu, Luo, Liu and Chen2019). Environmental and phenological factors, such as season, growth stage, soil fertility and soil nitrogen status, affect the nutritive characteristics of forage (Solontsi et al., Reference Solontsi, Maqubela, Niekerk, Swanepoel, Jordaan, Gulwa and Tokozwayo2022).
Despite their wide dispersion and use, studies evaluating qualitative and quantitative rangeland changes over time are scarce. Musco et al. (Reference Musco, Koura, Tudisco, Awadjihè, Adjolohoun, Cutrignelli and Calabrò2016) assessed the nutritional values of S. hamata and S. scabra during the vegetative developmental phase. These authors reported a high content of structural carbohydrates, followed by low energy levels and organic matter degradability. Understanding the factors involved in these variations is essential for implementing economically and environmentally sustainable animal production strategies.
The primary constraints of legumes as feedstuff sources are related to their fibre content, which can decrease ruminal degradability and protein availability (Castro-Montoya and Dickhoefer, Reference Castro-Montoya and Dickhoefer2020). Analysing the protein fractions that comprise the feed is crucial because it represents the fraction of the total N available to the animal. Nitrogen levels tied to cell wall compounds tend to increase with plant maturity and seasons of the year, mainly the fraction bound to acid detergent fibre (ADF) (Lagunes et al., Reference Lagunes, Pell, Blake, Lagunes and Rodríguez2018).
To better use Stylosanthes spp. in ruminant feeding systems, it is essential to understand the changes in nutritional value as cutting frequency increases since long harvest intervals lead to higher deposition of fibre material, diminishing the nutritional value of tissues (Klabi et al., Reference Klabi, Bell, Hamel, Iwaasa, Schellenberg and St-Arnaud2018). Recommendations for harvest regimes of different Stylosanthes genotypes are still poorly understood, so it is necessary to uncover their effects on the qualitative responses of this tropical legume. Therefore, this study aimed to evaluate harvest frequency and season of the year effects on the nutritional value and fractionation of carbohydrates and proteins of distinct Stylosanthes genotypes.
Materials and methods
Site description and weather conditions
The trial was carried out at the Sugarcane Experimental Station of Carpina – Universidade Federal Rural de Pernambuco (SESC/UFRPE) located in Carpina (07°51′03′′S, 35°15′17′′W and 180 m altitude), Pernambuco state, Brazil. The region's predominant climate is As, a rainy tropical climate with a dry summer, according to the Köppen-Geiger classification (Alvares et al., Reference Alvares, Stape, Sentelhas, Gonçalves and Sparovek2013), with 1863 mm rainfall and an average temperature of 25.5°C (Fig. 1).

Figure 1. Water balance, rainfall and average air temperature in 2017 and 2018 of Sugarcane Experimental Station of Carpina (SESC/UFRPE). *Def., hydric deficit; EXC., hydric excess; Rain, rainfall; Temp., average air temperature; ↑, months during which the forages were harvested.
The soil was classified as distrocohesive yellow argisol (Santos et al., Reference Santos, Jacomine, Anjos, Oliviera, Lumbreras, Coelho, Almeida, Araújo Filho, Oliveira and Cunha2018) or acrisol, according to the World Reference Base for Soil Resources (IUSS Working Group WRB, 2015), with a sandy loam textural class. Chemical properties of the 0–20 cm soil layer were: 5.7 pH, 0.065 cmolc/dm3 of K, 0.045 cmolc/dm3 of Na, 0.0 cmolc/dm3 of Al, 3.41 cmolc/dm3 of base sum, 7.76 cmolc/dm3 of cation exchange capacity, 43.94 cmolc/dm3 of base saturation and 2.7% of soil organic matter.
Establishment of the field experiment
The experimental area was established in November 2016 using seedlings produced from seeds. Four Stylosanthes spp. were evaluated: S. seabrana, S. scabra, S. mucronata and Stylosanthes spp. cv. Campo Grande (Campo Grande). The Campo Grande seedlings, consisting of a physical blend of 80% S. capitata and 20% S. macrocephala, were obtained from commercial seeds. The other evaluated genotypes originated from plants collected by Costa (Reference Costa2017) in the following municipalities: S. seabrana – Floresta-PE, S. scabra – Sertânia-PE and S. mucronata – Caetes-PE.
Basal fertilization was performed with 50 and 60 kg ha−1 of P2O5 and K2O, respectively. Weekly sprinkler irrigation was performed by applying 20 mm of water during the first month after planting because of the low rainfall when establishing the experimental field (November 2016). Annual maintenance fertilization was performed by applying 50 and 60 kg/ha of P2O5 and K2O at the end of each rainy season.
Treatments and experimental design
Treatments included four Stylosanthes genotypes subjected to three harvest frequencies (56, 77 and 98 days) from April 2017 to December 2018. The experimental design was a randomized complete block with a split-plot arrangement and four repetitions. In the main plot (1.5 × 4.5 m), the Stylosanthes genotypes were evaluated, whereas harvest frequencies were assessed in the split plots (1.5 × 1.5 m), 0.5 × 0.5 m distance in a row.
The harvests were divided into dry and rainy seasons according to rainfall and evapotranspiration information obtained from the meteorological station from SESC/UFRPE.
In April 2017, a uniform cut was made at a 20 cm stubble height to equal the treatments. Four plants were sampled from the net area of each split-plot for each frequency. Harvests were separated into dry and rainy periods based on rainfall and evapotranspiration indices. Three harvests were performed in 2017 (June, August and September) at the 56-day cut interval (one in the rainy and two in the dry season), while two cuts were made (one for each season) for the 77 (July and September) and 98-day frequencies (August and November). Owing to low rainfall indices from December 2017 onwards, harvests were suspended. In March 2018, another uniformity cut was performed, and harvests resumed. In 2018, four harvests were performed at the 56-day frequency (two in each period). For 77 and 98-day cut intervals, one harvest was made in the rainy and two in the dry season.
Plants harvested had their fresh weights determined. Then, the dry weight was obtained after drying the samples in a forced-air oven at 55°C until constant weight. The samples were ground in a Willey mill to pass 1 and 2 mm screens. Samples comprised four plants collected from each net plot area, which were identified and stored to determine dry matter (DM) yield by harvest, chemical composition analysis and assays of in vitro DM digestibility. The present work did not involve an ethics committee license.
Analysed variables
Concentrations of DM (930.15), mineral matter (MM) (942.05), ether extract (920.39) and CP (984.13) were analysed according to AOAC (2005). Contents of neutral detergent fibre (NDF), ADF and NDF free of ashes and proteins (NDFap) were obtained according to Van Soest et al. (Reference Van Soest, Robertson and Lewis1991), with modifications proposed by Senger et al. (Reference Senger, Kozloski, Sanchez, Mesquita, Alves and Castagnino2008). The modification involved autoclaving the samples at 110°C for 40 min. Condensed tannins (CT) and total phenolic compounds (TPC) were determined according to Hagerman and Butler (Reference Hagerman and Butler1978).
The ADF residue was washed with 72% sulfuric acid to solubilize cellulose and obtain the acid-digested lignin concentration (ADL) (Van Soest et al., Reference Van Soest, Robertson and Lewis1991).
Total and non-fibre carbohydrates (TC and NFC, respectively) were determined according to Sniffen et al. (Reference Sniffen, O'connor, Van Soest, Fox and Russell1992). The NFC was the fraction A + B1, while the fraction B2 (those slowly degraded in the rumen and often present in the plant cell walls) was derived from the difference between fraction C and NDFap. In turn, the fraction C (indigestible carbohydrates) was calculated by the following formula:
Protein fractionation was performed from the content of non-protein N (fraction A), neutral detergent insoluble N (fraction B3) and acid detergent insoluble N (fraction C) using the methodology described by Licitra et al. (Reference Licitra, Hernandez and Van Soest1996). With these results, we could calculate the proportion of fraction B1 + fraction B2, composed of true soluble nitrogen (fraction B1) plus soluble nitrogen into neutral detergent (fraction B2). The equation used is as follows:
The in vitro digestible dry matter (IVDDM) was determined according to Holden (Reference Holden1999) using the artificial incubator DAISYII (ANKOM® Technology). Samples were ground into 1 mm particles, stored in non-woven textile bags (100 g/m2) and kept in the apparatus for 72 h at 39°C. After 48 h of incubation, the samples were subjected to chemical digestion by adding 40 ml of HCl 6N solution and 8 g of pepsin into each of the four pots. The ruminant fluid was collected in the early morning from a rumen-fistulated cow.
Statistical analysis
Normality and homoscedasticity tests were performed to verify if data met variance analysis assumptions, and transformations were performed when necessary. Data were analysed using the PROC MIXED of the SAS University Edition. The fixed effects included genotype, harvest frequency, season and their interactions. Blocks, years and their interactions were random effects. The season of the year was analysed as a repeated measure over time. The means were compared using the PDIFF procedure adjusted by Tukey's test. For all variables, differences were considered significant at P ≤ 0.05 using the following statistical model:
where Yijk is the dependent variable; μ is the overall mean, τi is the genotype effect, γk is the random block effect, τγ is the error associated with the plot residue, βj is the harvest frequency effect, (τβ)ij is the interaction of fixed effects and (τβγ)ijk is the error associated with the split-plot.
When the effect of harvest frequencies was significant, polynomial regression analysis was performed. Differences were considered significant when P ≤ 0.05.
Results
Nutritional value
S. mucronata and Campo Grande showed 31.5 g/kg more DM than S. seabrana and S. scabra, with an average concentration of 378 g/kg DM (Fig. 2a). Plants had higher DM concentration in dry (440 g/kg) than in the rainy season (348 g/kg). The 98-day harvest frequency delivered higher forage DM contents than other frequencies (Fig. 2b).
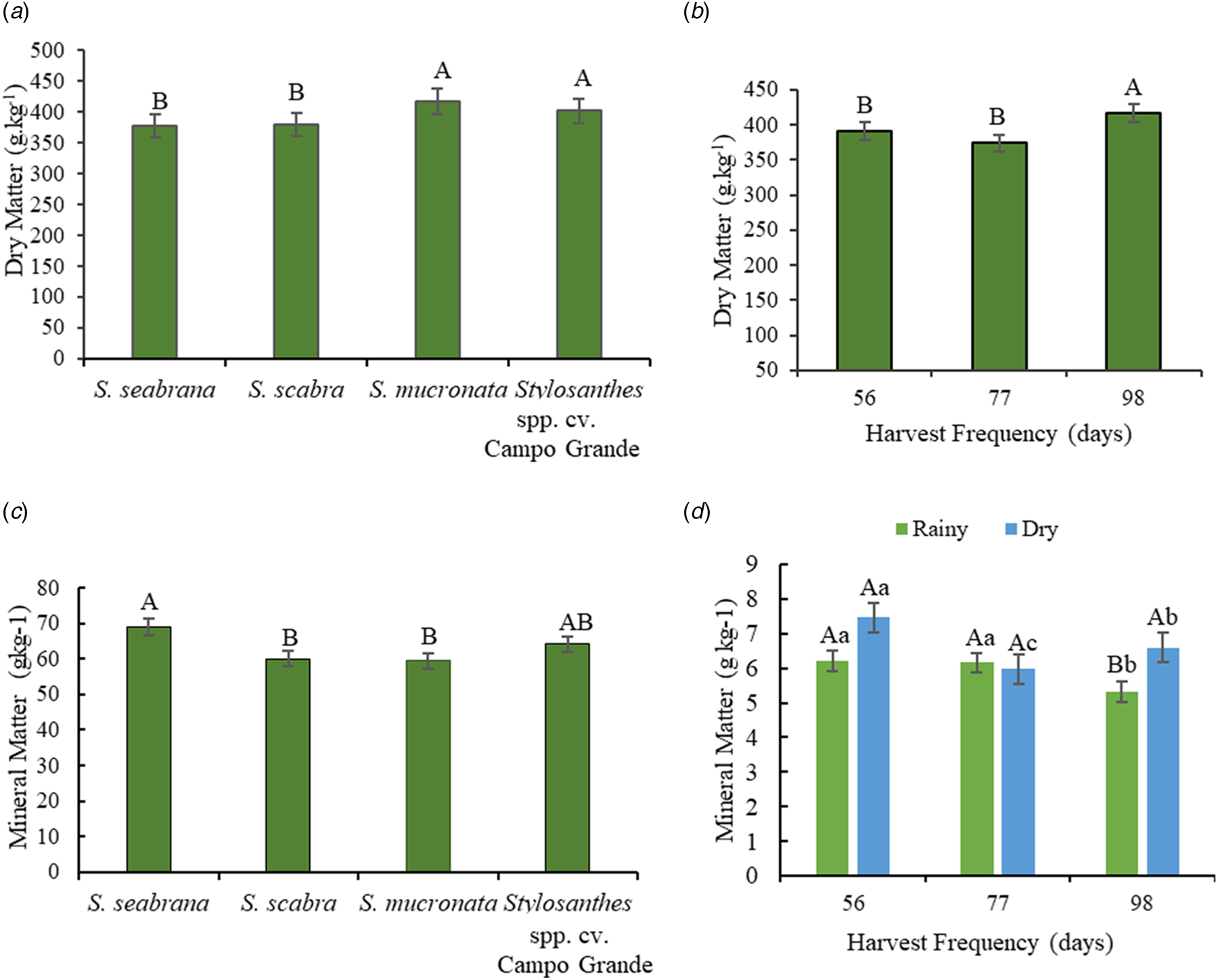
Figure 2. Dry matter content (DM, g/kg) (a), effect of harvest frequency on DM (b), mineral matter content (MM, g/kg of DM) (c), and effect of interaction between the harvest frequency and season of the year on MM (d) of Stylosanthes genotypes grown in the sub-wet tropical region of Pernambuco. Different letters compare genotypes (a and c) and harvest frequency (b). Bars indicate the standard error of the mean. Uppercase letters on the bar compare seasons of the year within harvest frequencies, and lowercase letters compare harvest frequencies within seasons of the year (d).
S. seabrana had 15% more MM than S. scabra and S. mucronata, but showed no differences with Campo Grande (Fig. 2c). The 56-day harvest frequency in both seasons resulted in higher MM contents (Fig. 2d).
Plants harvested in the rainy season had a higher CP content (184 g/kg DM) than those harvested in the dry period (153 g/kg DM). Nevertheless, no significant differences were found in the other factors evaluated.
Contents of NDF, ADF, IVDDM, CT and TPC of Stylosanthes genotypes were affected (P < 0.05) by the harvest frequency–season of the year interaction (Table 1). Harvest regimes of 56 and 98 days in the dry season resulted in the lowest NDF concentrations, respectively, showing a quadratic effect.
Table 1. Effects of interaction between harvest frequencies and seasons of the year on the NDF, ADF, IVDDM, CT, TP contents and proportions of carbohydrates' B2 fraction of Stylosanthes genotypes grown in the sub-wet tropical region of Pernambuco

NDF, neutral detergent fibre; ADF, acid detergent fibre; IVDDM, in vitro digestible dry matter; CT, condensed tannins; TPC, total phenolic compounds; carbohydrates fraction B2, carbohydrates slowly degraded in the rumen; SEM, standard error of the mean. Means followed by the same uppercase letters within a row, and the same lowercase letter within a column for the same variable, do not differ from each other by the Tukey's test (P < 0.05).
ns, non-significant.
*significant at 5% of error probability (NDF dry: Y = 65.62 + 11.06X–0.0710X 2, R 2 = 0.1009; ADF dry: Y = −199.78 + 14.38X–0.0917X 2, R 2 = 0.1697; IVDMD rainy: Y = 66.79 + 0.0620X–0.0015X 2, R 2 = 0.2213; IVDMD dry: Y = 120.43–1.5X + 0.0094X 2, R 2 = 0.1437; CT dry: Y = 152.45–3.01X + 0.0232X 2, R 2 = 0.1489; TP rainy: Y = 39.65 + 2.16X–0.0181X 2, R 2 = 0.1181; TP dry: Y = 164.92–3.16X + 0.0285X 2, R 2 = 0.3690; concentration of carbohydrates B2 fraction rainy: Y = 24.87 + 0.1037X, R 2 = 0.079).
For the IVDDM, 56 and 77-day harvest frequencies in the rainy season increased by 60 g/kg of digestible DM compared to the most prolonged cut interval (98 days). In the dry season, the highest IVDDM was observed at 56 days, with an increment of 40 g/kg in the digestible DM compared to the other frequencies. Within the 98-day cut interval, plants exhibited the highest IVDDM in dry than in the rainy season. Other harvest frequencies did not show significant alterations caused by the seasons. A quadratic effect was observed for both evaluation seasons of the year.
Harvest frequency did not affect CT concentration in the rainy season. Conversely, plants harvested in the dry season at the 98-day frequency had higher CT concentrations. The 98-day harvest frequency resulted in the lowest and highest TPC concentrations in rainy and dry seasons, respectively (Table 2), however, in the dry season showed no differences with the 77 harvest regime.
Table 2. Effects of interaction between Stylosanthes genotypes and seasons of the year on C fraction of carbohydrates (%)

Carbohydrates fraction C: indigestible carbohydrates. SEM, standard error of the mean. Means followed by the same uppercase letters within a row, and the same lowercase letter within a column, do not differ from each other by the Tukey's test (P < 0.05).
Genotypes differed (P < 0.05) in NDF, ADF and ADL contents (Figs 3a and 3b). The highest contents of NDF (520 g/kg DM) and ADF (375 g/kg DM) were found in S. mucronata and Campo Grande, while the lowest ADL concentrations were observed in these genotypes (40 g/kg DM) compared to S. seabrana and S. scabra. NDF, ADF and ADL concentrations in S. seabrana and S. scabra were 477, 343 (Fig. 3a) and 36 g/kg DM (Fig. 3b), respectively.
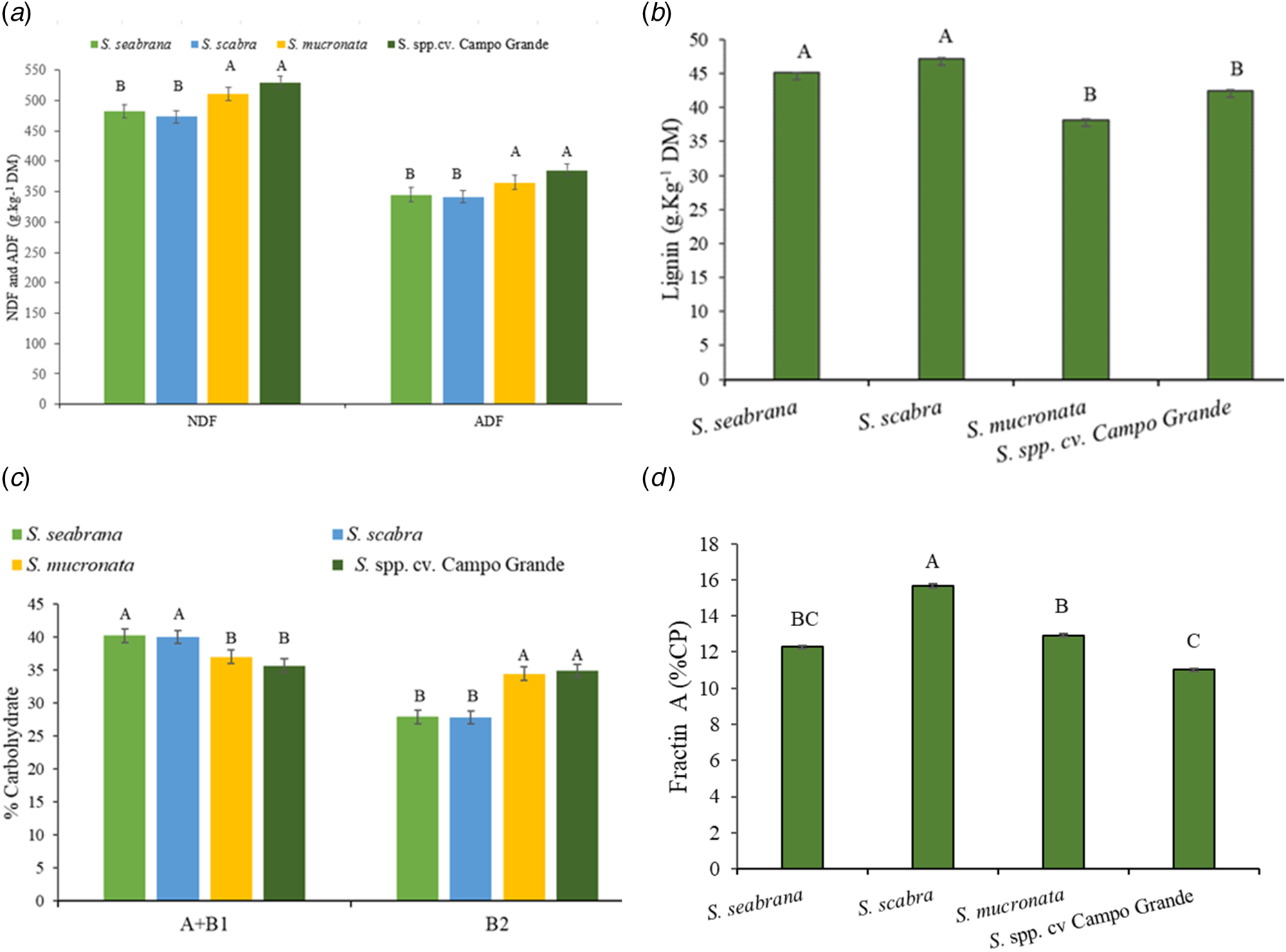
Figure 3. NDF, ADF (a) and ADL contents (g/kg of DM) (b), A1 + B1(non-fibre carbohydrates) and B2 fractions (carbohydrates slowly degraded in the rumen) of total carbohydrates (c), and protein A fraction (non-protein N) (d) of Stylosanthes genotypes grown in the sub-wet tropical region of Pernambuco. Uppercase letters compare the genotypes. Bars indicate the standard error of the mean.
Carbohydrates fractionation
Divergences inherent to the Stylosanthes genotypes were observed (P < 0.05) in A + B1 and B2 fractions of carbohydrates (Fig. 3c). S. seabrana and S. scabra presented the highest proportion of A + B1 and the lowest of B2. In addition, the dry season caused a higher proportion (P < 0.05) of the A + B1 carbohydrate fraction (395 g/kg TC) to the plants than in the rainy season (369 g/kg TC).
The B2 fraction was significantly affected (P < 0.05) by the interaction between harvest frequency and season of the year (Table 1). The 56-day frequency led to the lowest proportion of B2 fraction within the rainy season, while the longest harvest regime (98 days) provoked the lowest B2 fraction proportion of carbohydrates within the dry season.
Interaction of the harvest regime and Stylosanthes genotype affected (P < 0.05) the carbohydrates' C fraction (Table 2). Within the rainy season, S. seabrana and Campo Grande stood out with the highest proportion of C fraction, which was not recorded in the dry season, where all genotypes showed similar proportions of fraction C.
Protein fractionation
Stylosanthes genotypes differed (P < 0.05) in terms of protein fraction A (Fig. 3d). S. scabra showed the highest proportion (160 g/kg CP), while Campo Grande had the lowest (110 g/kg CP).
The Stylosanthes genotype–season of the year interaction affected the B1 + B2, B3 and C fractions of protein (P < 0.05) (Table 3). About 11% increment in B1 + B2 fraction in S. seabrana and S. mucronata was observed when comparing dry to the rainy season. Regardless of the season, Campo Grande stood out with the highest B1 + B2 proportion. For the B3 fraction, higher proportions were found in S. seabrana and S. mucronata than in the others during the rainy season, although no differences were observed for S. seabrana when compared with dry season. In contrast, only S. seabrana had the highest B3 proportion within the dry season. Regarding protein fraction C, S. scabra showed lower proportions in both seasons, while Campo Grande displayed it only in the rainy season.
Table 3. Effects of interaction between Stylosanthes genotypes and seasons of the year on protein fractions

Fraction B1 + B2, composed of true soluble nitrogen (fraction B1) plus soluble nitrogen into neutral detergent (fraction B2); fraction B3, nitrogen insoluble in neutral detergent; fraction C, nitrogen insoluble in acid detergent; SEM, standard error of the mean.
Means followed by the same uppercase letters within a row, and the same lowercase letter within a column for the same variable, do not differ from each other by the Tukey's test (P < 0.05).
Dry matter yield
DM production was affected by Stylosanthes genotype–cutting frequency of the year interaction (P < 0.05) (Table 4). No differences were observed between genotypes within the 56-day harvest regime. S. scabra and Campo Grande and S. seabrana had higher DM yield when harvested at 77 and 98-day cut intervals, respectively. The DM yield of S. scabra and S. seabrana showed a quadratic polynomial effect.
Table 4. Dry matter yield (t/ha of DM) of Stylosanthes genotypes as a function of different cutting frequencies
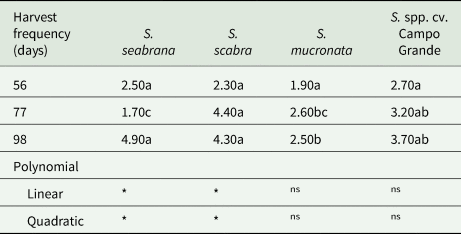
ns, not significant.
Means followed by the same lowercase letters within a row do not differ from each other by the Tukey's test (P < 0.05).
*significant at 5% probability (S. seabrana: Y = 24.17–0.64X + 0.0045X 2, R 2 = 0.3798; S. scabra: Y = −13.53 + 0.41X–0.0024X 2, R 2 = 0.2810).
Standard error of the mean: 0.5494.
Discussion
Nutritional value
Modifications caused by management practices, such as harvest frequency, can alter plants' growth patterns and structural traits. The highest DM contents observed in S. mucronata and Campo Grande (Fig. 2a) possibly resulted from morphological traits such as long branches and few leaves per plant (Diniz, Reference Diniz2020). Silva et al. (Reference Silva, Jobim, Nascimento, Ferreira, Silva and Três2013) reported dissimilar results in which Campo Grande showed 267 g/kg of DM at 100-day regrowth.
DM content in plants subjected to the 98-day harvest frequency (Fig. 2b) indicates advancement in the plant's maturity stage. C 3 plants like Stylosanthes species have slower growth than C 4 plants. Increasing the age of plants results in a proportional increase in DM concentration, leading to a decrease in the concentration of non-structural constituents and ultimately reducing the forage nutritional quality. Contrarily, the lower DM content observed in the rainy season was because of the greater water availability, which provided higher CP content and structural growth of the plants during this period.
Low DM content indicates that animals must ingest larger forage volume to meet nutritional requirements. Furthermore, the high moisture forage content accelerates the digesta passage rate throughout the gastrointestinal tract without satisfactory uptake of nutrients by animals (Gindri et al., Reference Gindri, Moraes and Teixeira2021).
Overall, in semiarid regions, most rangelands' productivity and nutritional value may decrease with advancing the physiological stage and depending on environmental conditions (Klabi et al., Reference Klabi, Bell, Hamel, Iwaasa, Schellenberg and St-Arnaud2018). The differences in CP content between the dry and rainy seasons were likely due to water deficit. Under water-restricted conditions, stomatal closure in plants reduces carbon absorption by limiting gas exchange (Carvalho et al., Reference Carvalho, Barreto, Prado, Habermann, Martinez and Branco2020). Furthermore, the limitation of photosynthesis and reduction in chlorophyll concentration occurs because of decreases in N absorption, which is required to synthesize proteins and other N compounds.
CP values found in the rainy and dry seasons (184 and 153 g/kg DM, respectively) underline the high biological value of Stylosanthes genotypes and their potential for use in animal feeding. The average CP values for both seasons were sufficient to meet the 7% requirement level recommended by NRC (2001), ensuring the minimum demanded by ruminants to stimulate voluntary forage intake and supply the necessary substrate for ruminal bacteria to accomplish fermentation.
The evaluated genotypes showed minor changes in nutritional value (Table 1) with the plant's advancing age (Akakpo et al., Reference Akakpo, Boer, Adjei-Nsiah, Duncan, Giller and Oosting2020), even though the forage plants are constantly maturing, and their quality is not static. These observations in nutritional value could be related to the photosynthetic C 3 metabolism of legume plants, which gives them a slower growth rate than grasses. Thereby, legumes can maintain their nutritional levels longer than grasses. These factors, combined with good acceptability, contribute to improved animal performance.
During the rainy season, the 98-day harvest frequency provided low-IVDDM materials (Table 1). The higher digestibility in the dry period could be explained by reduced cell growth, one of the most sensitive physiological processes to water deficit. Both cell elongation and cell wall synthesis are diminished in water-restriction conditions, so the plant stops growing and the forage harvested tends to be more digestible (Lopes and Lima, Reference Lopes and Lima2015).
Low CT levels in the genotypes suggest a potential to enhance livestock production by improving the inner microbial ecosystem. A higher concentration of TC and TP was observed by the frequency of 98 days in the dry season. These results demonstrate the importance of phenolic compounds for growth, reproduction and plant protection under adverse environmental conditions (Table 1). CT and TP biosynthesis are related to environmental conditions plus plant age and can vary with abiotic factors associated with changes in temperature, water content, photosynthetic radiation, UV exposure and mineral nutrient deficiency (Oliveira et al., Reference Oliveira, Santos, Muir, Cunha, Souza, Tedeschi, Neuamann, Mello and Meireles2022).
The lack of differences in some chemical constituents between harvest frequencies comprises an essential competitive advantage for using Stylosanthes legumes because the leading organic components' concentration of plant varies according to the plant's maturity. Besides, unlike other forage plants, Stylosanthes genotypes do not show marked declines in nutritional value as the plant's age advances.
NDF content of S. mucronata and Campo Grande (Fig. 3a) ranged from 511 to 530 g/kg DM, approaching the limiting value for consumption. NDF content is related to the top DM intake and roughages with concentrations near 550 g/kg DM limit feed and energy intakes (Tirado-Estrada et al., Reference Tirado-Estrada, Tirago-González, Medina-Cuéllar, Miranda-Romero, González-Reyes, Sánchez-Olmos and Castillo-Züñiga2020).
NDF concentrations associated with ADF content were observed in the S. mucronata, and Campo Grande (Fig. 3a) showed reduced digestibility. Silva et al. (Reference Silva, Jobim, Nascimento, Ferreira, Silva and Três2013) state that forages with ADF values close to or greater than 400 g/kg of DM are ingested less and have lower digestibility. Comparable results were reported by Silva et al. (Reference Silva, Oliveira, Aroeira, Chaves, Ponciano, Braga and Lima Júnior2015), who evaluated leaves and thin stems of S. humillis at the beginning of the reproductive phase and observed values of 170 g/kg of CP, 508 g/kg of NDF, 354 g/kg of ADF and 38 g/kg of ADL. Musco et al. (Reference Musco, Koura, Tudisco, Awadjihè, Adjolohoun, Cutrignelli and Calabrò2016) evaluated the nutritional value of S. scabra in the vegetative phase and observed 126 g/kg of CP, 496 g/kg of NDF, 482 g/kg of ADF, 80 g/kg of ADL and 600 g/kg of IVDDM. Such findings put the Stylosanthes genotypes as excellent protein sources for animal feed, but their high concentration of NDF and ADF may limit DM intake.
Carbohydrate fractionation
The highest proportion of the A + B1 fraction (sugars, organic acids, starch and pectin) in S. scabra and S. seabrana genotypes (Fig. 3c) was a consequence of the higher number of leaves and shorter branches (Diniz, Reference Diniz2020). The increased A + B1 fraction is beneficial for synthesizing starch and pectin (non-fibre carbohydrates). The higher proportion of A + B1 fraction in the dry (39.5%) than in the rainy season (37%) resulted from a lower growth rate in the dry period owing to a lower cell thickening and elongation that probably yielded a lower cell wall and higher cell content (Wilson and Mertens, Reference Wilson and Mertens1995). The A + B1 carbohydrate fraction contributed to the highest IVDDM values (Table 1) as it is the primary energy source for ruminal microorganisms and cell multiplication.
The highest proportion of C fraction in the dry season (Table 3) was observed in Campo Grande. The C fraction comprises a cell wall portion not digested in the gastrointestinal tract, and it is less frequent in plants' leaves and young tissues (Sniffen et al., Reference Sniffen, O'connor, Van Soest, Fox and Russell1992). Increases in the C fraction and reductions in A + B1 suppress the energy available for microorganisms that ferment fibre and non-fibre carbohydrates owing to the C fraction indigestibility, less potential consumption per unit of time and reduced animal production (Brandstetter et al., Reference Brandstetter, Costa, Silva, Araújo Neto, Silva, Neves and Oliveira2018). It is desirable to use forage species with low C fraction proportions, especially those native to semiarid or tropical climates. Silva et al. (Reference Silva, Jobim, Nascimento, Ferreira, Silva and Três2013) analysed the nutritional value of Campo Grande harvested at 100 days of regrowth and found proportions of 25.4, 29.5 and 45.4% for fractions A + B1, B2 and C fractions, respectively. These findings indicate that cropping conditions and harvest frequencies directly affect the proportion of carbohydrates in the genotypes.
Protein fractionation
The genotype S. scabra has the highest percentage of fraction A (non-protein N) (Fig. 3d), which suggests that it can serve as a viable N source for ruminal microorganisms. S. scabra stood out for the high amount of N in fraction A, which was associated with the availability of carbohydrates in fraction A + B1 (Fig. 3c). This fraction undergoes rapid ruminal degradation, characterized by synchrony in the degradation rates of these nutrients in the rumen.
The lowest proportions of C fraction were observed for S. scabra and Campo Grande (Table 3), demonstrating great N availability for ruminal microbiota. The protein C fraction in feedstuffs is crucial because it represents the total N unavailable to the animal since it comes from ADF-protein complexes. Thus, decreasing the C/CP total fraction ratio makes more CP available for animal metabolism, leading to improved protein digestibility.
Forage legumes are great roughage sources to improve the nutritional status of ruminants in tropical regions by providing forage with high nutritional value. Knowing the roughage's nutritive value is vital for formulating energy and protein-balanced rations (Gruber et al., Reference Gruber, Terler and Knaus2018). Legumes are forage with great nutritional value and can potentially mitigate enteric methane emissions (Beauchemin et al., Reference Beauchemin, Kreuzer, O'mara and McAllister2008; Suybeng et al., Reference Suybeng, Charmley, Gardiner, Malau-Aduli and Malau-Aduli2019). It is worth mentioning that, in addition to assessing nutritional value, the forage species' choice must consider aspects such as adaptation, yield and persistence. Furthermore, it is important to consider the potential of plants to stimulate feed intake and, consequently, improve animal performance. The bromatological traits of Stylosanthes spp. did not decline sharply owing to plant age advancement, indicating these genotypes are suitable for forage production systems in tropical or semiarid regions.
Dry matter yield
The lack of difference in DM yield of genotypes, comparing dry to rainy seasons, may have resulted from their adaptation to water deficit conditions. Oliveira et al. (Reference Oliveira, Queiróz, Romão, Silva and Brasileiro2016) reported that Stylosanthes species tolerate rainfall regimes varying from 280 to 1247 mm/year, values lower than those observed in our trial period.
S. mucronata had a low DM yield, likely due to its upright growth habit and morphological characteristics. These observations are consistent with a study by Costa (Reference Costa2017), who observed that S. mucronata had the lowest leaflet length and width averages. In addition, the study found a high correlation (0.82) between leaf DM production and the characteristic leaflet width for S. mucronata.
DM yield results differed from those of Muraina et al. (Reference Muraina, Olanite, Onifade, Ojo, Ewetola, Akinlolu and Akintunde2017), who evaluated S. guianensis cv. Cook and S. hamata cv. Verano subjected to two cropping methods (direct planting and minimum tillage) and obtained a DM production of 9.0 t/ha for S. guianensis in minimum tillage and 4.0 t/ha for the direct planting cropping. Lower productions were obtained for S. hamata, which presented values of 5.0 and 5.75 t/ha for minimum tillage and direct planting, respectively. However, according to FAO (2018), S. scabra has an average production of up to 9.0 t/ha in regions with high rainfall, with a leaf DM production ranging from 0.18 to 1.18 t/ha. S. scabra is widely used in Australia to enrich native pastures or intercrop grasses and in tropical regions in China and Africa as a protein supplement for animals reared by smallholder farmers (Mpanza and Hassen, Reference Mpanza and Hassen2015).
Conclusions
Stylosanthes genotypes showed little differences in nutrient contents. However, the adopted harvest frequencies, besides the seasons of the year, promoted some changes. The 56-day harvest regime may result in better levels of NDF, and IVDDM for the dry season, whereas the rainy season provides the most favourable nutritional traits.
The harvest frequencies evaluated did not affect the CP fractions. S. scabra, originating from the semiarid region of Pernambuco, has a high availability of CP owing to its low ratio of unavailability to total N.
Considering greater accumulation of forage, the S. scabra must be harvested with a collection frequency of 77 days, while S. seabrana requires less frequent harvests (98 days).
Acknowledgements
The National Council for Scientific and Technological Development (CNPq) received financial support. The Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE), for the doctoral scholarship.
Author contributions
W. P. S. Diniz, M. V. F. Santos, M. V. Cunha and M. A. Lira Junior conceived and designed the study, performed statistical analyses and wrote the manuscript. W. P. S. Diniz, L. S. Santos, O. F. Oliveira conducted data gathering. D. E. Simões Neto, G. G. Leal and A. C. L. Mello executed the work and revised the content.
Funding statement
This study was partially funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001.
Competing interests
None.
Ethical standards
Not applicable.