Growing numbers of studies have revealed that vegetable and fruit consumption is inversely associated with colorectal cancer risk( Reference Aune, Lau and Chan 1 ). Concomitantly, there has been increasing interest in phytochemicals( Reference Macdonald and Wagner 2 , Reference Miller and Snyder 3 ), such as flavonoids( Reference Kocic, Kitic and Brankovic 4 ), polyphenols( Reference Kumar, Shibata and Helm 5 ) and phytosterols( Reference Woyengo, Ramprasath and Jones 6 ), which could be responsible for the protective effect of vegetable and fruit against colorectal cancer. Phytosterols are derivatives of the parent molecule 4-desmethyl sterol and are found exclusively in plant food. They are 28- or 29-carbon alcohols that structurally resemble 27-carbon cholesterol( Reference Darnet and Rahier 7 ). The most common phytosterols in the human diet are β-sitosterol, campesterol, stigmasterol. All phytosterols contain a double bond at carbon–5; when this double bond is saturated under certain conditions, these compounds are referred to as plant stanols, the two most common of which are β-sitostanol and campestanol( Reference Bradford and Awad 8 ). Oils, nuts, legumes, cereals, vegetables and fruits are the main dietary sources of phytosterols( Reference Racette, Lin and Ma 9 , Reference Han, Yang and Feng 10 ).
Regarding their bioactivity, phytosterols have been proposed not only to lower serum cholesterol levels( Reference Wu, Fu and Yang 11 ) but also to reduce cancer risk by mediating cell cycle arrest, inducing cellular apoptosis( Reference Choi, Kong and Kim 12 ) and reducing cellular oxidative stress( Reference Baskar, Al and Gabriel 13 ), all of which have been experimentally verified. Moreover, epidemiological studies have reported that phytosterol consumption could be beneficial in reducing the risk of cancer in the lung( Reference Mendilaharsu, De Stefani and Deneo-Pellegrini 14 ), breast( Reference Ronco, De Stefani and Boffetta 15 ), oesophagus( Reference De Stefani, Brennan and Boffetta 16 ), stomach( Reference De Stefani, Boffetta and Ronco 17 ) and ovaries( Reference McCann, Freudenheim and Marshall 18 ). Regarding the anticancer effect of phytosterols on colorectal cancer, Nair et al.( Reference Nair, Turjman and Kessie 19 ) found that Seventh-day Adventists who consumed high levels of phytosterols in their diets had a decreased risk of developing colorectal cancer compared with the general population. On the other hand, a case–cohort study conducted in the Netherlands found no reduction in colorectal cancer risk in relation to a high intake of any phytosterol( Reference Normen, Brants and Voorrips 20 ).
It was reported that total vegetable and fruit consumption was 440 g/d among men and 502 g/d among women in the Chinese population( Reference Yu, Zhang and Gao 21 ), which is higher than the consumption of people from European countries (397 g/d)( Reference Leenders, Siersema and Overvad 22 ). To date, no study has focused on the relationship between the intake of plant-origin nutrients, phytosterols and colorectal cancer risk in the Chinese population. Therefore, the present work aims to examine this issue. As a previous investigation concluded that a high consumption of vegetables and fruit is negatively associated with colorectal cancer( Reference Luo, Fang and Lu 23 ), we hypothesised that a higher intake of phytosterols from plant foods could decrease the risk of colorectal cancer.
Methods
Study subjects
This is an ongoing case–control study that began in July 2010. Potential case subjects were consecutively recruited from patients admitted to the surgical units of the Sun Yat-sen University Cancer Center, Guangzhou, China. Eligible cases were required to be patients with incident, primary, histologically confirmed colorectal cancer diagnosed no more than 3 months before the interview, aged 30–75 years, and that either were natives of Guangdong province or had lived in Guangdong for at least 5 years. Subjects were excluded if they could not understand or speak Mandarin/Cantonese or had a history of any cancer. Furthermore, subjects with an energy intake that was too low or too high (<2510 or >14 644 kJ/d (<600 or >3500 kcal/d) for women, <3347 or >17 573 kJ/d (<800 or >4200 kcal/d) for men)( Reference Nimptsch, Zhang and Cassidy 24 ) were not included in the analysis. From July 2010 to June 2016, 2012 eligible cases were identified and 1805 were successfully interviewed, with a response rate of 89·71 %; 207 patients did not complete the investigation, mainly due to fatigue, communication barriers and refusal. Three subjects with an energy intake that was too low or too high were not included in the analysis. Finally, 1802 cases were included in the analysis.
Two control groups were used in this case–control study. The first control group was recruited from the in-patients admitted to three affiliated hospitals of the Sun Yat-sen University during the same period, with eye disorders, ear–nose–throat diseases, trigeminal neuralgia, varicose veins, osteoarthritis, degenerate joint disease, orthopaedics, facial paralysis and acute appendicitis. The second control group was recruited from residents of the same community via advertisements, written invitations or referrals. The eligibility criteria for the controls were the same as described for the cases except that they had no history of colorectal cancer. They were frequency-matched by age (5-year interval) and sex to the cases. In total, 1059 hospital-derived controls were identified and 924 were successfully interviewed in the study, yielding a participation rate of 87·25 %. In addition, 889 community-derived controls were interviewed.
We assumed that people with higher phytosterols intake was 25 % among the general population, the estimated OR between phytosterols intake and colorectal cancer risk was 0·67( Reference Normen, Brants and Voorrips 20 ), the significant P value was <0·05 (α=0·05), the power of test was 90 % (β=0·1), and the response rate was 80 %, thus leading to the calculated sample size of 979 cases.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures involving human subjects were approved by The Ethical Committee of School of Public Health, Sun Yat-sen University. All the participants signed informed consent forms before the interview.
Data collection
Both cases and controls were interviewed face-to-face by trained interviewers using a structured questionnaire that collected information on socio-demographic characteristics, family history of cancer, lifestyle (e.g. active and passive smoking, alcohol consumption and physical activity) and body measurements (weight and height). For female subjects, menstrual and reproductive histories were also obtained. Relevant medical data such as diagnosis and histological findings were extracted from the hospital medical records. In this study, smokers were defined as people who had smoked at least one cigarette a day for more than 6 months consecutively or accumulatively in their lifetime( 25 ). Passive smokers were defined as non-smokers who reported being exposed to the smoke exhaled by smokers for at least 15 min/d over a week. Regular drinking was defined as the consumption of alcohol at least once per week during the past year. The level of physical activity was assessed based on self-reported occupational, household and leisure activity in the previous year. Frequency (d/week) and duration (h/d) data were collected for household and leisure activity. According to labour intensity, occupational activity was classified as follows: (a) not working or retired; (b) long time sitting; (c) low intensity; (d) moderate intensity; or (e) vigorous intensity, with examples provided. Household and entertainment activities were combined in this study. They were categorised into light physical activity (walking, etc.), moderate physical activity (mountaineering, jogging, playing table tennis, etc.) and vigorous physical activity (running, playing football/basketball, etc.). The mean metabolic equivalent task (MET)-hours value of each activity was acquired by estimating the average of all comparable activities in the Compendium of Physical Activities( Reference Ainsworth, Haskell and Whitt 26 , Reference Ainsworth, Haskell and Herrmann 27 ). MET-hours per week over the past 12 months was calculated as follows: number of days per week×number of hours per day×MET for a specific type of activity=MET-hours per week. Postmenopausal status was defined as at least 12 months since the last menstrual cycle. BMI was calculated as the ratio of weight (kg) to squared height (m2).
The subjects completed an interviewer-administered FFQ that included eighty-one food items and assessed their dietary habits. The main food groups included cereal products, legumes, vegetables, fruits, red and processed meat, poultry, fish and other seafood, egg, dairy products and nuts. Dietary information was requested from the cases covering the year before diagnosis and from controls covering the year before interview. The participants were asked to report how often they consumed each food and the average amount eaten each time. Food photographs were used to help participants to quantify the portions consumed. Information on cooking oil intake was also collected. The subjects were asked to report how much cooking oil was consumed by the whole family in 1 month, the number of family members and which kind of oils they preferred to use (such as peanut oil, rapeseed oil, maize oil and blend oil). Then the quantity of cooking oil for each subject consumed per day was calculated. Phytosterol intake was estimated by summing the amount of food consumed and the phytosterol content, which was estimated according to a previous report in China( Reference Han, Yang and Feng 10 ). In total, five frequently occurring phytosterols were measured: the unsaturated plant sterols β-sitosterol, campesterol and stigmasterol, and the saturated plant stanols β-sitostanol and campestanol. In the present study, all plant-derived foods were considered to be sources of phytosterols, including plant oils, cereal products, legumes, nuts, vegetables and fruits. The 2002 Chinese Food Composition Table was used to calculate energy and other nutrient intakes( Reference Yang, Wang and Pan 28 ). As part of the study, we also collected information on whether study participants had changed their dietary habits in the past 5 year before the interview. Study participants were considered as having significant dietary changes if they reported ‘Yes’ to the following questions: ‘Compared with the previous years, have you changed your dietary habits in the past five year?’
The FFQ has been validated and tested for reproducibility( Reference Zhang and Ho 29 ), and has been used in previous studies( Reference Luo, Fang and Lu 23 ). The energy-adjusted Pearson’s correlation coefficients comparing the FFQ and the six 3-d dietary records were 0·25–0·65 for nutrients and 0·30–0·68 for food groups. The correlation coefficients for the reproducibility of the FFQ were 0·46–0·71 for nutrients and 0·36–0·66 for food groups.
Statistical analysis
SPSS 21.0 (SPSS Inc.) was used to conduct the data analysis. The χ 2 test was used to test the difference between the cases and controls for the categorical variables, and the t test or Wilcoxon’s signed-rank test was used for the continuous variables. Dietary phytosterol intake was adjusted based on total energy intake of men and women, using the regression residual method( Reference Willett, Howe and Kushi 30 ). Phytosterol consumption was categorised into quartiles (Q1–Q4) based on the distribution among controls for men and women separately. Unconditional logistic regression models were used to estimate the OR and 95 % CI for the associations between phytosterol intake and colorectal cancer risk, using the lowest quartile as the reference group. According to the comparison of the characteristics of the cases and controls or previous reported confounders, the following variables were selected for multivariable-adjusted models: age, sex, marital status, residence, education, occupation, income level, BMI, smoking status, passive smoking, alcohol drinking, family history of cancer, occupational physical activity, household and leisure-time activities, intake of dairy products, red and processed meat. All confounding factors were included as categorical variables except for age, BMI, household and leisure-time activities, intake of dairy products, red and processed meat which were treated as continuous variables. Consumption of phytosterols, dairy products, red and processed meat in the multivariable model was adjusted for energy intake according to the residual method. Tests for trend were performed by entering the categorical variables as continuous variables in the multiple regression models.
Stratified analysis by sex was conducted. The interaction effect between phytosterols intake and sex was performed. Interaction terms were calculated as the products of the stratified factor (sex, dichotomous) and the phytosterols intake (continuous). Subgroup analysis by cancer site (colon or rectal cancer) and by sources of controls (community-derived controls and hospital-derived controls) was conducted. Sensitivity analysis was also performed by excluding study subjects who had dietary changes during the past 5 years, because of the possibility that pre-diagnosis symptoms might alter the dietary intake. In addition, the Pearson’s correlation coefficient between intakes of total phytosterols and total dietary fibre was calculated. In this study, all P values were two-sided and a P value <0·05 was deemed to denote statistical significance.
In the present study, the sample size of 1003 cases and 906 controls in two quartiles (Q1 and Q4) gave us greater than 99 % power to detect the OR of 0·52 for the association between total phytosterols intake and colorectal cancer risk. We also had greater than 99 % power to detect the OR of 0·48, 0·43 and 0·60 for β-sitosterol, campesterol and campestanol, respectively. However, the power was less than 65 % for stigmasterol and β-sitostanol.
Results
Among the 1802 cases, 1011 were men and 791 were women. In total, 1091 were diagnosed with colon cancer and 711 with rectal cancer. The distribution of their socio-demographic and several relevant characteristics are shown in Table 1. Compared with the controls, more cases lived in rural area, were less educated, had higher incomes, had a higher frequency of smoking, passive smoking and regular drinking experiences, engaged in more occupational activities, engaged in fewer household and leisure activities and tended to have a family history of cancer. No significant differences were found between the case and control subjects in age, sex, marital status, occupation, BMI, age at menarche and menopausal status.
Table 1 Demographic and selected risk factors of colorectal cancer cases and controls in Chinese populationFootnote * (Mean values and standard deviations; medians and 25th, 75th percentiles)

MET, metabolic equivalent task.
* Continuous variables were evaluated using t tests or Wilcoxon rank-sum tests. Categorical variables were evaluated using χ 2 tests.
† Among female subgroup.
The total intake of five phytosterols was 202·6 (sd 76·3) mg/d for cases and 225·9 (sd 79·2) mg/d for controls; the difference between these values was statistically significant. Compared with the controls, the cases had a higher intake of red and processed meat, and a lower intake of total energy, β-sitosterol, campesterol, campestanol and dairy products. No significant difference was found in dietary intake of stigmasterol and β-sitostanol between cases and controls (Table 2).
Table 2 Intakes of energy, phytosterol and foods among case and control subjects in Guangdong, ChinaFootnote * (Mean values and standard deviations; medians and 25th, 75th percentiles)

* Wilcoxon’s rank-sum test comparing the median consumption levels between cases and controls.
† The consumption was adjusted for total energy intake by the regression residual method.
The OR and 95 % CI of colorectal cancer for phytosterols are shown in Table 3. For total phytosterols, the highest quartile intake showed a risk reduction of 50 % compared with the lowest quartile (OR 0·50; 95 % CI 0·41, 0·61, P trend<0·01). For the specific phytosterols, after controlling for the potential confounders, the OR of the highest quartile intake compared with the lowest quartile intake were 0·46 (95 % CI 0·37, 0·57, P trend<0·01) for β-sitosterol, 0·42 (95 % CI 0·34, 0·52, P trend<0·01) for campesterol and 0·67 (95 % CI 0·54, 0·83, P trend<0·01) for campestanol. However, stigmasterol was found to be associated with an increased risk of colorectal cancer, with an adjusted OR of 1·26 (95 % CI 1·03, 1·54, P trend=0·01) comparing the highest with the lowest quartile. No statistically significant association was found between β-sitostanol intake and colorectal cancer risk.
Table 3 OR of colorectal cancer according to quartiles (Q) of phytosterol intake (Odds ratios and 95 % confidence intervals)

* OR was adjusted for age, sex, marital status, residence, education, occupation, income level, BMI, smoking status, passive smoking, alcohol drinking, family history of cancer, occupational physical activity, household and leisure-time activities, dietary intake of dairy products, red and processed meat.
Stratified analysis by sex showed that the intake of total phytosterols, β-sitosterol and campesterol was inversely associated with colorectal cancer risk in both sexes, whereas no significant association was found between β-sitostanol intake and colorectal cancer risk neither in men nor women. Stigmasterol intake was found to be positively associated with colorectal cancer risk only among women. Compared with the lowest quartile, the highest quartile had an adjusted OR of 1·50 (95 % CI 1·11, 2·03, P trend<0·01). No significant association was found between stigmasterol intake and colorectal cancer risk among men. Campestanol intake was found to be related to a decreased risk of colorectal cancer only among men (Table 4).
Table 4 OR of colorectal cancer according to quartiles (Q) of phytosterol intake by sex (Odds ratios and 95 % confidence intervals)
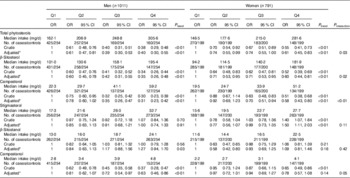
* OR was adjusted for age, marital status, residence, education, occupation, income level, BMI, smoking status, passive smoking, alcohol drinking, family history of cancer, occupational physical activity, household and leisure-time activities, dietary intake of dairy products, red and processed meat.
The results of the subgroup analysis by cancer site were similar to the main analysis in all colorectal cancer cases. The consumption of total phytosterols, β-sitosterol, campesterol and campestanol appeared to be inversely associated with the risk of both colon cancer and rectal cancer. For the intake of stigmasterol and β-sitostanol, both of them were observed to be related to an increased risk of colon cancer. Compared with the lowest quartile, the highest quartile had an adjusted OR of 1·41 (95 % CI 1·12, 1·79, P trend<0·01) and 1·35 (95 % CI 1·07, 1·71, P trend<0·01), respectively. However, no significant association was found between the consumption of stigmasterol or β-sitostanol and the risk of rectal cancer (Table 5). Furthermore, subgroup analysis by community-derived controls and hospital-derived controls was conducted. The inverse associations between the intake of total phytosterols, β-sitosterol and campesterol and colorectal cancer risk were more evident when using the hospital-derived controls than using the community-derived controls. Stigmasterol intake was positively associated with colorectal cancer risk when only using community-derived controls, whereas β-sitostanol intake was positively related to colorectal cancer risk when only using hospital-derived controls (Table 6). Sensitivity analysis by excluding participants who had dietary changes during the past 5 years (146 cases and 204 controls) did not materially change the results (data not shown). The energy-adjusted Pearson’s correlation coefficient between intake of total phytosterols and total dietary fibre was 0·41 (P<0·01) (data not shown).
Table 5 OR of colorectal cancer according to quartiles (Q) of phytosterol intake by cancer site (Odds ratios and 95 % confidence intervals)

* OR was adjusted for age, sex, marital status, residence, education, occupation, income level, BMI, smoking status, passive smoking, alcohol drinking, family history of cancer, occupational physical activity, household and leisure-time activities, dietary intake of dairy products, red and processed meat.
Table 6 OR of colorectal cancer according to quartiles of phytosterol intake in two control groups (Odds ratios and 95 % confidence intervals)

* OR was adjusted for age, sex, marital status, residence, education, occupation, income level, BMI, smoking status, passive smoking, alcohol drinking, family history of cancer, occupational physical activity, household and leisure-time activities, dietary intake of dairy products, red and processed meat.
Discussion
In this case–control study, a significant inverse association was found between the risk of colorectal cancer and the consumption of total phytosterols, β-sitosterol, campesterol and campestanol, whereas stigmasterol intake was related to an increased risk of colorectal cancer. Stratified analysis by sex showed that the positive association of stigmasterol intake with colorectal cancer risk was found only among women. Subgroup analysis by cancer site showed that stigmasterol and β-sitostanol intake was positively associated with colon cancer risk.
It was reported that the Seventh-day Adventists who consumed high levels of phytosterols in their diets had a decreased risk of developing colorectal cancer compared with the general population( Reference Nair, Turjman and Kessie 19 ). Moreover, a series of case–control studies performed in Uruguay revealed that dietary phytosterol intake exerts a potential protective effect on lung( Reference Mendilaharsu, De Stefani and Deneo-Pellegrini 14 ), breast( Reference Ronco, De Stefani and Boffetta 15 ), oesophageal( Reference De Stefani, Brennan and Boffetta 16 ) and stomach cancer( Reference De Stefani, Boffetta and Ronco 17 ). These studies recruited 100–500 newly diagnosed patients with specific cancers. Total phytosterol intake was inversely associated with the risk of lung cancer for all cell types (OR 0·50; 95 % CI 0·31, 0·79, P trend<0·01), breast cancer (OR 0·37; 95 % CI 0·24, 0·57, P trend<0·01), oesophageal cancer (OR 0·27; 95 % CI 0·10, 0·70, P trend<0·01) and stomach cancer (OR 0·33; 95 % CI 0·17, 0·65, P trend=0·01). These findings are similar to our current results. However, in two other case–control studies from the USA, there was no significant association between total phytosterol intake and the risk of prostate( Reference Strom, Yamamura and Duphorne 31 ) and ovarian( Reference McCann, Freudenheim and Marshall 18 ) cancer. Instead, the consumption of stigmasterol was found to be positively associated with prostate cancer risk (OR 2·09; 95 % CI 1·19, 4·43, P trend=0·03) but inversely associated with ovarian cancer risk (OR 0·42; 95 % CI 0·20, 0·87, P trend<0·05). These two studies included eighty-three prostate cancer cases and 124 ovarian cancer cases. Moreover, in a case–cohort study conducted in the Netherlands, no reduction in colorectal cancer risk( Reference Normen, Brants and Voorrips 20 ) was related to a high intake of any phytosterol.
Several possible explanations might account for these inconsistent results. First, dietary patterns vary between different countries. The amount of phytosterol consumption might be different due to differences in phytosterol content in food. In our study, the mean intake of total phytosterols among the controls was 225·9 mg/d, which was derived from plant oils, cereal products, vegetables and fruits, legumes and nuts. In the Uruguayan case–control study( Reference Mendilaharsu, De Stefani and Deneo-Pellegrini 14 ), the mean consumption of total phytosterols among controls was 144 mg/d and the main food sources were maize, legumes, bananas and apples. The average American diet( Reference Racette, Lin and Ma 9 ) contained 154 mg of phytosterols for adults consuming 8368 kJ/d (2000 kcal/d). The Netherlands study mentioned above reported that the daily phytosterol intake was 307 mg for men and 263 mg for women( Reference Normen, Brants and Voorrips 20 ). The total consumption of β-sitosterol and stigmasterol among the non-vegetarians in American general population was 77·9 mg/d( Reference Nair, Turjman and Kessie 19 ). Second, factors such as the type of phytosterol (plant sterol or plant stanol), the phytosterol sources and the cooking methods used may affect the bioavailability and bioactivity of phytosterols( Reference Racette, Lin and Ma 9 ). A previous investigation using animal models found that intestinal absorption of phytosterols was selective: campesterol was absorbed better than β-sitosterol and stigmasterol was only minimally absorbed( Reference Ling and Jones 32 ). In addition, refined grain products contain fewer phytosterols than their whole grain counterparts( Reference Han, Yang and Feng 10 ) and boiling may variably affect the phytosterol content of vegetables( Reference Normen, Johnsson and Andersson 33 ).
The potential mechanisms of the protective effect of most of the phytosterols against colorectal cancer include inhibition of the cell cycle progression, induction of cellular apoptosis and the reduction of cellular oxidative stress. In a study of the effects of β-sitosterol on the growth of HCT116 human colon cancer cells, Choi et al.( Reference Choi, Kong and Kim 12 ) found a dose-dependent growth inhibition coupled with an increase in a sub-G1 cell population. β-Sitosterol induced cell cycle arrest by regulating the expression of apoptosis-related proteins. Moreover, Baskar et al.( Reference Baskar, Al and Gabriel 13 ) reported that β-sitosterol inhibited the depletion of antioxidant enzymes and restored the levels of nonenzymatic antioxidants in a 1,2-dimethylhydrazine-induced colon carcinogenesis rat model. This suggests that β-sitosterol possesses antioxidant properties that can protect cells from damage by reactive oxygen species. In addition, previous prospective studies reported that a high level of serum total cholesterol( Reference Yao and Tian 34 ) and a high dietary cholesterol intake( Reference Jarvinen, Knekt and Hakulinen 35 ) was associated with an increased risk of colorectal cancer. As phytosterols are structurally similar to cholesterol, phytosterols can compete with cholesterol absorption in the intestines, leading to lower serum cholesterol levels( Reference Ellegard, Andersson and Normen 36 ) and inhibiting the proliferation of cancer cells( Reference Ramprasath and Awad 37 ).
The result of the positive association between stigmasterol intake and colorectal cancer risk was contrary to our hypothesis. Probably, it was only a chance finding. Although one case–control study from the USA( Reference Strom, Yamamura and Duphorne 31 ) reported positive association between stigmasterol intake and prostate cancer risk and one case–cohort study from the Netherlands( Reference Normen, Brants and Voorrips 20 ) reported positive association between stigmasterol intake and colon cancer among men, it was not possible to draw any firm conclusions at this time. More studies, especially with prospective study design, are needed to clarify this association.
Stratified analysis by sex showed that the intake of stigmasterol was positively related to the risk of colorectal cancer only among women. Similarly, one case–cohort study conducted in the Netherlands( Reference Normen, Brants and Voorrips 20 ) also observed a positive association between the consumption of stigmasterol and proximal colon cancer among women (OR 2·52; 95 % CI 1·34, 4·75). This sex difference may be due to dietary complexities. It was reported that typical dietary patterns differed between men and women( Reference Northstone 38 ), suggesting that different combinations of food groups or nutrients in men’s and women’s diets may have distinct effects on the carcinogenesis of colorectal cancer. As shown in our study, the amount of phytosterols intake among men was higher than that among women, which may partly explain the stronger inverse association found in men. Moreover, tumour carcinogenesis may be regulated by sex hormones. An animal study indicated that high intake of plant sterols accelerated intestinal tumourigenesis in female (Apc)Min mice( Reference Marttinen, Pajari and Paivarinta 39 ), and reduced the concentration of free cholesterol in intestinal mucosa only in male mice. A significant positive correlation was found between tumour number and the level of β-oestrogen receptors in female mice. One study in Korea reported that the ratio of the α- and β-oestrogen receptors was important with regard to colorectal cancer risk( Reference Kim and Kim 40 ). However, to date, the mechanism underlying this sex difference has not been clarified. Therefore, whether the association between phytosterols and colorectal cancer risk is modified by sex still needs further investigation.
Subgroup analysis by cancer site presented a positive association between intake of stigmasterol, β-sitostanol and the risk of colon cancer. Similar to these results, one case–cohort study in the Netherlands( Reference Normen, Brants and Voorrips 20 ) observed that higher consumption of stigmasterol was related to an increased risk of colon cancer in men (OR 1·84; 95 % CI 1·14, 2·96), and an increased risk of proximal colon cancer in women (OR 2·52; 95 % CI 1·34, 4·75). Furthermore, animal study( Reference Quilliot, Boman and Creton 41 ) found that feeding the rats with a mixture of phytosterols as the human diet had no protective effect on the colon carcinogenesis. The probable reason of which was the phytosterols supplement had a negative effect on bacterial activity( Reference Quilliot, Boman and Creton 41 ). Nevertheless, this issue needs to be justified with further studies.
Both hospital-derived and community-derived controls were used in this study to strengthen the association between dietary phytosterols intake and colorectal cancer risk. The results showed that the inverse associations between the intake of total phytosterols, β-sitosterol and campesterol, and colorectal cancer risk were more evident when using the hospital-derived controls than using the community-derived controls. One possible reason might be that the control subjects admitted to the hospitals were much younger than the community residents. The average age was about 51·0 years for hospital-derived controls and 61·5 years for community-derived controls, respectively. It is well-known that the incidence of cancer increases with age( Reference Chen, Zheng and Zhang 42 ). Therefore, it was likely that the protective effect of phytosterols intake was stronger when using the younger hospital-derived controls than by using older community-derived controls. The positive association of stigmasterol intake with colorectal cancer risk was observed when using only community-derived controls, and the positive association of β-sitostanol intake with colorectal cancer risk was found when using only hospital-derived controls. This might be a chance finding. More studies are needed to clarify this association.
It was reported that a higher dietary fibre intake was associated with a reduced risk of colorectal cancer( Reference Aune, Chan and Lau 43 ). So, dietary fibre may be a potential confounder when examining the association between phytosterols intake and colorectal cancer risk. The intake of dietary fibre and total phytosterols was significantly correlated in the present study, with the correlation coefficient of 0·41. Therefore, to avoid the over-adjustment, dietary fibre was not included in the multivariable model. Moreover, it was reported that about 60 % of dietary fibre was derived from vegetables and fruits in Chinese population( Reference Zhong, Fang and Pan 44 ). However, vegetables and fruits only contributed 13·5 % to the total phytosterols intake( Reference Han, Yang and Feng 10 ). About 40 % of the total phytosterols intake in Chinese people was from edible oil( Reference Han, Yang and Feng 10 ), which was free from dietary fibre. Therefore, the inverse association between phytosterols intake and colorectal cancer risk was probably not confounded by dietary fibre.
The present study has the following strengths. First, a validated FFQ was used to assess the frequency and quantity of phytosterol intake. Second, we adjusted several potential confounders, including dietary and non-dietary factors, in the analysis. In addition, the sample size is larger than in previous relevant case–control studies: we therefore have enough power to detect small associations with the risk of colorectal cancer. Furthermore, this is the first study to examine the relationship between phytosterol intake and colorectal cancer risk in the Chinese population, which might help to produce targeted dietary guidance for this group.
The present study has several potential limitations. First, selection bias is difficult to rule out in hospital-based case–control studies. Although the colorectal cancer patients were recruited from only one hospital, Sun Yat-sen University Cancer Center, it is the biggest cancer centre in Southern China. The colorectal patients at this centre and those from other two big hospitals in Guangdong or in mainland China therefore shared similar clinical characteristics. Moreover, the high participation rate (90 % for cases and 87 % for hospital-derived controls) also helped to reduce selection bias in our results. Second, as in all case–control studies, recall bias is inevitable. To minimise this bias, we included only newly diagnosed cases and interviewed them as soon as possible after diagnosis. The average time interval between the diagnosis of colorectal cancer and study interview was 11·0 d for the case subjects. In addition, food photographs with usual portion sizes were also provided to help the participants to estimate their intake more accurately. Third, random measurement error of diet is also of concern in the estimation of usual intake, since this misclassification bias is likely to be nondifferential, leading the OR to the null. This measurement error is therefore of less importance and our results may tend to be conservative. Fourth, although various dietary and non-dietary factors were adjusted in our multivariable analysis, residual confounding factors may still remain. Fifth, there is a concern that colorectal cancer cases might change their dietary habits due to pre-diagnosis symptoms and lead to the lower nutrients and food intake. However, sensitivity analysis by excluding study subjects with dietary changes showed no substantial change. Therefore, the pre-diagnosis symptoms of colorectal cancer, even if they might have, should not be a serious problem.
In conclusion, this study shows that dietary intakes of total phytosterols, β-sitosterol, campesterol and campestanol are inversely associated with colorectal cancer risk in a Chinese population. The positive association between stigmasterol intake and colorectal cancer risk observed in the present study needs to be further justified.
Acknowledgements
The authors gratefully acknowledge the cooperation of the study participants; without them the study would not have been possible.
This study was supported by Guangdong Natural Science Foundation (no. 2014A030313188, 2016A030313225). The funders had no role in the design, analysis or writing of this article.
The authors’ responsibilities were as follows: H. J. conducted the data collection, analysed the data and writing of this paper. M. X., M.-S. L. and W.-Q. H. participated in the data collection and data entry. Y.-J. F. and Z.-Z. P. were responsible for connecting and coordinating the field work. Y.-M. C. provided significant advice regarding the analyses and interpretation of the data. C.-X. Z. constructed the project design, supervised and contributed to the manuscript writing.
The authors declare that there are no conflicts of interest.









