Anxiety symptoms are common, and if excessive in magnitude, duration and frequency, can lead to clinical anxiety (Mallorquí-Bagué et al., Reference Mallorquí-Bagué, Bulbena, Pailhez, Garfinkel and Critchley2016; Mehta et al., Reference Mehta, Simonsick, Penninx, Schulz, Rubin, Satterfield and Yaffe2003). Both symptomatic and clinical anxiety co-occur with cardiovascular health problems (Allgulander, Reference Allgulander2016; Celano et al., Reference Celano, Daunis, Lokko, Campbell and Huffman2016; Chang et al., Reference Chang, Chen, Lee, Chen, Yang and Chen2016; Emdin et al., Reference Emdin, Odutayo, Wong, Tran, Hsiao and Hunn2016; Janszky et al., Reference Janszky, Ahnve, Lundberg and Hemmingsson2010; Pratt et al., Reference Pratt, Druss, Manderscheid and Walker2016; Vogelzangs et al., Reference Vogelzangs, Seldenrijk, Beekman, van Hout, de Jonge and Penninx2010). Longitudinally, anxious individuals have an elevated risk of coronary heart disease (CHD), independent of demographic variables (e.g., age), biological risk factors (e.g., family history) and lifestyle/health behaviors (e.g., exercise; Roest et al., Reference Roest, Martens, de Jonge and Denollet2010). Somatic symptoms of anxiety, such as heart palpitations, are also associated with an increased CHD risk in women (Nabi et al., Reference Nabi, Hall, Koskenvuo, Singh-Manoux, Oksanen, Suominen and Vahtera2010), highlighting a physiological, autonomic pathway in which anxiety may link to cardiovascular events (Celano et al., Reference Celano, Daunis, Lokko, Campbell and Huffman2016).
Autonomic dysfunction, an imbalance between parasympathetic and sympathetic control, may contribute to this cardiovascular burden. To test this, cardiovascular autonomic functioning has been measured using three indices: baroreflex sensitivity (BRS), interbeat interval (IBI) and heart rate variability (HRV). BRS reflects efficiency in responding to blood pressure (BP) changes. Short-term regulation of BP is achieved through baroreceptors, which detect an increase in BP, resulting in a reduction of heart rate through inhibition of sympathetic activity and activation of parasympathetic flow. The inverse occurs when BP is decreasing (Shaffer et al., Reference Shaffer, McCraty and Zerr2014; Swenne, Reference Swenne2013). There is limited research on the anxiety–BRS relationship and existing studies mainly take a clinical perspective (Mussgay & Rüddel, Reference Mussgay and Rüddel2004).
Nonetheless, anxiety symptoms have been associated with lowered BRS by up to 36%, independent of demographic variables and existing cardiovascular health predictors (Virtanen et al., Reference Virtanen, Jula, Salminen, Voipio-Pulkki, Helenius, Kuusela and Airaksinen2003; Watkins et al., Reference Watkins, Blumenthal and Carney2002; Watkins et al., Reference Watkins, Grossman, Krishnan and Sherwood1998). The association is apparent over and above depression (Watkins et al., Reference Watkins, Grossman, Krishnan and Blumenthal1999) and recorded in response to stress, argued to induce a shift in autonomic reactivity (Ginty et al., Reference Ginty, Kraynak, Fisher and Gianaros2017). Following a stress-inducing task, young adults scoring high on trait anxiety display lower BRS compared to their low trait anxiety peers (Sanchez-Gonzalez et al., Reference Sanchez-Gonzalez, Guzik, May, Koutnik, Hughes, Muniz and Fincham2015). Furthermore, this highly anxious group had an attenuated BRS comparable to a middle-aged sample, suggesting cardiovascular outcomes similar to that produced with aging. Anxiety symptoms may therefore associate with reduced parasympathetic activity both at baseline and in response to stress.
Varying IBIs, the period in between successive heartbeats, is a marker of healthy cardiovascular autonomic functioning (Costa et al., Reference Costa, Davis and Goldberger2017). Typically measured as the time between ‘R’ peaks on an electrocardiogram (ECG), IBI is also referred to as the ‘RR interval.’ Clinical anxiety studies suggest shorter IBIs, indicative of increased heart rate with low variability (Hoehn-Saric et al., Reference Hoehn-Saric, McLeod and Zimmerli1991; Thayer et al., Reference Thayer, Friedman and Borkovec1996).
HRV can be defined as the overall fluctuation of heart period over time (Chalmers et al., Reference Chalmers, Quintana, Abbott and Kemp2014). HRV can be indexed using several methods, including based on IBI time series (e.g., mean interbeat interval, mIBI), or frequencies (low, LFIBI, 0.04–0.15 Hz; and high, HFIBI, 0.15–0.40 Hz) and nonlinear techniques (e.g., Poincaré plots). Being a common marker of psychological wellbeing, cardiovascular health and mortality (Chalmers et al., Reference Chalmers, Quintana, Abbott and Kemp2014; Kemp & Quintana, Reference Kemp and Quintana2013), HRV has been negatively associated with anxiety phenotypes. For example, HRV as measured in short time periods in the high-frequency band has been associated with generalized anxiety (Chang et al., Reference Chang, Chang, Tzeng, Kuo, Lu and Huang2013b; Yeragani et al., Reference Yeragani, Tancer, Seema, Josyula and Desai2006), panic disorder (Chang et al., Reference Chang, Chang, Tzeng, Kuo, Lu and Huang2013a; Wang et al., Reference Wang, Yeon, Hwang, Lee, Kweon, Lee and Lee2013) and social anxiety (Gaebler et al., Reference Gaebler, Daniels, Lamke, Fydrich and Walter2013; Pittig et al., Reference Pittig, Arch, Lam and Craske2013). As with BRS and IBI studies, research focus is on clinical anxiety, which does not represent its dimensional, quantitative nature in the population (Bjelland et al., Reference Bjelland, Lie, Dahl, Mykletun, Stordal and Kraemer2009; Kircanski et al., Reference Kircanski, LeMoult, Ordaz and Gotlib2017).
There is also sparse research on what underlies this autonomic dysfunction. According to neurobiological models (Friedman, Reference Friedman2007; Thayer & Lane, Reference Thayer and Lane2000), anxiety reflects poor inhibition of cognitive (e.g., worry), affective (e.g., panic), behavioral (e.g., avoidance) and physiological (e.g., increased heart rate) responses, reducing autonomic and physiological flexibility (Thayer et al., Reference Thayer, Yamamoto and Brosschot2010). Sex differences have also been reported, whereby women show decreased parasympathetic activity in comparison to men (Fiol-Veny et al., Reference Fiol-Veny, De la Torre-Luque, Balle and Bornas2018; Koenig et al., Reference Koenig, Rash, Campbell, Thayer and Kaess2017), though findings are inconclusive, with other studies indicating increased heart variability in women (Snieder et al., Reference Snieder, van Doornen, Boomsma and Thayer2007). There is also limited behavioral genetic research on the common genetic (pleiotropy) and environmental influences that could link the two domains as they appear in the normal population. Twin studies are imperative in understanding this relative contribution to individual differences in traits, as done with anxiety symptoms previously (Ask et al., Reference Ask, Torgersen, Seglem and Waaktaar2014; López‐Solà et al., Reference López‐Solà, Fontenelle, Alonso, Cuadras, Foley, Pantelis and Harrison2014; Nivard et al., Reference Nivard, Dolan, Kendler, Kan, Willemsen, Beijsterveldt and Boomsma2015; Petkus et al., Reference Petkus, Gatz, Reynolds, Kremen and Wetherell2016). Previous studies, using the same sample as used here, suggest genetic influences on BRS, high frequency band (HFIBI) and mIBI, plus on the relationship between neuroticism and BRS (Riese et al., Reference Riese, Rijsdijk, Ormel, van Roon, Neeleman and Rosmalen2006, Reference Riese, Rosmalen, Ormel, Van Roon, Oldehinkel and Rijsdijk2007). Yet, these parameters have not been investigated in the context of anxiety symptoms.
This behavioral genetics study uses a genetically sensitive twin design to explore: (1) how anxiety symptoms correlate with the three cardiovascular autonomic measures (mIBI, HFIBI, BRS), (2) the extent to which individual differences in anxiety symptoms and cardiovascular autonomic measures are determined by latent genetic and environmental factors and (3) the genetic and environmental underpinning of the associations between the two domains.
Materials and Methods
Participants
This study capitalizes on the Twin Interdisciplinary Neuroticism Study (TWINS; Riese et al., Reference Riese, Rijsdijk, Snieder and Ormel2013), of the Groningen Twin Register (GTR) in the north of the Netherlands. Monozygotic (MZ) and dizygotic (DZ) twins were identified for the GTR based on being born between 1972 and 1992 from the same mother with identical birth dates. In the current study, we used data from a subset of female twin pairs (N = 250) aged 18−30 who participated in a laboratory session as part of TWINS. Individuals with existing cardiovascular health problems were not considered for inclusion of the study. The study was given ethical approval by the Ethics Committee at the University Medical Center Groningen, and all individuals provided written consent (METc 2000/060e).
Measures
Anxiety symptoms
We included four measures of anxiety symptoms for each twin. This included a mix of both state and trait measures to gauge an overall anxiety symptoms’ composite. First, a trait anxiety sum score was derived from four items in the eight-item Hopkins’ Symptom Checklist (HSCL), a validated psychometric tool to measure general psychological distress (Derogatis et al., Reference Derogatis, Lipman, Rickels, Uhlenhuth and Covi1974). Second, a single-item state anxiety score was measured by the Profile of Mood States (POMS) questionnaire (McNair, Reference McNair1971). Third was a single-item state anxiety measure from the Dutch version of the state-trait anxiety inventory (STAI-DY; Defares et al., Reference Defares, van der Ploeg and Spielberger1980). Finally, we included the HSCL anxiety sum score from the co-twin sister, also derived from four items in the HSCL (Twin 1 reporting on Twin 2 and vice versa). This is done to control for self-report bias and decrease variance in self-reported mental health (Kendler et al., Reference Kendler, Prescott, Jacobson, Myers and Neale2002; Riese et al., Reference Riese, Rosmalen, Ormel, Van Roon, Oldehinkel and Rijsdijk2007). All measures were treated as continuous, except for the POMS variable, which was entered as a dichotomous variable (as it is originally ordinal data).
Cardiovascular autonomic functioning
Participants were instructed to abstain from intensive physical activity (including sports) and alcohol consumption 24 h before testing and to fast (including coffee and tea consumption) from 10:00 pm on the evening before visiting the lab. Measurement of cardiovascular autonomic functioning has been described in detail elsewhere (Riese et al., Reference Riese, Rosmalen, Ormel, Van Roon, Oldehinkel and Rijsdijk2007, Reference Riese, Rijsdijk, Snieder and Ormel2013) and outlined in Supplementary Material 1. Briefly, BRS, HRV and mean IBI were measured in an experimental task with four standardized conditions: Rest (R1), stress with visual feedback (S1), stress with auditory feedback (S2) and rest (R2). Participants completed the tasks in a seated posture. In the stress conditions, participants completed a modified version of the ‘emotion face dot probe task’ (Mogg & Bradley, Reference Mogg and Bradley1999), whereby participants indicate whether they saw three or four dots previously occupied by a pair of faces. Visual feedback was presented as the correct answer at the center of the screen for 1000 ms. In the auditory feedback condition, participants’ wrong answers were met with 100 dB of white noise for 500 ms.
Cardiovascular measurements were collected in a sitting position after participants relaxed for 10 min, with each condition lasting approximately 5 min. An ECG was recorded using Ag/AgCl electrodes (3M™ Red Dot™, St Paul, MN, USA) and a custom-made ECG-amplifier and trigger device (ETC-3, DataLab, Faculty of Behavioural and Social Sciences, University of Groningen, The Netherlands). A Portapres device continuously measured beat-to-beat BP from the finger (FMS, Finapres Medical Systems BV; Amsterdam, the Netherlands). As respiration is known to influence BRS, changes in respiration signals were recorded with a flexible band placed on the upper thorax connected to an amplifier. ECG, finger BP and respiration were digitized using a data acquisition board (Keithley DAS-12, Keithley Instruments, Inc., USA) at 100 Hz. Custom-made PreCar 3.0 (Greaves-Lord et al., Reference Greaves-Lord, Tulen, Dietrich, Sondeijker, Roon, Oldehinkel and Huizink2010) software was used for R-peak detection (at ±2 ms accuracy) and artefact correction (i.e., IBI time series with supraventricular extra systoles were excluded).
mIBI (mean of the interbeat intervals, ms), HFIBI (power of interbeat intervals in the high frequency band 0.15–0.40 Hz, ms2) and BRS (gain or modulus, between systolic BP and IBI, in the frequency band 0.07–0.14 Hz, ms/mmHg) were calculated using the CARSPAN spectral analysis program (Mulder, Reference Mulder1988; Riese et al., Reference Riese, Rosmalen, Ormel, Van Roon, Oldehinkel and Rijsdijk2007; Robbe et al., Reference Robbe, Mulder, Rüddel, Langewitz, Veldman and Mulder1987), a method that has been previously used (Althaus et al., Reference Althaus, Van Roon, Mulder, Mulder, Aarnoudse and Minderaa2004; Dietrich et al., Reference Dietrich, Riese, van Roon, Engelen, Ormel, Neeleman and Rosmalen2006; Lefrandt et al., Reference Lefrandt, Hoogenberg, van Roon, Dullaart, Gans and Smit1999; Van Roon et al., Reference Van Roon, Mulder, Althaus and Mulder2004). More details on exclusion criteria can be found in Supplementary Material 1.
Statistical Analyses
Prior to statistical analyses, the effects of age, body mass index (BMI, kg/m2), medication-use, systolic and diastolic BP were regressed out of the cardiovascular autonomic variables in SPSS version 12.0.2 (SPSS Inc., Chicago, IL, USA) to take these confounders into consideration without losing statistical power. Of the MZ twins (N = 148), 15 individuals reported medication use (1 = antihypertensive, 14 = other, not cardioactive, medication). Of the DZ twins (N = 102), 32 individuals used medication (2 = antihypertensives, 30 = other, not cardioactive, medication). Those using cardioactive medications were excluded from the analysis. The effect of medication is found to be marginal (Riese et al., Reference Riese, Rijsdijk, Ormel, van Roon, Neeleman and Rosmalen2006), we therefore decided to account for its effect by regressing this out prior to analyses.
As for BP, this was regressed out due to its known influence on vascular stiffness (including carotid artery stiffness) and can thus influence BRS (Mukai et al., Reference Mukai, Gagnon, Iloputaife, Hamner and Lipsitz2003). We also regressed out the effects of age from the anxiety variables in R statistical environment and subsequently used residuals in the analysis.
Twin Model Fitting Analysis
We analyzed the relationships between anxiety and the three cardiovascular autonomic function measures in a multivariate twin model. The classical twin design rests on the comparison between MZ and DZ twins; MZ twins share 100%, whereas DZ twins share on average 50% of their segregating DNA. We initially estimated similarity in MZ and DZ twin pairs within and across traits (twin correlations). Using biometrical structural equation modeling (SEM), variance of traits is further decomposed into three latent factors: additive genetic influences (A); common/shared environmental influences (C), which contribute to twin pair similarity (e.g., environmental factors affecting both twins in one family) and nonshared environmental factors (E), which contribute to differences between twins within one pair (including random measurement error).
Through standardization, the A, C and E factors represent proportion of variance. For example, heritability (a 2) of a trait is the proportion of variance in that trait due to genetic differences in the population. The same principle applies for standardizing environmental influences (c 2 and e 2). Covariance between traits is also decomposed into etiological correlations (denoted r A, r C and r E), which suggest the extent to which the A, C and E factors underlying variance for one trait also affect the other. Using this etiological information, the phenotypic correlation (rPh) between anxiety and cardiovascular autonomic measures can also be decomposed.
Our multivariate model features a latent anxiety factor (LANX), ascertained by the twins’ self-reported anxiety and co-twin sisters’ report (four measures). The latent BRS, HFIBI and mIBI factors (LBRS, LHF, LIBI) were each determined by four measurements during the experimental conditions. In addition to specific measurement error, we also modeled a ‘rater-bias’ component for the anxiety variables. This considers the additional covariance between a twin’s self-report and what is reported by the co-twin and separates rater bias and unreliability from the latent anxiety factor. The analyses in this article follow previous procedures using the same sample (Riese et al., Reference Riese, Rosmalen, Ormel, Van Roon, Oldehinkel and Rijsdijk2007) with model-fitting conducted in the OpenMx package in R (Neale et al., Reference Neale, Hunter, Pritikin, Zahery, Brick, Kirkpatrick and Boker2016; Neale & Miller, Reference Neale and Miller1997). The full model-fitting procedure is further detailed in Supplementary Material 2.
Results
Table 1 presents general characteristics of the sample and Table 2 details means (SD) for the four experimental conditions.
Table 1. General characteristics of the twin sample with means (SD)

Note: The Profile of Mood states variable is ordinal in nature and we have therefore reported proportions.
Table 2. Means (SD) for BRS, mIBI and HFIBI in each of the four experimental conditions
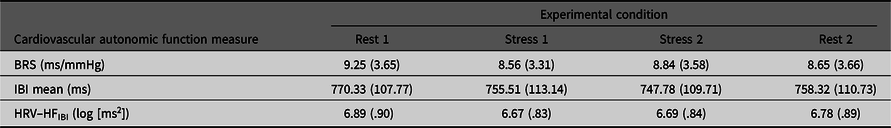
Note: BRS, Baroreflex sensitivity; mIBI, mean IBI; HFIBI, Heart rate variability with IBI power in the 0.15–0.40 Hz frequency band.
Phenotypic Factor Model
The phenotypic factor model (Figure 1) obtains correlations between the latent anxiety and the three cardiovascular autonomic factors (−2 Log L = 11457.38, df = 3542, AIC = 4373.381). Latent anxiety significantly negatively correlated with BRS (r = −0.24, 95% CI [−.42, −.05]). The relationship between latent anxiety and mIBI was also negative but nonsignificant (r = −.15, 95% CI [−.33, .03]) and the same with HFIBI (r = −.16, 95% CI [−.34, .03]). Table 3 outlines these phenotypic (rPh) and other intraclass correlations.
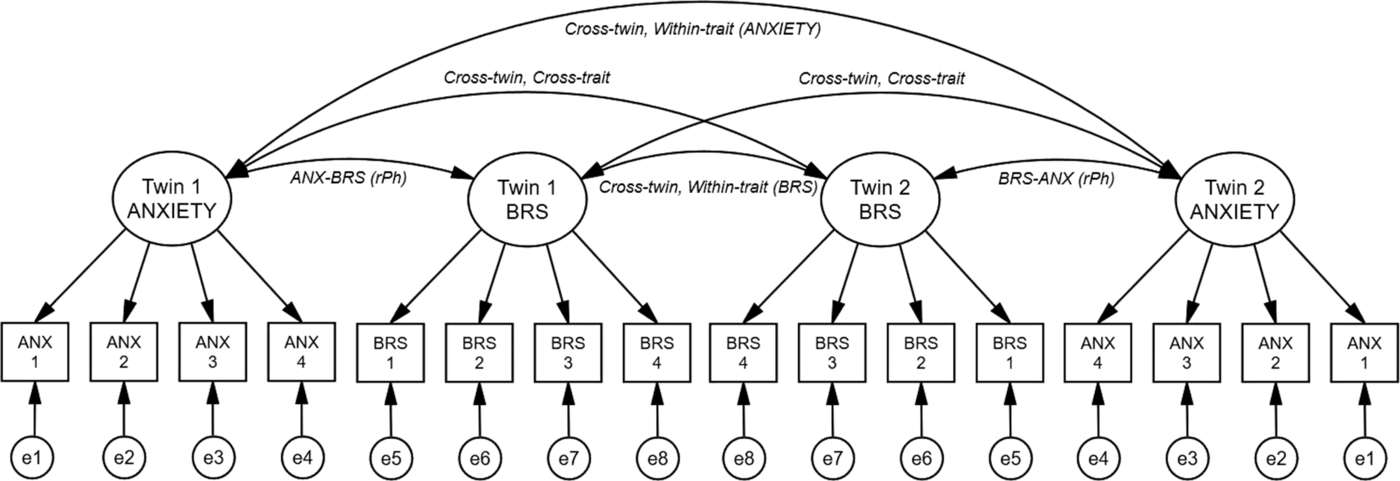
Fig. 1. Phenotypic factor model (including a rater bias component). Note: Phenotypic associations between latent anxiety and baroreflex sensitivity (BRS) factors (for a twin pair). For simplicity, HRV (HFIBI) and interbeat interval (mIBI) were omitted from this figure. Latent (unobserved) factors are depicted in circles, observed (measured) variables shown in rectangles. Twin 1/2 ANXIETY, latent anxiety factor for twin 1/2; Twin 1/2 BRS, latent baroreflex sensitivity factor for twin 1/2. Anx 1, Profile of Mood states anxiety; Anx 2, State anxiety; Anx 3, Hopkin’s Symptom checklist anxiety; Anx 4, Co-twin sibling report of anxiety via the Hopkins symptom checklist. BRS 1–4, four measurements of BRS during the experimental task. Arrows running from latent factors to measured variables indicate path loadings, paths between latent factors represent the phenotypic correlations. ANX–BRS (rPh), phenotypic correlation between latent factors. Cross-twin, within-trait (ANXIETY/BRS), correlations across twins, within latent anxiety and BRS factors; cross-twin, cross-trait, correlations across twins and across latent anxiety and BRS factors; e1-e8, specific unique environmental effects on the measured variables.
Table 3. Twin correlations within and across traits (95% CI) for MZ and DZ twins separately
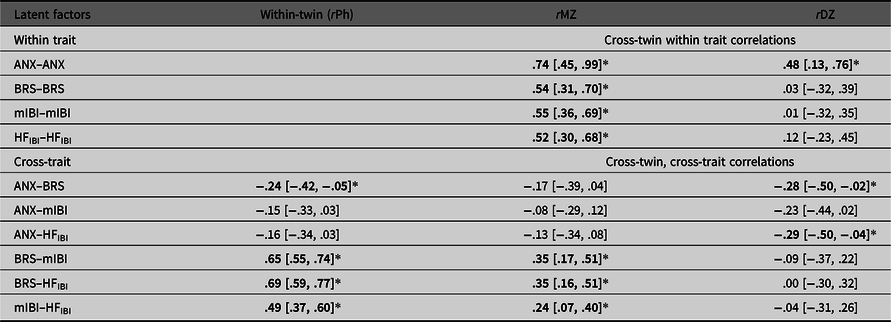
Note: Correlations derived from the Phenotypic Common Pathway model. rPh, phenotypic correlation, that is, the within-twin cross-trait correlations; rMZ, monozygotic twin correlation; rDZ, dizygotic twin correlation; ANX, Anxiety; BRS, Baroreflex sensitivity; mIBI, latent interbeat-interval factor; HFIBI, heart rate variability with IBI power in the 0.15–0.40 Hz frequency band. HFIBI values were log transformed. *Significant correlations in bold type (indicated by the 95% CI not spanning zero).
Genetic Factor Model
The full genetic SEM model, estimating all parameters (−2LL = 11408.13, df = 3510, AIC = 4388.129), was used as a comparison for nested submodels to determine the model with best fit. The final model fixed the A and C specific effects (on measured variables) to zero, apart from one A specific effect on the state anxiety variable (as it was too substantial to drop from the model). There was no significant reduction in fit between the full and final model, and a lower AIC observed (Δ−2LL [Δdf] = 23.35[31], p = .84). We henceforth report results of the final model (Figure 2).

Fig. 2. Genetic factor model (including a rater bias component). Note: The genetic model depicting genetic and environmental contributions to Anxiety, BRS, IBI and HRV (For an individual). Circles depict latent (unobserved) factors, rectangles are observed (measured) variables. ANXIETY, latent anxiety factor; BRS, latent baroreflex sensitivity factor; IBI, latent IBI factor; HRV, latent heart rate variability factor. ANX 1, Profile of Mood States anxiety; ANX 2, State Anxiety; ANX 3, Hopkin’s Symptom checklist anxiety; ANX 4, Co-twin sibling report of anxiety via the Hopkins symptom checklist. A, Additive genetic effects; C, Shared environmental effects; E, Unique environmental effects. e1–e16, Unique environmental effects specific to observed variables; a2, genetic-specific effect on state anxiety. Arrows represent path loadings. Paths running between latent A factors represent genetic correlations. For simplicity, C and E correlations as well as the rater bias component are not shown. Table 5 details the full account of etiological correlations between factors.
Standardized variance components (a 2, c 2 and e 2) of each latent factor were estimated (Table 4), with heritability (a 2) estimates being moderate and significant for mIBI. Genetic correlations were not significant between latent anxiety and any of the cardiovascular autonomic measures (Table 5), with BRS (r g= −.18, 95% CI [−1, 1]); with mIBI (r g = −.13, 95% CI [−1, 1]) or with HFIBI (r g = −.13, 95% CI [−1, 1]). We did, however, find that the phenotypic relationship between anxiety–BRS was mostly explained by shared environmental influences (58%). Rater bias components were nonsignificant for all the anxiety variables.
Table 4. Standardized variance components (95% CI) of latent factors

Note: Contribution of genetic (a 2), common environmental (c 2) and unique environmental (e 2) influences on the variance of the latent anxiety and the three autonomic factors. BRS, Baroreflex sensitivity; mIBI, latent interbeat-interval factor; HFIBI, heart rate variability.
* Bold type indicates significance (indicated by the 95% CI not spanning zero).
Table 5. Genetic and environmental correlations (95% CI) between latent factors
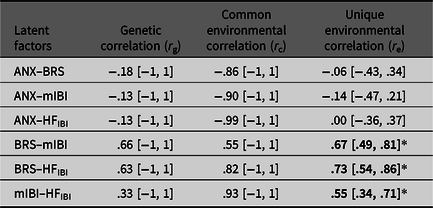
Note: *Significant correlations shown in bold (indicated by the 95% CI not spanning zero).
Discussion
This study investigated the genetic and environmental etiology and relationships between a latent anxiety and three cardiovascular autonomic function factors; BRS, mIBI and HRV in the HFIBI. We report a significant negative correlation between the latent anxiety and BRS factors, mostly driven by shared environmental influences. We did not obtain significant genetic or environmental correlations.
Etiology of Anxiety and Autonomic Measures
We report moderate heritability estimates for the latent anxiety and cardiovascular autonomic measures (35−44%) with the estimate for mIBI being significant. Heritability of the anxiety factor is consistent with previous literature (López‐Solà et al., Reference López‐Solà, Fontenelle, Alonso, Cuadras, Foley, Pantelis and Harrison2014; Nivard et al., Reference Nivard, Dolan, Kendler, Kan, Willemsen, Beijsterveldt and Boomsma2015). Although the estimates for the autonomic measures are lower than those of a previous study using the same sample, reporting heritability estimates ~50% (Riese et al., Reference Riese, Rosmalen, Ormel, Van Roon, Oldehinkel and Rijsdijk2007), the confidence intervals are wide in both studies, indicating the need for larger sample sizes to increase certainty around the point estimates. There are also other possible reasons for this: first, our study fits a latent anxiety factor rather than neuroticism, a different psychological construct, which can change the correlation structure of the twin model. Our cross-twin, cross-trait correlations (Table 3) suggest a higher influence of shared environmental effects explaining covariance between factors, whereas this pattern is reversed in the previous paper (where MZ correlations were higher than DZ, suggesting more genetic influence), although again with wide confidence intervals.
Second, we report a different final model compared to the previous paper and have kept the substantial specific genetic influence on state anxiety as opposed to dropping the parameter completely. Third, our analyses used OpenMx (Neale et al., Reference Neale, Hunter, Pritikin, Zahery, Brick, Kirkpatrick and Boker2016), a relatively new modeling package with a different optimizer than that used in the previous study. Taken together, replication of this study in a larger sample is required to improve precision in estimating genetic and environmental effects.
Relationships between Anxiety and Cardiovascular Autonomic Measures
Higher scores on a latent anxiety factor were correlated with lower BRS, suggesting reduced ability to respond to, and regulate BP with increasing levels of anxiety. Negative correlations were also found between anxiety–IBI and HFIBI but did not reach significance. This may highlight a specific link not only between anxiety–BRS but also necessitates further evidence, as this is currently the first twin study combining the three cardiovascular autonomic measures with anxiety. Nevertheless, our results lend support to the neurovisceral integration model, whereby autonomic flexibility may be reduced with elevated levels of anxiety and stress (Friedman, Reference Friedman2007). We did not, however, find evidence for pleiotropic genetic effects. Aside from sample size, our participants are relatively healthy in terms of anxiety symptoms, creating a restricted range of scores, possibly decreasing power further. The relationship could also be largely environmentally driven, as supported by previous work on cardiovascular autonomic functioning with genetic effects being minimal (Osztovits et al., Reference Osztovits, Horváth, Littvay, Steinbach, Jermendy, Tárnoki and Jermendy2011). The phenotypic relationship between anxiety and BRS was mostly accounted for by shared environmental influences. These are environments that make twins similar, such as the home environment, school attended and peer groups. These environments may foster an anxious profile for the twins reducing BRS or vice versa. Nevertheless, this should be interpreted with caution, given that the shared environmental correlation is nonsignificant and difficulty in pinpointing exact environmental factors from this study alone.
Strengths
Our study has several strengths. This is, to our knowledge, the first multivariate twin analysis combining anxiety symptoms (both state and trait anxiety) with all three cardiovascular autonomic measures (mIBI, HFIBI, BRS). Anxiety was investigated in a dimensional, symptom-based context as opposed to a diagnostic perspective, tapping into the anxiety spectrum rather than a relatively restricted range of scores. Second, our study offers a behavior genetic perspective. Although previous work suggests negative associations between anxiety symptoms and measures of autonomic function, they do not employ twin analyses into the phenotypic, genetic and environmental relationships. Although we were likely underpowered to detect such genetic and environmental effects, our design allowed us to also test these parameters. Our finding of a negative association between anxiety and BRS directly supports previous research and adds to the role of autonomic dysfunction with anxiety phenotypes (Sanchez-Gonzalez et al., Reference Sanchez-Gonzalez, Guzik, May, Koutnik, Hughes, Muniz and Fincham2015; Virtanen et al., Reference Virtanen, Jula, Salminen, Voipio-Pulkki, Helenius, Kuusela and Airaksinen2003; Watkins et al., Reference Watkins, Blumenthal and Carney2002). Third, we controlled for various confounders, including BMI and medication, and as our study was made up of a homogeneous female sample, results were not confounded by sex and a wide age span.
Limitations and Future Directions
First, we were limited by our small female sample. Although eliminating sex- and age-specific confounds, replication is required in larger samples for increased statistical power. It is also worth including males, considering sex differences in anxiety and autonomic functions reported at the phenotypic and genetic level (Koenig et al., Reference Koenig, Rash, Campbell, Thayer and Kaess2017; McLean & Anderson, Reference McLean and Anderson2009). Also, both anxiety (Lee et al., Reference Lee, Gatz, Pedersen and Prescott2016) and cardiovascular health (North & Sinclair, Reference North and Sinclair2012; Paneni et al., Reference Paneni, Diaz Cañestro, Libby, Lüscher and Camici2017) are influenced by age, prompting further research with different age groups. Furthermore, genetic and environmental influences can be age dependent, such that new age-specific factors may emerge over time (Franić et al., Reference Franić, Middeldorp, Dolan, Ligthart and Boomsma2010). A longitudinal twin design can best decipher the stability and change in such genetic and environmental influences.
Second, we measured autonomic functioning in a laboratory setting, with 5-min recordings for each condition. While this may not reflect everyday autonomic functioning, experimental tasks are a widely used, accurate method to investigate autonomic performance (Chalmers et al., Reference Chalmers, Heathers, Abbott, Kemp and Quintana2016; Riese et al., Reference Riese, Rijsdijk, Ormel, van Roon, Neeleman and Rosmalen2006, Reference Riese, Rosmalen, Ormel, Van Roon, Oldehinkel and Rijsdijk2007). Short-term measurements of autonomic functions have been found to be highly reliable, especially with healthy adults (Sandercock et al., Reference Sandercock, Bromley and Brodie2005). Future studies, however, may use the growing work on ambulatory assessment of anxiety and cardiovascular functioning in real time through mobile, wearable technology. This can provide data that are both longitudinal and reflective of everyday tasks. Third, as autonomic functions were measured at one time-point in the TWINS study, we were limited by a cross-sectional design. Although causal inference (e.g., between anxiety and BRS) is not possible here, future work may focus on designs that are closer to establishing causality, including longitudinal research and combining causal inference methods with the twin design (Minică et al., Reference Minică, Dolan, Boomsma, de Geus and Neale2018).
Fourth, our findings are based on European participants. Results may differ across non-Western samples, especially considering culture-specific anxiety syndromes (Koydemir & Essau, Reference Koydemir, Essau, Hodes, Gau and De Vries2018) and differences in cardiovascular health according to ethnicity (El-Gabalawy et al., Reference El-Gabalawy, Mackenzie, Pietrzak and Sareen2014; Li et al., Reference Li, Snieder, Su, Ding, Thayer, Treiber and Wang2009). Further cross-cultural research that incorporates genetically sensitive designs may decipher similarities and differences in anxiety and cardiovascular health markers.
Finally, our results are preliminary. Although we find that environmental influences shared between twins mostly explain the overlap between anxiety and lower baroreflex control, genetic influences should not be ruled out. More recently, genome-wide association studies (GWAS) have begun highlighting common genetic variants associated with anxiety (Alves et al., Reference Alves, Moura, Correia, Nardi, Alves, Moura and Nardi2017; Gottschalk & Domschke, Reference Gottschalk and Domschke2017; Purves et al., Reference Purves, Coleman, Meier, Rayner, Davis, Cheesman and Eley2019) and cardiovascular functions (Nolte et al., Reference Nolte, Munoz, Tragante, Amare, Jansen, Vaez and de Geus2017; Sigurdsson et al., Reference Sigurdsson, Waldron, Bortsov, Smith and Maixner2018). This approach provides insight into genetic etiology at a molecular level and paves the way for polygenic risk scores to identify individuals at-risk for anxiety and autonomic dysfunction. There is, therefore, scope to expand on the twin design reported here. Future clinical applications may involve screening individuals with anxiety for cardiovascular autonomic dysfunction to identify and prevent future cardiovascular complications.
Conclusion
In conclusion, higher scores on a latent anxiety factor were associated with lower BRS and shared environmental factors may possibly underlie this. The ability to respond to and regulate BP may therefore be compromised by increasing levels of anxiety symptoms. Higher anxiety was also related to lower interbeat interval (mIBI) and HRV (HFIBI), but these associations were not significant. We did not find evidence for pleiotropic effects (i.e., relationships due to shared genetic influences), although further research with larger sample sizes is required to determine these findings. Our results suggest a link between anxiety and lower baroreflex control and add to the literature on the governing role of autonomic dysfunction in the associations between anxiety and cardiovascular health.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/thg.2020.47.
Acknowledgments
The TWINS was supported by the Netherlands Organisation for Health Research and Development (ZonMw 904-57-130), and the UK–Netherlands Partnership Program in Science (BR 56-481 and BR 96-229) which is jointly run and financed by the British Council and the Netherlands Organization for Scientific Research (NWO). The authors thank the twins for their participation in our research.
Author contributions
ZN and FR conceived the study. FR and HR were involved in the TWINS study formulation and data preprocessing. ZN performed the twin modeling analysis supervised by FR. ZN and FR wrote the manuscript. AR provided expertise regarding the cardiovascular autonomic measures. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.









