INTRODUCTION
From 2007 to 2009, the southern part of The Netherlands faced large seasonal outbreaks of Q fever in small ruminants and humans [Reference Dijkstra1]. With more than 4000 human cases it was the largest epidemic of Q fever ever reported [Reference Speelman2]. Before the outbreak in 2007, Q fever was reported sporadically in The Netherlands with 5–20 human cases annually [Reference Schimmer3]. After the peak in 2009, the incidence declined to 504 cases in 2010 and 81 cases in 2012 due to veterinary control measures focused on dairy goats and dairy sheep [Reference Hogerwerf4].
Q fever is a zoonosis with no evidence for person-to-person transmission and has a worldwide reservoir in many different animal species [Reference Porter5]. In The Netherlands dairy goat farms, and to a lesser extent dairy sheep farms that experienced clinical signs with abortion waves were implicated as the predominant source of the outbreak [Reference van der Hoek6]. Infection of humans takes place through inhalation of aerosols, contaminated with the causative bacterium of Q fever, Coxiella burnetii. The bacteria are in high loads excreted in abortion, and the birth material of goats and sheep [Reference Angelakis and Raoult7]. The bacterium is very persistent in the environment and can be dispersed several kilometres by wind [Reference Arricau-Bouvery8–Reference Tissot-Dupont10]. Clinical manifestations of acute Q fever are influenza-like illness, pneumonia and hepatitis, but in 50–60% of the cases the infection remains asymptomatic [Reference Angelakis and Raoult7, Reference Tissot-Dupont and Raoult11].
The epidemic in The Netherlands generated a lot of information on Q fever but there are still questions to be answered; the transmission pathways of C. burnetii to humans and the maintenance of the bacterium in the animal reservoirs are not completely understood [Reference Roest12]. In The Netherlands, seroprevalence of Q fever in animals is highest in cattle, but similar seroprevalence figures are reported from other European countries and those countries report very few human Q fever cases [Reference Roest12, 13]. Concerning the influence of environmental factors, previous studies have indicated that vegetation density and soil conditions play a role in the transmission of Q fever to humans [Reference van der Hoek6]. An environmental factor that might play a role in the airborne transmission of C. burnetii, but that has not been investigated before, is the amount of particulate matter in the air [Reference de Bruin14, Reference Hogerwerf15].
The large number of human Q fever cases in The Netherlands and uncertainty about the importance of a number of environmental risk factors, prompted an analysis at the national level. The purpose of this ecological study is to investigate the spatial association between reported Q fever cases and air quality (particulate matter), vegetation density, and animal density. The findings of the study are expected to contribute to a better understanding of the factors that play a role in the transmission of C. burnetii and other airborne zoonotic diseases from animals to humans.
METHODS
Study design
In this geographical ecological study, data were gathered and combined from different existing databases. The four-digit postal code is the smallest unit in The Netherlands for which reliable routine data is available, this was therefore selected as the spatial unit of analysis (4005 postal code areas in total). In The Netherlands, a median postal code area covers an area of 5·3 km2 (range 0·1–132 km2).
Human data
In The Netherlands, acute Q fever is a notifiable disease. Data on Q fever cases were derived from the national infectious diseases surveillance system (Osiris). Notification criteria are a clinical presentation of Q fever combined with a positive laboratory result. The clinical presentation is further defined as fever, pneumonia or hepatitis. Limited patient information is available in Osiris including the four-digit postal code of residential address and day of first clinical symptoms. Population size and urbanization level for each postal code area were obtained from Statistics Netherlands [16].
Veterinary data
Exact numbers of ruminants for each postal code area were available from the registration system of the Ministry of Economic Affairs, Agriculture & Innovation for November 2008 (goats, sheep), November 2009 (goats, sheep) and November 2011 (goats, sheep, cattle). Data for the missing years, 2007 and 2010 for goats and sheep and 2007–2010 for cattle were extrapolated based on available data at municipality level from Statistics Netherlands [16]. In October 2009 mandatory monitoring of C. burnetii DNA in bulk tank milk on dairy goat and dairy sheep farms with more than 50 animals was implemented [Reference Dijkstra1]. Locations of bulk tank milk-positive farms were available at the website of the Food and Consumer Product Safety Authority [17]. Information on locations of farms that experienced Q fever-induced abortion waves were provided by the Animal Health Service. Proximity to an infected farm was calculated from the centroid of a postal code area. In addition, the numbers of goat and sheep farms within 5 km of a postal code area were calculated. A previous study had shown that the risk of infection is highest within 5 km of an infected farm [Reference Schimmer18].
Environmental risk factors
Different environmental datasets were used; particulate matter, land use, and vegetation density. Data on particulate matter with an aerodynamic diameter smaller than 10 μm (PM10) were available from the Dutch Air Quality Monitoring Network at the National Institute for Public Health and the Environment. The level of particulate matter is measured in μm/m3 on a yearly basis at a 1 × 1 km raster level (averaged to postal code area for this study). The land use data was obtained from the Dutch land use database, LGN6 version 2008. This raster dataset with a resolution of 25 m is based on a combination of satellite imagery and ancillary data [Reference Hazeu19]. This dataset is converted from 39 classes in the original dataset to six main land use classes relevant for this study: agriculture, forest, infrastructure, water, buildings, and nature. The class ‘forest’ includes all areas with coniferous and deciduous trees. The ‘nature’ class consists of coastal, heath, swamp and bog areas and if forest is situated within these areas it is included in the class ‘nature’ not the ‘forest’ class. Infrastructure includes major roads and railroads whereas land used for buildings consist of urban areas, buildings in the countryside or green in urban areas [Reference Hazeu19]. Vegetation density is measured by the normalized difference vegetation index (NDVI), which is obtained from the MODIS (moderate resolution imaging spectroradiometer) sensor of the NASA Terra and Aqua satellites. The grid with a spatial resolution of 250 m was aggregated to postal code area level and divided into a low and high vegetation index, based on the threshold value of 0·67 identified by previous research [Reference Brandsma20]. One single image, day 113 of each year (end of April), is used because research indicates that during mid-May incidence of C. burnetii is highest [Reference van der Hoek6]. The average incubation period is 21 days, therefore most transmission would have taken place 3 weeks prior to the highest peak in May [Reference Porten21].
Data analysis
Q fever cases between 2007 and 2011 were included. All analyses were stratified by year of onset of Q fever illness because of slight differences in notification criteria, vaccination and monitoring over the years. In addition, the period 2007–2009, the years with most Q fever cases reported, was analysed separately. A spatial regression analysis was conducted to assess the spatial association between Q fever incidence, environmental variables, and animal densities. Potential risk factors, as described above, for the transmission of Q fever were investigated using a univariate logistic regression analysis. For this analysis the postal code areas were divided into two groups, to distinguish between areas where it is assumed that transmission of Q fever to humans took place (defined as areas with more than one notification) and areas where none or very few cases were observed [Reference Hunink22]. Some of the explanatory variables were dichotomized or categorized with cut-off points based on the literature, the mean, the median or percentiles depending on the distribution of the variable. Density of dairy goats was dichotomized in <1 or ⩾1 dairy goats/km2 to distinguish between areas with and without dairy goats. Most of the dairy goats are kept at commercial farms with large numbers of animals per farm. In 2009, only 10% of the farms had fewer then 10 animals. The average number of dairy goats at a farm was 819 in 2009 (range 1–6251 animals per farm). Furthermore, the cumulative incidence was calculated per 100 000 person-years from 2007 to 2011. These incidences were analysed with a multilevel Poisson regression model. This analysis accounted for correlated cases within postal code areas by using a random effect with a compound symmetry correlation structure. Spatial correlation between postal code areas was not taken into account. Variables in the univariate logistic regression analysis with a P value of <0·2 were included in the multivariate logistic regression model. These variables were tested for collinearity by Spearman's correlation coefficient and a backward approach was applied. In this model, a P value of <0·05 was considered as statistically significant. Odds ratios/relative risks are presented with their 95% confidence intervals (CIs). The statistical and spatial analyses were performed using SPSS statistical software version 19 (IBM, USA) and R statistical software package 2.14.0 (www.r-project.org). ArcGIS, a geographical information system, was used for data pre-processing and compilation of maps (ArcGIS 9.3.1, ESRI, USA).
RESULTS
Descriptive analysis
Between January 2007 and December 2011, 4109 symptomatic laboratory-confirmed cases of Q fever were notified. In Figure 1, the geographical distribution of Q fever incidence per 100 000 persons is shown. The cumulative incidence of human Q fever is not evenly distributed, with higher incidence in the southern part of the country. Similarly, most farms that experienced abortion waves due to C. burnetii or tested positive for C. burnetii in bulk tank milk were also situated in this area. Between 2007 and 2011, there was transmission of Q fever to humans in 392 postal code areas. In 2009, the largest number of postal code areas was affected (257 areas) and in 2011 only five postal code areas experienced more than one notified Q fever case.

Fig. 1 [colour online]. Cumulative incidence (×100 000 population) (2007–2011) of notified Q fever cases (n = 4109) in The Netherlands by postal code area (n = 4005) and location of farms affected by Q fever.
The geographical distributions of livestock are shown in Figure 2. Cattle are widespread over the country with high numbers in the eastern part of The Netherlands. The number of sheep is greatest in the northern and northwestern parts of The Netherlands. The number of goats is particularly high in the east and south but low in the west. The distribution of dairy goats (not shown on the map) is comparable to goats, with especially high numbers in the southern part of the country. Visual comparison of Figures 1 and 2 suggests an association between (dairy) goats and the cumulative Q fever incidence.

Fig. 2 [colour online]. Number of ruminants per km2 at municipal level in 2009 for (a) cattle, (b) sheep, (c) goats.
Univariate analysis of risk factors
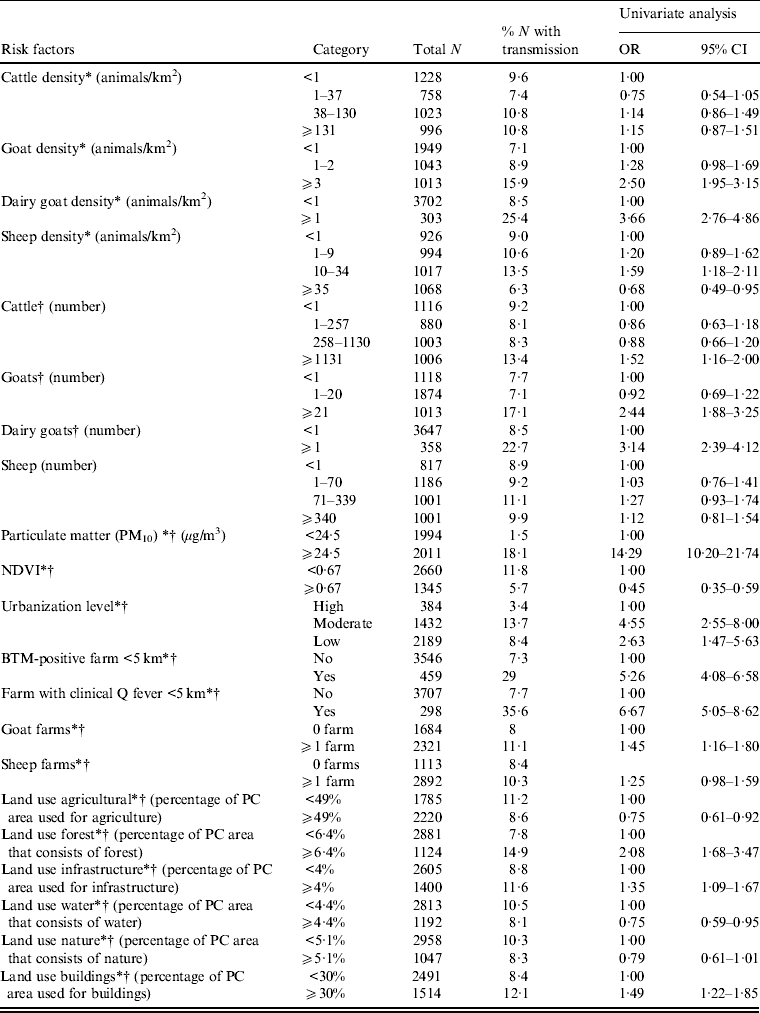
In the univariate analysis, the strongest risk factor for Q fever transmission is a high level of particulate matter (Table 1). Presence of an infected dairy goat farm or bulk tank milk-positive farm at <5 km, and high numbers and density of (dairy) goats are also clearly associated with human Q fever transmission. For cattle only high numbers but not high density is associated with Q fever. There is no association between sheep numbers and human Q fever, but high sheep density is associated with low transmission. Other risk factors are low or moderate urbanization level, and a high percentage of surface area covered with forest, infrastructure and building. High vegetation density as measured by NDVI is associated with low transmission. Dividing the variable particulate matter in four categories (based on percentiles) shows a very high risk for areas with a particulate matter concentration ⩾25·7 μg/m3 compared to areas with a particulate matter concentration <21·8 μg/m3 (Table 2).
Table 1. Univariate logistic regression analysis of risk factors associated with Q fever transmission, 2007–2011, at the four-digit postal code level (n = 4005)

OR, Odds ratio; CI, confidence Interval; NDVI, normalized difference vegetation index; BTM, bulk tank milk; PC, postal code.
* Included in multivariate model with animal density.
† Included in multivariate model with number of animals.
Table 2. Univariate logistic regression analysis of particulate matter and Q fever transmission, 2007–2011

OR, Odds ratio; CI, confidence interval.
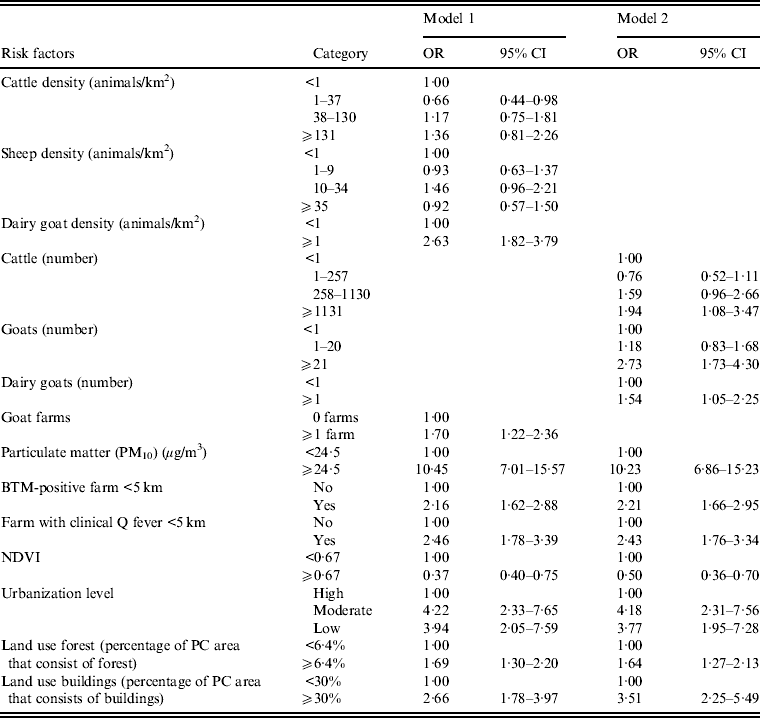
Multivariate analysis of risk factors
Animal densities and animal numbers are highly correlated with each other (Spearman's correlation coefficient of 0·72 for goat numbers and density, P < 0·001). We therefore used two different multivariate models; one model with animal densities (model 1) and a model with the number of animals per postal code area (model 2). High particulate matter remained a very strong risk factor for Q fever in both models (Table 3). Goat density, goat numbers and presence of an infected farm at <5 km remained independent risk factors. Other risk factors independently associated with Q fever transmission at the postal code level are high number (but not density) of cattle, moderate or low urbanization level and postal code areas with relatively more buildings. A high NDVI remained a significant protective factor (Table 3, model 1). Stratified analysis for the years 2008, 2009 and 2007–2009 did not show important different results (data not shown).
Table 3. Multivariate logistic regression analysis of risk factors associated with Q fever transmission, 2007–2011, at the four-digit postal code level (n = 4005), including a model with animal densities (model 1) and a model with the number of animals (model 2)

OR, Odds ratio; CI, Confidence interval; NDVI, normalized difference vegetation index; BTM, Bulk tank milk; PC, postal code.
Blank cells indicate variable not included in analysis.
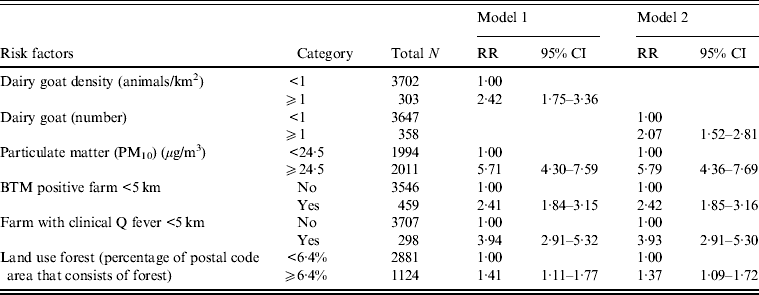
Multilevel Poisson analysis of risk factors
Multilevel Poisson analysis was performed to adjust for variations within postal code areas. The strongest association found between the risk factors and Q fever incidence in the univariate analysis were the presence of an infected farm within 5 km and a high level of particulate matter (see Supplementary material). In the multivariate model with animal densities, shown in Table 4 (model 1), a high level of particulate matter, a farm with clinical Q fever within 5 km and more than one dairy goat/km2 were risk factors for high Q fever incidence. In the model with the number of animals, a high level of particulate matter was the strongest independent risk factor, with relative risks marginally higher than in the model with animal densities, except for land use forest (Table 4, model 2). High numbers and density of cattle are significant risk factors in the univariate model but not in the multivariate model.
Table 4. Multivariate Poisson multilevel model of risk factors associated with Q fever cumulative incidence, 2007–2011, with postal code area as unit of analysis (n = 4005)

RR, Relative risk; CI, confidence interval; BTM, Bulk milk tank.
Blank cells indicate variable not included in analysis. Adjusted for urbanization level.
DISCUSSION
To our knowledge, this study provides the first analysis of environmental determinants for Q fever at the national level for the entire duration of the Q fever epidemic in The Netherlands. The study shows a strong association between high particulate matter concentration in a postal code area and human Q fever incidence.
It is plausible, from a physical and biological point of view that particulate matter plays a role in transmission of C. burnetii and other bacterial zoonotic pathogens from animals to humans. Recent research in goat farms has shown that DNA of C. burnetii can be detected in inhalable airborne dust samples [Reference de Bruin14, Reference Hogerwerf15]. The bacteria attach to fine dust particles and the particles clump together by diffusion and air movements. This allows for efficient airborne transmission in the immediate environment of infected farms. The risk for infection will decline with increasing distance because of deposition of the larger particles. It is unlikely that the relevant particulate matter originates from the infected goat farms themselves. Particulate matter emissions are much greater from poultry farms and pig farms than from goat farms, and in The Netherlands agriculture contributes only 4·5% to the total amount of particulate matter in the air [Reference Velders23]. However, there are large regional differences in concentrations and composition of particulate matter and in rural agricultural areas the contribution of animal husbandry to total particulate matter concentrations is larger than in urban areas, where emissions from traffic play an important role. Literature on health effects of particulate matter almost entirely focus on urban areas although a recent study suggested that people with prolonged exposure to fine particulate matter in a rural area in the south of The Netherlands are more vulnerable for acquiring pneumonia, one of the major manifestations of Q fever [Reference Smit24, Reference Heederik25].
The role of vegetation in the transmission of Q fever from animals to humans has been reported before [Reference van der Hoek6]. Risk for transmission to humans is reduced when higher vegetation densities occur in the direct surroundings of an infected farm. Vegetation is known to reduce the production of dust from erodable surfaces and to remove dust from the air. The weak associations that are found between relatively more forest or buildings in a postal code area and transmission of Q fever seem counterintuitive. However, land use classes were generalized from the original land use dataset and differences between some classes, such as forest and nature are difficult to interpret in the context of C. burnetii transmission [Reference van der Hoek6]. Seasonality for the land use data, which is especially relevant for agricultural uses, was not taken into account in this dataset. The vegetation index, NDVI, is therefore a better indicator because this data is available on a monthly basis. In this study, the vegetation index at the end of April was used, since the incidence of Q fever is highest in mid-May and the mean incubation period is 21 days [Reference van der Hoek6, Reference Porten21]. Conflicting results of relatively more buildings and low or moderate urbanization level can be explained by differences in measurements. The first is measured by percentage of postal code area that consists of buildings (dichotomized) and urbanization level is measured by address density and categorized into three groups.
The present study confirms the previously reported importance of goats in the Q fever epidemic in The Netherlands [Reference van der Hoek6, Reference Schimmer26, Reference Veenstra27] with an assumed linear relationship between number of bacteria that are excreted by goats and risk of infection in humans [Reference Sauter28]. However, the role of cattle remains unclear. While seroprevalence in cattle herds is more than 50% in The Netherlands and other European countries, cattle is generally considered not to play an important role in human Q fever [Reference Roest12, Reference Tilburg29]. We found high cattle numbers but not density, to be a risk factor for Q fever transmission. However, effect estimates were inconsistent and low compared to the effect of goat numbers and density. Results of limited genotyping studies showed that the Coxiella genotype in cattle is different from that in humans and goats [Reference Tilburg29, Reference Tilburg30]. In cattle, clinical manifestations such as abortion waves due to C. burnetii are less common than in goats and sheep [Reference Porter5] and shedding of Coxiella is relatively low. It might well be that people are only infected when in close (occupational) contact with infected cattle. This could also explain the high prevalence of antibodies against C. burnetii in dairy cattle herds in many countries that rarely report clinical human Q fever [Reference Muskens31].
A geographical ecological design such as used in the present study can be useful for decision-making as health policy is usually conducted at the municipality, regional or national level rather than the individual level. Furthermore, the influence of environmental variables is difficult to assess at the individual level. However, a major limitation of the ecological design is that no causal inference can be made between exposures (risk factors) and outcome (Q fever) at the individual level. Furthermore, some potentially important risk factors were not included in the present study, such as smoking behaviour and animal densities other than goats, sheep and cattle. Smoking is a well-established risk factor for Q fever but was not available at postal code level [Reference Veenstra27, Reference Karagiannis32]. Data at a higher level of aggregation (Municipality Health Service level) showed ambiguous results (data not shown). We did not include poultry and pig densities in the analysis as a role for pigs and poultry in Q fever has never been reported. However, PM10 emissions are much higher from poultry and pig farms than from cattle, goat or sheep farms. According to Statistics Netherlands there are about 95 million birds and more than 12 million pigs in the country, compared to four million head of cattle, one million sheep and 400 000 goats.
Residual variations of the random effects in Poisson analysis, with high log-relative risk in the southern part of The Netherlands, suggest that the risk factors in the final model (with animal densities) do not explain the whole Q fever epidemic (data not shown). Culling of pregnant dairy goats on infected farms in 2010 and intensified vaccination of dairy goats since 2009 could have influenced the results of this study; however, stratified analysis for the years 2008, 2009 and 2007–2009 did not show significantly different results compared to 2007–2011. Small differences between the logistic and the Poisson analyses could be explained by the fact that some information is lost when dichotomizing the outcome variable (Q fever notifications) in the logistic regression. Therefore, in case of different outcomes between the two analyses the results of the Poisson analysis may be preferred above the logistic regression. Finally, the use of a multilevel Poisson regression model with a random effect reduces the correlation of cases within postal code areas. This is appropriate for Q fever because there is no person-to-person transmission and the outcome of exposure in one individual is independent of outcome in other individuals [Reference Haus-Cheymol33].
The present study did not take into account the differences in particulate matter composition between urban and rural areas and did not include density of poultry farms and pig farms in the analysis. These are important issues for a large project that will start in 2013, looking at health effects of intensive animal husbandry in the south of The Netherlands. Further quantification of the role of particulate matter in the transmission pathways of zoonotic diseases and attribution of different sources of particulate matter is needed to provide a sound evidence base for possible policy measures to reduce particulate matter concentrations, such as prevention of emission from farms (e.g. by installing air scrubbers in stables), planning vegetation barriers, and keeping safe distances between farms and residential areas.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268813000460.
ACKNOWLEDGEMENTS
We thank Ben Bom (Expertise Centre for Methodology and Information Services of the National Institute for Public Health and the Environment) for helping with the mapping and veterinary data; Jan van de Kassteele (Expertise Centre for Methodology and Information Services of the National Institute for Public Health and the Environment) for his statistical support. FutureWater for providing the vegetation data; Frederika Dijkstra (Centre for Infectious Disease Control of the National Institute for Public Health and the Environment) for providing the surveillance data; Piet Vellema (Animal Health Service) for providing information on locations of farms with clinical Q fever; and the municipal health services for providing human notification data.
DECLARATION OF INTEREST
None.