Significant resources are allocated for prevention, management, and eradication of invasive plants to ameliorate their negative ecological impact. Although most land managers desire to permanently remove (i.e., eradicate) invasive plants, in most cases eradication is not a feasible option for established populations (Rejmánek and Pitcairn Reference Rejmánek and Pitcairn2002). Therefore, invasive plant populations are managed in an ongoing effort to reduce population sizes and their contribution to future generations. Unfortunately, management itself can cause negative externalities, such as reduced native plant cover (Rinella et al. Reference Rinella, Maxwell, Fay, Weaver and Sheley2009) and establishment of secondary invaders (Pearson et al. Reference Pearson, Ortega, Runyon and Butler2016; Skurski et al. Reference Skurski, Maxwell and Rew2013). Therefore, in certain circumstances, managing to mitigate impacts may not result in a net positive impact to the ecosystem.
However, in some instances, local extirpation of an invasive population is possible (Simberloff Reference Simberloff2003). Assuming the invasive plant was responsible for the undesirable ecosystem effects (i.e., a driver of change; MacDougall and Turkington Reference MacDougall and Turkington2005), a return to a pre-invasion state should follow once the invasive plant is removed. Thus, invader eradication is often assumed to be analogous with restoration (Corbin and D’Antonio Reference Corbin and D’Antonio2012). Unfortunately, there is very little evidence of successful eradication leading immediately to an ecosystem functioning as an uninvaded state. Therefore, although restoration is most often the goal of land managers, as Corbin and D’Antonio (Reference Corbin and D’Antonio2012: 117) explain, “Even where local eradication is achieved, removal by itself is unlikely to allow restoration of broader community or ecosystem characteristics.” This lack of restoration following invader removal could be attributed to the invaders not being the drivers of change, though increasing evidence suggests otherwise (Vilà et al. 2011), or their changes being persistent.
Although the physical presence of the invader may be removed through management, residual impacts may remain. Instead of an instantaneous return to an uninvaded ecological state, invader-mediated changes may persist, resulting in a “shadow” of the invader. For example, although a species that increases soil pH may have been locally eradicated, this increase in pH may remain long after the plant material is removed. Impact persistence, termed “legacy effects,” can vary in temporal persistence (Marchante et al. Reference Marchante, Marchante, Freitas and Hoffmann2015) and may have unequal effects across an ecosystem (Cuddington Reference Cuddington2011). In some instances, legacy effects may persist in perpetuity unless an intervention is made (Hobbs et al. Reference Hobbs, Higgs and Harris2009). Despite the importance of these temporal dynamics, our understanding of legacy effects is limited but may have implications for achieving management goals.
In addition to the “back end” of invasion leading to potential legacy effects, even less is known about the accrual of invasive plant impacts following establishment of a nascent invasion. Over time, nascent invasions will increase in size and density, two factors that we know influence the magnitude of ecological impacts in some circumstances (Barney et al. Reference Barney, Tekiela, Dollete and Tomasek2013). However, invasive plant impacts have been studied almost exclusively using established populations, usually of unknown age, precluding identification of how impacts change with time (Kumschick et al. Reference Kumschick, Gaertner, Vila, Essl, Jeschke, Pysek, Ricciardi, Bacher, Blackburn, Dick, Evans, Hulme, Kuhn, Mruga, Pergl, Rabitsch, Richardson, Sendek and Winter2015). Understanding whether impacts occur immediately or accrue slowly with population age will aid development of appropriate management objectives based on impact dynamics (Barney Reference Barney2016).
Temporal effects of invasive plant impacts are an important yet poorly understood element of invasion dynamics with potential implications for management and restoration. First, because management itself can have negative impacts, and invasive plants can have legacy effects, the choice to manage invasive plants should be carefully considered and driven by an understanding of invader impacts (Barney Reference Barney2016). Some studies have shown legacy effects in single-metric impacts (e.g., Grove et al. Reference Grove, Parker and Haubensak2015; Holdredge and Bertness Reference Holdredge and Bertness2010), but no study to date has looked at both biotic and abiotic characteristics of the environment following invader removal or impact accrual following establishment. In fact, few studies consider temporal effects at all either on the front end or back end of an invasion (unpublished data). Here we determine whether Japanese stiltgrass (Microstegium vimineum) has temporal impacts on biotic and abiotic environmental characteristics and, if so, at what rate they change following both removal and establishment.
Materials and Methods
Three sites were established across the Virginia section of the Ridge and Valley at Pandapas Pond Recreation Area in Montgomery County, VA (37.281088°N, 80.475236°W), Peaks of Otter Recreation Area in Jefferson National Forest, Bedford County, VA (37.442586°N, 79.612103°W), and Babbling Springs Recreation Area in George Washington National Forest, Rockbridge County, VA (37.926001°N, 79.605503°W), as described in Tekiela and Barney (Reference Tekiela and Barney2015). The experimental design of Barney et al. (Reference Barney, Tekiela, Barrios-Garcia, Dimarco, Hufbauer, Leipzig-Scott, Nuñez, Pauchard, Pyšek, Vítková and Maxwell2015) was followed in accordance with the Global Invader Impact Network (GIIN) and described in Tekiela and Barney (Reference Tekiela and Barney2015); however, a summary of methods follows. In 2013, at each location, four treatments were established: two randomly placed within the invasion and two within the uninvaded site. Within the invasion we installed spatially paired 1.75 by 1.75 m invaded (IN) and removal (RE) plots (i.e., 3.50 by 1.75 m) for a total of 22 replicates. IN plots were left unmanipulated for the duration of the study. RE plots were managed in June of each year to remove all M. vimineum individuals by hand pulling, and then remanaged in July to remove any new seedlings. Within the uninvaded site, we installed uninvaded (UN) and seeded (SE) plots. UN plots were left unmanipulated to represent an uninvaded forest understory. SE plots were sown with M. vimineum seed at the same density as the surrounding invasion in May 2013.
Data collection occurred in late July of 2013 through 2015, approximately at peak growing season, to capture the greatest influence of M. vimineum. Each vascular plant in each plot was identified to species, and the percent ground cover was assessed to the nearest 1%. Five 1-cm-diameter by 10-cm-deep soil samples were collected in each plot and homogenized. These samples were then dried, sieved (4-mm sieve), and analyzed for soil macro- (N, P, and K) and micro- (Ca, Mg, Zn, Mn, Cu, Fe, and B) nutrients, pH, and cation exchange capacity. Soil moisture was measured using three subsamples of an electronic soil moisture probe, and soil infiltration rate was calculated using a randomly placed single 10-cm-diameter ring driven 10 cm into the ground and infiltrated with 600 ml of water. Additionally, light penetration was calculated by measuring photosynthetic active radiation (PAR) above and below the forest understory layer across three equidistant transects within each plot.
To test the effect of removals and seeding on individual ecosystem metrics, plant richness, native richness, and invasive richness were measured. An analysis of variance (ANOVA) was run on each dependent variable using removal year (1, 2, or 3), treatment, site, and the interaction of treatment by removal year as fixed effects (Bolker et al. Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009). Means were separated using post hoc Tukey’s honest significant difference (HSD).
We also wanted to compare the similarities between plant communities in both species identity and abundance, which is not possible with standard richness or biodiversity metrics. Similarity metrics can compare unique species among plots, but they only consider presence/absence of species and are not capable of considering abundance/prevalence (Ricotta and Szeidl Reference Ricotta and Szeidl2006). Biodiversity metrics are capable of describing the relative abundance within an area but are not capable of making species composition comparisons. Both elements are important for comparing plant community composition. Therefore, we developed a new method to estimate the similarity of species composition, similar to a TWINSPAN analysis (Hill Reference Hill1979), that explicitly accounts for species identity and abundance by describing the “plant community distance” between each treatment.
First, all resident plant species percent cover data, excluding data for M. vimineum, were subjected to a principal component analysis that was varimax rotated to center. Then, a factor analysis was used to reduce the complexity of the data by removing any components with variance explained <1. This reduced the plant community from 148 species to 60 components. Because of the flexibility of this analysis, we used the same method to incorporate and reduce complexity of all abiotic metrics, which reduced 15 ecosystem properties to 6 components to also test the similarity between overall ecosystem characteristics.
We analyzed plant community and ecosystem metric data sets separately. The center of mass (COM) for IN and UN treatments was calculated independently for each year by each site in multivariate space by averaging the plots in each treatment (i.e., IN or UN) across each component (6 or 10 respective to site). Then, Euclidean distances were calculated to measure the distance between individual plots and the COM (i.e., correct removal year and site) of each treatment within multivariate space for plant community composition and ecosystem properties separately. This means, for example, when comparing the COM of IN to IN plots, the average distance describes the variation within the treatment. ANOVA was performed on the log-transformed Euclidean distance of each treatment to the COM of IN and UN plots, because these two are the “original state” references using removal year, treatment, and site as main effects and the interactions treatment by removal year. Means were separated using post hoc Tukey’s HSD.
Results
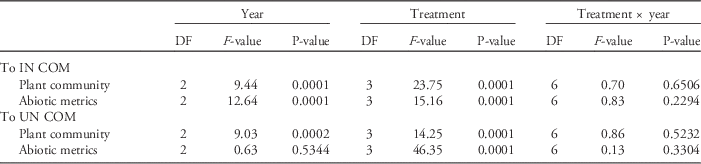
For metrics evaluated independently, a significant treatment by year interaction term would mean the relationship among treatments changed from year to year, which is what we predicted. While there were no significant interactions for any of the abiotic metrics, there were treatment differences for all abiotic metrics except CEC, P, Fe, and soil infiltration (Table 1). Of the metrics that varied among treatments, pH, K, Zn, Cu, and soil moisture did not show a significant difference between IN and RE plots, while Ca, Mg, Mn, B, and light penetration did show a significant difference between IN and RE plots (Figure 1). Only Zn showed a difference between IN and SE plots (Figure 1).
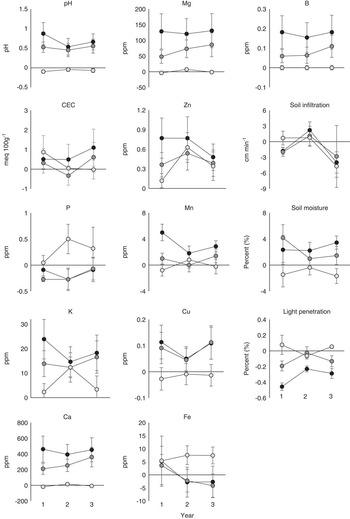
Figure 1 Difference between individual abiotic metrics of invaded (IN, black circles), removed (RE, dark gray circles), and seeded (SE, light gray circles) plots compared with uninvaded (UN) plots for each year after initial removal and seeding.
For all measurements of plant richness, treatment effects did not change over the course of the experiment (Table 1). RE plots had the greatest total plant richness, and IN plots had fewer species than UN plots (Table 1; Figure 2). RE and UN plots had greater native richness compared with SE and IN plots (Table 1; Figure 2), and RE and IN plots had the greatest invasive richness, while UN had lowest invasive richness (Table 1; Figure 2).
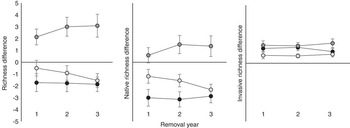
Figure 2 Difference between total richness, native richness, and invasive richness of invaded (IN, black circles), removed (RE, dark gray circles), and seeded (SE, light gray circles) plots compared with uninvaded (UN) plot richness for each year after initial removal and seeding.
When the abiotic metrics were integrated and their distances measured to the COM of the reference UN and IN plots, it was found that their relationships did not change over the course of the experiment (Table 2). IN plots were least similar to the COM of UN plots, while SE plots were most similar to the COM of UN across all years (Table 2; Figure 3). UN and SE plots were equally dissimilar to the COM of IN, and RE plots were not significantly different from IN plots (Figure 1).

Figure 3 The Euclidean distances to the multidimensional center of mass (COM) for the plant community and abiotic metrics of both uninvaded (UN) and invaded (IN) plots for each year. UN plots are white, seeded (SE) plots are light gray, removal (RE) plots are dark gray, and IN plots are black. Greater distances suggest less similarity to the respective COM.
Table 2 Statistics from ANOVA of the integrated plant community and abiotic metrics comparing distance of plots to multidimensional center of mass (COM) of invaded (IN) and uninvaded (UN) plots.Footnote a
a See Figure 2 for distance to COM means.
As with the abiotic metrics, the integrated plant communities showed no treatment variation over the course of the experiment (Table 2). Additionally, there was no difference among SE, UN, and IN plot richness in relation to the COM of UN; only RE plots were different (Figure 3). IN plots were most similar to the COM of IN; however, SE plots were more similar to the COM of IN than RA plots (Figure 3).
Discussion
Consistent with other studies on the ecological impacts of M. vimineum to forest understories (e.g., Ehrenfeld et al. Reference Ehrenfeld, Kourtev and Huang2001; Kourtev et al. Reference Kourtev, Ehrenfeld and Huang1998, Reference Kourtev, Ehrenfeld and Häggblom2003; McGrath and Binkley Reference McGrath and Binkley2009; Tekiela and Barney Reference Tekiela and Barney2015), we found that most metrics were different in the invaded patch compared with the uninvaded forest. However, few studies have investigated the temporal dynamics of invader impacts. In our 3-yr study, the differences between the invaded and uninvaded plots remained stable. While the age of the populations we studied is unknown, the stability we observed suggests the invasions are mature (i.e., not increasing or decreasing in local density or impacts). This impact stability provides an important baseline for gauging temporal trends following invader removal and establishment.
Following annual invader removal, where M. vimineum was assumed to make no contribution to observed impacts, greater than 50% of the individual abiotic parameters remained close to invaded levels. However, although those metrics that did change shifted within the first year toward those of the uninvaded plots, the predicted gradual return to an uninvaded state was not seen (Cuddington Reference Cuddington2011). Similar to the abiotic variables, plant community richness changed immediately and dramatically following invader removal. Removal plots gained four to five more species than invaded plots, and two to three more than uninvaded plots. This may have resulted from either the small disturbance imposed by the removal of M. vimineum or a competitive release. Skurski et al. (Reference Skurski, Maxwell and Rew2013) found that different removal methods lead to different disturbances, which themselves may affect observations. Not only does the removal create a minor soil disturbance, but it also modifies soil surface light exposure, which has been shown to affect germination (Baskin and Baskin Reference Baskin and Baskin1998). Many of the species that emerged in removal plots were weedy species themselves (e.g., ladysthumb [Polygonum persicaria L.] and clearweed [Pilea pumila (L.) Gray]) that may have taken advantage of the disturbance. We cannot parse whether the additional recruitment in the removal plots was a result of reduced competition from M. vimineum, increased light availability and soil moisture, or the disturbance from management. Nevertheless, these new species clearly either recruited from a suppressed seedbank that was “released” or from surrounding vegetation following invader removal.
Contrary to our expectations, there was little evidence for temporal variation in abiotic impacts following the establishment of M. vimineum. In fact, abiotic factor levels in the seeded plots remained similar to uninvaded levels, suggesting these edaphic factors have both slow turnover rates and that the differences between invaded and uninvaded plots accrued over many years or at least in a greater time span than our study. Additionally, M. vimineum cover in the seeded plots remained at very low density for years 1 and 2 (~1%) and only began to become established in the 3rd year (20%). For many invasive plants, the impact magnitude has been shown to scale with invader cover. Various linear and nonlinear relationships have been identified in many species (Pearson et al. Reference Pearson, Ortega, Eren and Hierro2015; Tekiela and Barney Reference Tekiela and Barney2015; Thiele et al. Reference Thiele, Isermann, Kollmann and Otte2011). In fact, M. vimineum has been shown to have a potential impact threshold for many factors at ~40% cover. In other words, impacts were not observed until 40% invader cover was reached, which was not achieved in the seeded plots within the first 3 yr (Tekiela and Barney Reference Tekiela and Barney2015). Thus, the small changes we observed for most factors could also be explained by the very low M. vimineum cover.
However, when abiotic factors were integrated using multivariate techniques, much of the individual metric noise was reduced and a clear pattern emerged. Removal plots became more similar to uninvaded plots, while becoming less similar to invaded plots. Similarly, seeded plots became more similar to invaded plots. In short, removing the invader made the abiotic characteristics become more associated with an uninvaded state, while seeding an uninvaded area made it look more similar to an invaded state. Surprisingly, these shifts happened within the first year, with no additional change over the next 2 yr. This immediate change followed by persistent legacy effects has also been observed in Scotch broom [Cytisus scoparius (L.) Link] and was suggested to be due to the nitrogen pulse left by the carcasses of this nitrogen-fixing invader (Grove et al. Reference Grove, Parker and Haubensak2015). However, in our study, M. vimineum was hand managed at the seedling stage, and carcasses were removed; thus, these dramatic legacy litter effects were not possible. Although we did not examine the soil microbial community, long-lasting legacies in the microbial community (see Elgersma et al. Reference Elgersma, Ehrenfeld, Yu and Vor2011; König et al. Reference König, van Kleunen and Dawson2016) could have changed the nutrient-cycling characteristics of this system and been responsible for the soil legacies that did not appear to change in 3 yr.
The resident plant community showed minor compositional changes due to the presence of M. vimineum. In fact, removing M. vimineum may have done more harm than good and further distanced the resident plant community from an uninvaded or invaded landscape. The plant community—incorporating richness, identity, and abundance—became entirely novel with respect to both reference communities. In this case, other ruderal weedy species that were not well represented in either invaded or uninvaded plots now dominate where invader removals occurred. In the context of legacy effects, this is a worst-case scenario, as the legacy in fact makes the new plant community even less like the uninvaded landscape. One invader was replaced by a variety of additional weedy species, an occurrence that has been described as the “bane of weed management” (Pearson et al. Reference Pearson, Ortega, Runyon and Butler2016). This negative response to management is often seen in the systems of the western United States, where the removal of invasive perennial forbs does little to restore the native plant community and instead encourages establishment of new invasive annual grasses (Skurski et al. Reference Skurski, Maxwell and Rew2013). This is additionally concerning for M. vimineum invasions, because re-establishment of this primary invader is likely, potentially leaving an even poorer-quality community when management is terminated (DeMeester and Richter Reference DeMeester and Richter2009). If a return to an uninvaded state is desirable, active restoration involving reseeding of desirable species may be the only viable option, although many species of the desired native community may not have readily available commercial seed stocks.
In contrast, seeding an uninvaded area with M. vimineum did not change the resident plant community in 3yr. It remained similar to the uninvaded resident plant community and dissimilar to the invaded plant community. This may not be surprising, considering the M. vimineum cover was so low and its residence time short. Dostál et al. (Reference Dostál, Müllerová, Pyšek, Pergl and Klinerová2013) showed that newly established giant hogweed (Heracleum mantegazzianum Sommier & Levier) imposed greater reductions in species richness early in the invasion process, but those authors’ time frame was much longer, and H. mantegazzianum had much higher cover early in the invasion, further suggesting this is likely a function of cover not time. Thus, we would expect the plant community to begin to shift in the nascent invasion as M. vimineum expanded and became denser.
Here we have shown that important temporal effects do exist following both removal and establishment of M. vimineum—a dominant forest understory invader in the eastern United States. Importantly, the accrual and loss of changes differed between biotic and abiotic components and occurred at different timescales. Many responses occurred immediately after our interventions and will now either persist in a different state in perpetuity or are shifting to the new state at too slow a rate to identify within 3 yr. Most concerning from a management perspective is the shift of the resident plant community to a novel condition not seen in either the invaded or uninvaded states. If management efforts only replace a problematic invader with other weedy species, the cost of management may outweigh its gains. Temporal dynamics in relation to density of invasions is an important factor to consider when elucidating invader impacts and designing management plans.
Acknowledgments
We would like to thank Dr. Dan Atwater, Elise Benhase, Eugene Dollete, Stacy Fanning, Morgan Franke, Matthew Ho, Rose Peterson, Ryan Schmitt, and Larissa Smith for help in the field. This project is a component of the GIIN.








